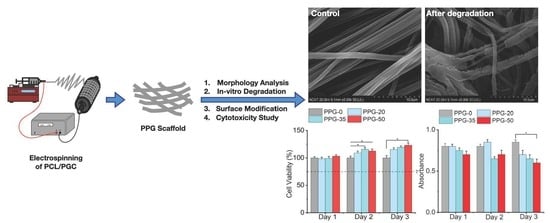
Preparation and Characterization of Poliglecaprone-Incorporated Polycaprolactone Composite Fibrous Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PCL/PGC Solution Preparation
2.3. Electrospinning of PPG Nanofibers
2.4. Surface Morphology Analysis
2.5. X-ray Diffraction Analysis
2.6. Contact Angle Measurement
2.7. Mechanical Testing
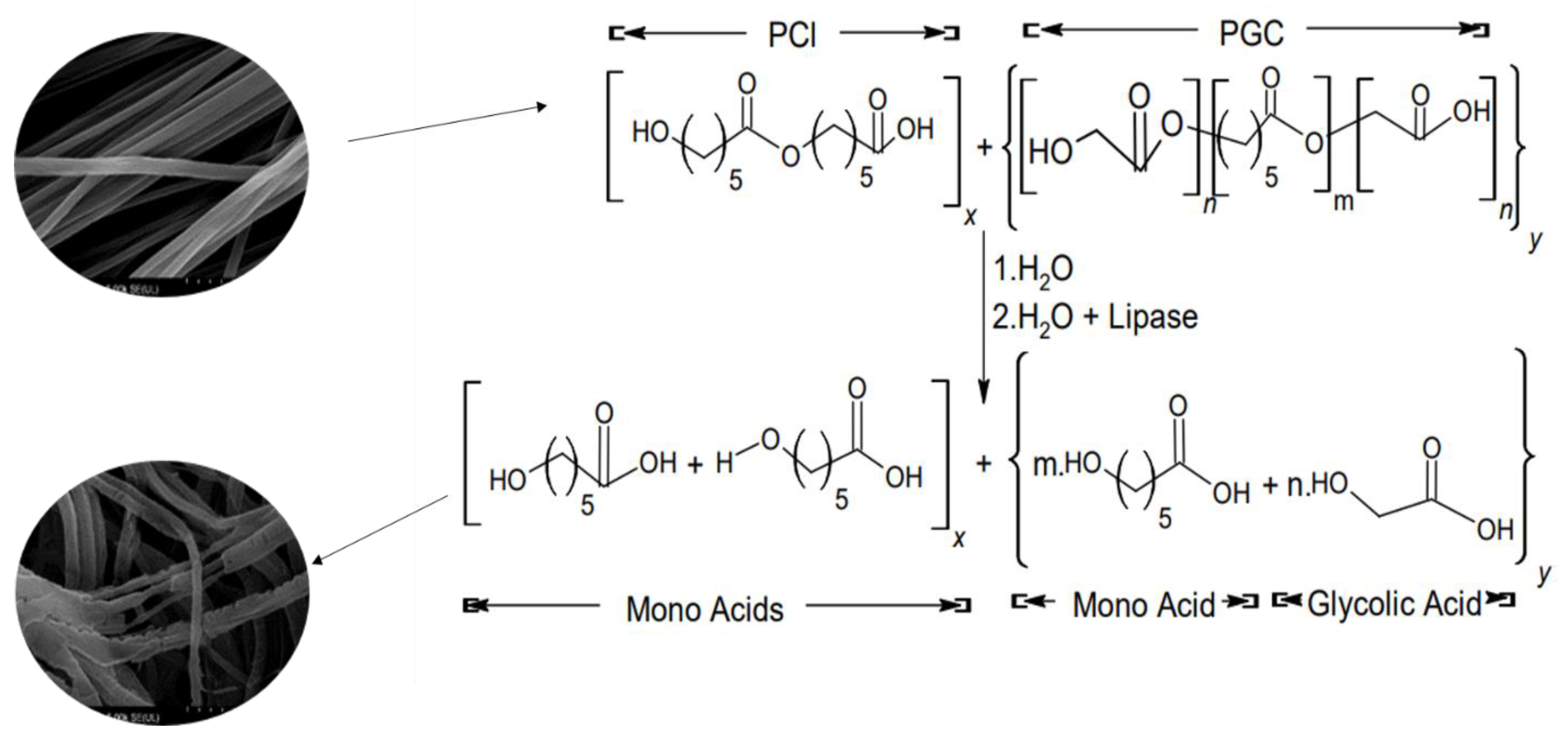
2.8. In Vitro Degradation
2.9. Surface Modification
2.10. Cell Attachment, Viability, and Toxicity Analysis
3. Results and Discussion
3.1. Morphological Analysis of PPG Nanofibers
3.2. X-ray Diffraction
3.3. Contact Angle Analysis
3.4. Mechanical Testing
3.5. In Vitro Degradation
3.6. Surface Modification
3.7. Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible synthetic polymers for tissue engineering purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Sadraei, S.M.; Mansoori, R.; Chauhan, N.P.S.; Sargazi, G. Nanomaterials supported by polymers for tissue engineering applications: A review. Heliyon 2022, 8, e12193. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Socci, M.C.; Rodríguez, G.; Oliva, E.; Fushimi, S.; Takabatake, K.; Nagatsuka, H.; Felice, C.J.; Rodríguez, A.P. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering 2023, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, L.; Sigen, A.; Gao, Y.; Zhou, D.; Greiser, U.; Creagh-Flynn, J.; Zhang, H.; Dong, Y.; Cutlar, L. Injectable hyperbranched poly (β-amino ester) hydrogels with on-demand degradation profiles to match wound healing processes. Chem. Sci. 2018, 9, 2179–2187. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Leonés Gil, A.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-based blends: Processing optimization for their scalable production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Schindler, C.; Williams, B.L.; Patel, H.N.; Thomas, V.; Dean, D.R. Electrospun polycaprolactone/polyglyconate blends: Miscibility, mechanical behavior, and degradation. Polymer 2013, 54, 6824–6833. [Google Scholar] [CrossRef]
- Dhanasekaran, N.P.D.; Muthuvelu, K.S.; Arumugasamy, S.K. Recent Advancement in Biomedical Applications of Polycaprolactone and Polycaprolactone-Based Materials. In Encyclopedia of Materials; Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 795–809. [Google Scholar]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt Electrowriting of PLA, PCL, and Composite PLA/PCL Scaffolds for Tissue Engineering Application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-Caprolactone)-Based Electrospun Nano-Featured Substrate for Tissue Engineering Applications: A Review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef]
