1. Introduction
Marine biofouling is the growth and accumulation of micro and macro-organisms on wet surfaces in saline environments and it is a global problem disturbing aquatic industries. Biofouling can reduce the speed of ships, boost fuel consumption, and stimulate corrosion, which provokes mechanical deterioration of static structures [
1]. Biofouling takes place on almost all surfaces starting with the attachment of organic molecules pursued by adherence of bacteria, diatoms, and microalgae to create a biofilm that is followed by the adhesion of macro-organisms, such as barnacles, bivalves, and macro-algae [
2,
3]. Priyanka et al. [
4] have demonstrated that photocatalytic material can prevent biofouling by generating reactive oxygen species under visible light irradiation, which in a marine environment can lead to cell death in a variety of contaminants [
5,
6,
7].
The corrosion of metals in natural seawater is a consequence of interactions between metal, living organisms, and seawater composition [
8]. When a metal is submerged into seawater, corrosion, and biofouling take place on almost the same time proportions [
9]. This is usually pursued by the development of biofilm within days after submersion [
10] and after a continued exposure, macrobiota like invertebrate larvae may combine with the biofilm and settle on the surface [
11].
To solve this problem, we address two functional properties: photocatalysis, which is related to the surface properties to avoid the initial step of attachment, and corrosion, to protect the steel used in the mechanical system. Cathodic protection systems might be able to polarize all underwater steel structures to a potential between −800 and −1100 mV vs. the Ag/AgCl/seawater reference electrode, and to preserve the potential in this interval throughout the life of the structures [
12].
Titanium is the most abundant transition metal on earth and its metal oxide TiO
2 is a wide band-gap semiconductor with high chemical stability, low-cost, non-toxicity, and high photoelectric conversion productivity. In recent years, titanium dioxide (TiO
2) has been extensively investigated for its remarkable UV-photocatalytic and self-cleaning properties, which, for example, can promote the decomposition of organics present into harmless products under UV light irradiation [
13]. The extent of the photo-activity depends on an extensive variety of properties, such as morphology, crystallinity, and surface area.
TiO
2 possesses unique electronic properties combined with the possibility of easy nanostructuring and chemical stability. Typically, when the surface of a semiconductor material is exposed to photons having energy greater than or equal to its band-gap energy, photo-electrons e
–, and photo-holes h
+ are produced [
14]. As the photocatalyst is simultaneously capable of adsorbing reactants and absorbing photons, the photogenerated e
– and h
+ are thus capable of reducing and oxidizing pollutants adsorbed on the photocatalyst’s surface [
15].
Nano and microstructures play an important role in enhancing the performance of devices, mainly due to the increase of the specific surface area [
16] as for photocatalyzed reactions. TiO
2 structures that have a high mesoporosity and surface area are mostly attractive, as these increase the efficiency of photocatalysis phenomena taking place at the interfaces. Morphology, structure, phase, and dimensionality adjust physical and chemical properties of the TiO
2 nanostructures. Tian [
16] demonstrated that 3D flower-like TiO
2 showed the highest UV light absorption when integrated in dye-sensitized solar cells (DSSC) compared to nanospheres and nanorod structures; even the photocatalytic activity (97% degradation efficiency) for flowers was higher than for spheres or rod-like structures (60% and 55%, respectively). These are mainly due to the fact that 3D hierarchical flower-like structures are easily accessible to light and reactants thanks to an increased effective surface area.
Zhu et al. [
17] also demonstrated that anatase TiO
2 materials having flowerlike morphologies with a high specific surface area presented an excellent performance when tested in photodegradation of methylene blue.
Aerosol assisted metal–organic chemical vapor deposition (AA-MOCVD) has been already demonstrated to deposit TiO
2 films having hierarchical TiO
2 microflowers. Biswas et al. [
18] showed that AA-MOCVD working at atmospheric pressure allows the synthesis of TiO
2 microflowers physically adhered to a thin film of TiO
2. We propose using these microstructures as a TiO
2 active layer in the bilayer coating.
Aluminum and its alloys are extensively used in many industrial applications as long as their open circuit potential is more negative than that of steel implying its use as sacrificial materials for the protection of steel. Aluminum-based alloy coatings have been extensively studied and proposed as potential candidates for galvanic corrosion protection of mechanical steel parts [
19,
20,
21,
22,
23,
24,
25]. For instance, transition metals (TM) can be added to mechanically reinforce aluminum. The low solubility of TM in aluminum allows the deposition of extended supersaturated solid solution of TM in aluminum. Moreover, a good thermal stability due to the low diffusivity of TM in aluminum is expected. Addition of high content of TM conducts typically to high mechanical properties compared to pure aluminum but with the detriment of sacrificial properties [
26,
27]. The ideal solution in terms of protection of steels agrees to a compromise between mechanical and corrosion properties.
Zirconium is a transition metal, which is known as a great grain refiner in traditional aluminum alloys [
28]. It boosts mechanical properties [
28] and improves considerably the corrosion resistance in deaerated borate-boric acid solution containing Cl
− [
29]. Different to traditional methods where the Zr solid solubility is very low, non-equilibrium processing methods like rapid solidification [
30], mechanical alloying [
31], and sputtering technique [
29,
32,
33] offer the possibility to achieve a supersaturated single solid solution with high Zr contents. In order to improve the efficiency of aluminum anodes, they are typically alloyed with other elements to encourage depassivation (breakdown of the oxide film) and/or shift the operating potential of the metal towards a more negative direction. Addition of alloying elements like Zr is characterized by an increase of the corrosion potential. Moreover, in the same time, increasing Zr content in Al allows one to lower the corrosion current density, which is associated with better corrosion resistance [
34]. Al–Zr with 4 at.% Zr appears as the best compromise between the good corrosion behavior and the sacrificial character of the coating.
In this work, we propose a bilayer film consisting of a sputtered deposited crystalline Al–Zr film containing 4 at.% of Zirconium with a thickness of about 3 µm as a sacrificial anode coating, and a TiO2 film deposited by the Aerosol CVD technique responsible for enhanced photocatalytic properties. The proposed functionalized coating is a promising candidate for an anti-fouling system in sea water.
3. Results and Discussion
Polished high-speed steel (HSS) substrates were systematically covered with a previously optimized atomic composition Al–Zr coating [
34]. Then, TiO
2 films were deposited at different temperatures to determine the deposition conditions allowing the formation of flower-like microstructures, as described by Biswas et al. [