- Patel, H.N.; Garcia, R.; Schindler, C.; Dean, D.; Pogwizd, S.M.; Singh, R.; Vohra, Y.K.; Thomas, V. Fibro-porous poliglecaprone/polycaprolactone conduits: Synergistic effect of composition and in vitro degradation on mechanical properties. Polym. Int. 2015, 64, 547–555. [Google Scholar] [CrossRef]
- Yaseri, R.; Fadaie, M.; Mirzaei, E.; Samadian, H.; Ebrahiminezhad, A. Surface Modification of Polycaprolactone Nanofibers through Hydrolysis and Aminolysis: A Comparative Study on Structural Characteristics, Mechanical Properties, and Cellular Performance. Sci. Rep. 2023, 13, 9434. [Google Scholar] [CrossRef]
- Vilar, G.; Tulla-Puche, J.; Albericio, F. Polymers and Drug Delivery Systems. Curr. Drug Deliv. 2012, 9, 367–394. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Li, D.; Yin, Y.; Zhou, F. Electrospun PHB/Chitosan Composite Fibrous Membrane and Its Degradation Behaviours in Different pH Conditions. J. Funct. Biomater. 2022, 13, 58. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and Characterization of PCL/Gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yun, S. Il Chitosan-reinforced PHB hydrogel and aerogel monoliths fabricated by phase separation with the solvent-exchange method. Carbohydr. Polym. 2022, 284, 119184. [Google Scholar] [CrossRef] [PubMed]
- Molea, G.; Schonauer, F.; Bifulco, G.; D’angelo, D. Comparative Study on Biocompatibility and Absorption Times of Three Absorbable Monofilament Suture Materials (Polydioxanone, Poliglecaprone 25, Glycomer 631). Br. J. Plast. Surg. 2000, 53, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Langley-Hobbs, S.J. Sutures and general surgical implants. In Feline Soft Tissue and General Surgery; Elsevier Ltd.: New York, NY, USA, 2014; pp. 105–116. [Google Scholar]
- Manivasagam, G.; Reddy, A.; Sen, D.; Nayak, S.; Mathew, M.T.; Rajamanikam, A. Dentistry: Restorative and Regenerative Approaches; Narayan, R.B.T.-E., Ed.; Elsevier: Oxford, UK, 2019; pp. 332–347. [Google Scholar]
- Niles, J.; Williams, J. Suture Materials and Patterns. In Pract. 1999, 21, 308–320. [Google Scholar] [CrossRef]
- Trott, A.T. Chapter 8—Instruments, Suture Materials, and Closure Choices. In Wounds Lacerations, 4th ed.; Trott, A.T., Ed.; WB Saunders: Philadelphia, PA, USA, 2012; pp. 82–94. [Google Scholar]
- Khiste, S.V.; Ranganath, V.; Nichani, A.S. Evaluation of Tensile Strength of Surgical Synthetic Absorbable Suture Materials: An in Vitro Study. J. Periodontal Implant. Sci. 2013, 43, 130–135. [Google Scholar] [CrossRef]
- Zhang, X.; Thomas, V.; Xu, Y.; Bellis, S.L.; Vohra, Y.K. An in vitro regenerated functional human endothelium on a nanofibrous electrospun scaffold. Biomaterials 2010, 31, 4376–4381. [Google Scholar] [CrossRef]