18] In this previous study, they proved that AACVD (aerosol-assisted chemical vapor deposition) allows the deposition of dense TiO
2 layers containing hierarchical microflowers crystallized in the anatase phase and presenting a milky texture as a result of light scattering from the flowers. It was demonstrated that by adjusting the deposition conditions, it is possible to obtain TiO
2 films containing TiO
2 hierarchical microflowers on the surface [
18]. Nevertheless, the formation of these hierarchical structures is obtained for a limited deposition condition window. These conditions are often reactor dependent and have to be tuned. To find the optimal deposition conditions, the solution concentration and solution feeding rate were fixed and deposition temperature varied between 500 and 550 °C. A feeding rate between 3 and 4 mL/min allows the formation of microflowers.
3.1. Morphology of Al–Zr and TiO2 Films
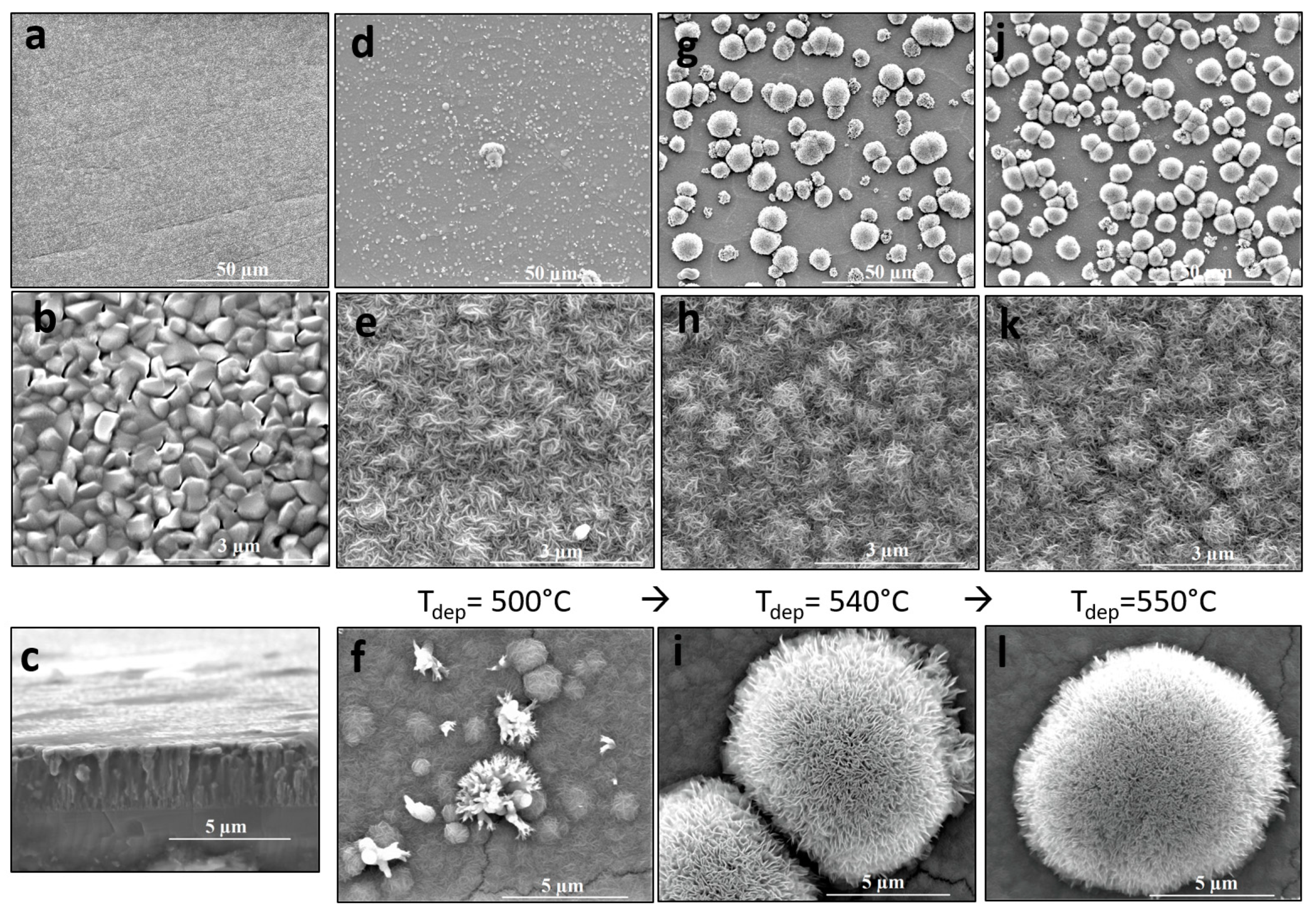
The morphology of as-deposited Al–Zr films observed by SEM is shown in
Figure 1a,b for low and high magnifications conditions in the upper and lower pictures, respectively, and in cross-section represented in
Figure 1c. For low Zr contents like in our case and in our deposition conditions, the coatings grow with a columnar morphology forming big grains of around 500 nm of diameter. The Al–Zr coating thickness is 2.7 µm. The hardness of this film is 2.3 Gpa and Young’s modulus E is 94.46 Gpa. This material is susceptible to limit cracking from the top layer (
Figure 1f) to the Al–Zr/steel interface. Nanoindentation experiment was impossible on the TiO
2 top layer surface because of its particular morphology.
When TiO
2 is deposited by AACVD as a second layer on the Al–Zr coating, two kinds of features appear. On one hand, a film consisting in small needle-like (dendritic microspheres) grains of 50 nm covered the Al–Zr grains in a very conformal way. This morphology can be observed in
Figure 1e,h,k), for the three deposition temperatures used. The Al–Zr grains can be still identified even if TiO
2 film thicknesses are around 1.4 µm. On the other hand, microflowers started to grow for deposition temperatures higher than 500 °C. At 500 °C, represented in
Figure 1d–f, some sparse flowers can be observed but much smaller than those shown in
Figure 1g–l) for films deposited at 540 and 550 °C, respectively. For films deposited at 540 and 550 °C, the flowers density increases. Flowers having an average diameter of about 7 µm can be described by a homocentric association of TiO
2 nanopetals forming a corolla as if growing from the receptacle of a flower. The main characteristics of these microstructure is that petals have a nanometric thickness (~5 nm) and are formed by nanocrystal of anatase phase as we show in the next section.