- Zhang, X.; Thomas, V.; Vohra, Y.K. Two ply tubular scaffolds comprised of proteins/poliglecaprone/polycaprolactone fibers. J. Mater. Sci. Mater. Med. 2010, 21, 541–549. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Zhao, Y.; Ma, P.; Zha, S.; Chen, P.; Lu, H.; Jiang, X.; Wan, S.; Luo, J. Dual-peptide-functionalized nanofibrous scaffolds recruit host endothelial progenitor cells for vasculogenesis to repair calvarial defects. ACS Appl. Mater. Interfaces 2019, 12, 3474–3493. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun Composite Mats of Poly [(D, L-lactide)-co-glycolide] and Collagen with High Porosity as Potential Scaffolds for Skin Tissue Engineering. Macromol. Mater. Eng. 2009, 294, 611–619. [Google Scholar] [CrossRef]
- Malakpour-Permlid, A.; Buzzi, I.; Hegardt, C.; Johansson, F.; Oredsson, S. Identification of Extracellular Matrix Proteins Secreted by Human Dermal Fibroblasts Cultured in 3D Electrospun Scaffolds. Sci. Rep. 2021, 11, 6655. [Google Scholar] [CrossRef]
- Nelson, M.T.; Short, A.; Cole, S.L.; Gross, A.C.; Winter, J.; Eubank, T.D.; Lannutti, J.J. Preferential, enhanced breast cancer cell migration on biomimetic electrospun nanofiber ‘cell highways’. BMC Cancer 2014, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Poyraz, Ş.; Altınışık, Z.; Çakmak, A.S.; Şimşek, M.; Gümüşderelioğlu, M. Random/aligned electrospun PCL fibrous matrices with modified surface textures: Characterization and interactions with dermal fibroblasts and keratinocytes. Colloids Surf. B Biointerfaces 2022, 218, 112724. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. Part A 2009, 90A, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.N.; Vohra, Y.K.; Singh, R.K.; Thomas, V. HuBiogel incorporated fibro-porous hybrid nanomatrix graft for vascular tissue interfaces. Mater. Today Chem. 2020, 17, 100323. [Google Scholar] [CrossRef]
- Saudi, S.; Bhattarai, S.R.; Adhikari, U.; Khanal, S.; Sankar, J.; Aravamudhan, S.; Bhattarai, N. Nanonet-nano fiber electrospun mesh of PCL–chitosan for controlled and extended release of diclofenac sodium. Nanoscale 2020, 12, 23556–23569. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Bhattarai, N.; Ashie, M.D.; Tettey, F.; Yusa, S.; Nakashima, K. Single-Micelle-Templated Synthesis of Hollow Barium Carbonate Nanoparticle for Drug Delivery. Polymers 2023, 15, 1739. [Google Scholar] [CrossRef]
- Tatum, S.D.; Saudi, S.; Tettey, F.; Bhandari, R.K.; Bhattarai, N. A Novel Hydrogel-Bronchial Epithelial Cell Spheroids for Toxicological Evaluation. Biomed. Sci. Instrum. 2021, 57, 4. [Google Scholar] [CrossRef]
- Yin, H.; Gao, M.; Leoni, L.; Han, H.; Zhang, X.; Fu, Z. The therapeutic role of monocyte chemoattractant protein-1 in a renal tissue engineering strategy for diabetic patients. PLoS ONE 2013, 8, e57635. [Google Scholar] [CrossRef]
- Pratten, D.H.; Craig, R.G. Wettability of a Hydrophilic Addition Silicone Impression Material. J. Prosthet. Dent. 1989, 61, 197–202. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef]
- Lacroix, D.; Prendergast, P.J. A mechano-regulation model for tissue differentiation during fracture healing: Analysis of gap size and loading. J. Biomech. 2002, 35, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.A.; Prendergast, P.J. Discriminating the loosening behaviour of cemented hip prostheses using measurements of migration and inducible displacement. J. Biomech. 2002, 35, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.N.; Thai, K.N.; Chowdhury, S.; Singh, R.; Vohra, Y.K.; Thomas, V. In vitro degradation and cell attachment studies of a new electrospun polymeric tubular graft. Prog. Biomater. 2015, 4, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hetemi, D.; Pinson, J. Surface functionalisation of polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Park, C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef]
- Bikmulina, P.; Kosheleva, N.; Efremov, Y.; Antoshin, A.; Heydari, Z.; Kapustina, V.; Royuk, V.; Mikhaylov, V.; Fomin, V.; Vosough, M. 3D or not 3D: A guide to assess cell viability in 3D cell systems. Soft Matter 2022, 18, 2222–2233. [Google Scholar] [CrossRef]








| Nanofiber Sample | Proportion of PCL:PGC (w/w) | Concentration (%) |
|---|---|---|
| PPG-0 | 100:0 | 14 |
| PPG-20 | 100:20 | 14 |
| PPG-35 | 100:35 | 14 |
| PPG-50 | 100:50 | 14 |
| Nanofiber Sample | Averaged Contact Angle Measurement + S.D. |
|---|---|
| PPG-0 | 137 ± 10.4 |
| PPG-20 | 54 ± 3.5 |
| PPG-35 | 47 ± 4.4 |
| PPG-50 | 45 ± 6.1 |
| Fiber Composition | Modulus of Elasticity (MPa) | Ultimate Tensile Strength (MPa) |
|---|---|---|
| PPG-0 | 14.6 ± 5.34 | 3.64 ± 0.07 |
| PPG-20 | 23.9 ± 3.32 | 4.12 ± 0.05 |
| PPG-35 | 28.4 ± 4.91 | 4.88 ± 0.05 |
| PPG-50 | 36.3 ± 9.76 | 6.11 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tettey, F.; Siler-Dearring, J.; Moody, A.; Bhattarai, N. Preparation and Characterization of Poliglecaprone-Incorporated Polycaprolactone Composite Fibrous Scaffolds. Fibers 2023, 11, 82. https://doi.org/10.3390/fib11100082
Tettey F, Siler-Dearring J, Moody A, Bhattarai N. Preparation and Characterization of Poliglecaprone-Incorporated Polycaprolactone Composite Fibrous Scaffolds. Fibers. 2023; 11(10):82. https://doi.org/10.3390/fib11100082
Chicago/Turabian StyleTettey, Felix, Jaclynn Siler-Dearring, Alexis Moody, and Narayan Bhattarai. 2023. "Preparation and Characterization of Poliglecaprone-Incorporated Polycaprolactone Composite Fibrous Scaffolds" Fibers 11, no. 10: 82. https://doi.org/10.3390/fib11100082
APA StyleTettey, F., Siler-Dearring, J., Moody, A., & Bhattarai, N. (2023). Preparation and Characterization of Poliglecaprone-Incorporated Polycaprolactone Composite Fibrous Scaffolds. Fibers, 11(10), 82. https://doi.org/10.3390/fib11100082