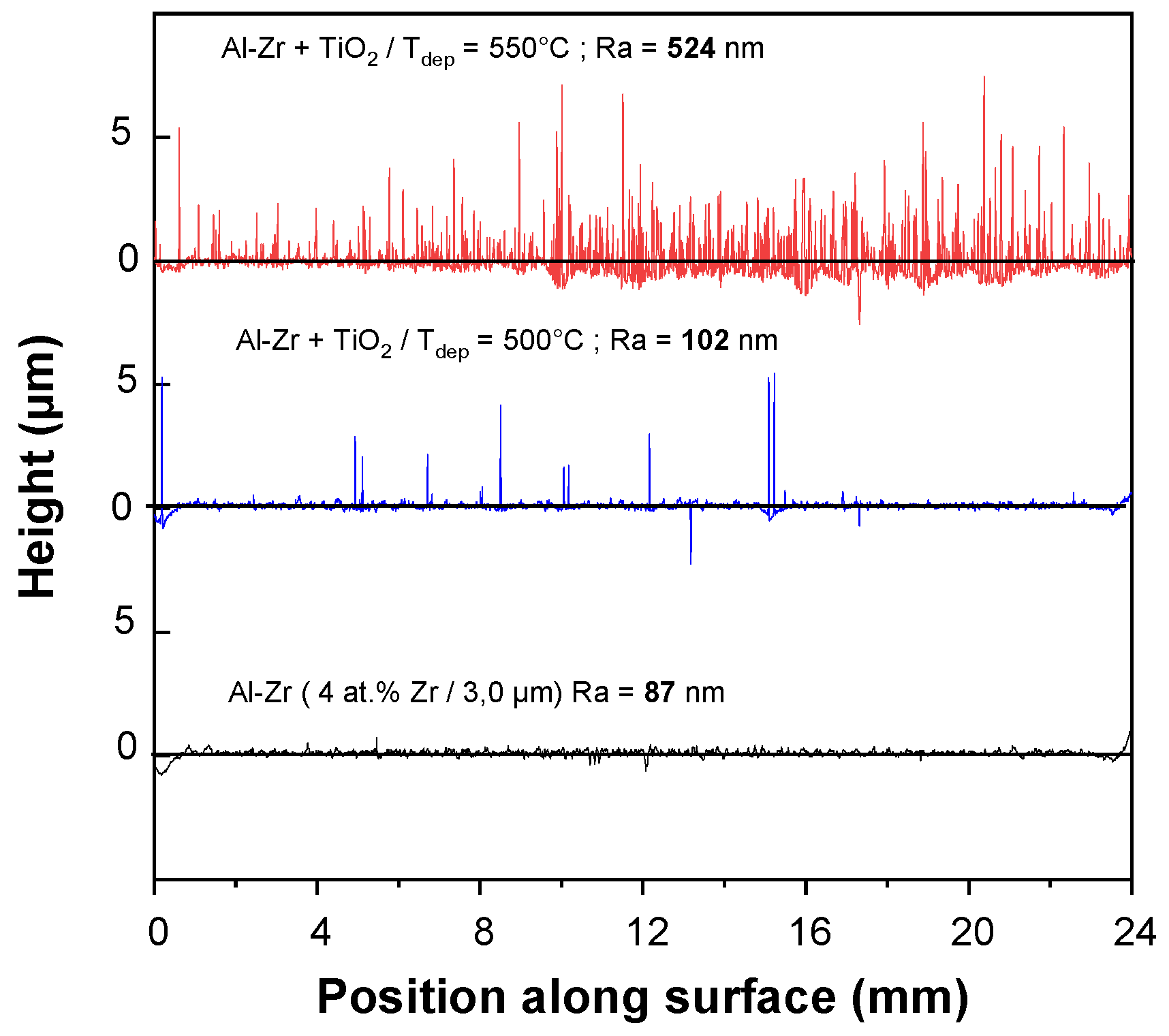
Figure 2 presents the surface roughness profiles for the HSS/Al–Zr sample and for the TiO
2/AlZr bilayer sample deposited at 500 and 550 °C.
Ra values were measured using contact mode, where the measuring instrument was in direct contact with the surface.
Al–Zr monolayer film presents a rough surface with a Ra value of 87 nm. The roughness increases when TiO2 layer is added and also when the deposition temperature increases. The TiO2 film deposited at 500 °C, reproduces the Al–Zr baseline, with sparse peaks corresponding to the presence of microflowers. For TiO2 deposited at 540 °C, the density of microflowers is so high that provokes a very high roughness with an average Ra = 524 nm. The highest variation in height corresponds to structures of 7 µm, which are associated to the height of the biggest microflowers dispersed at the top of the layer.
3.2. Structure Analysis
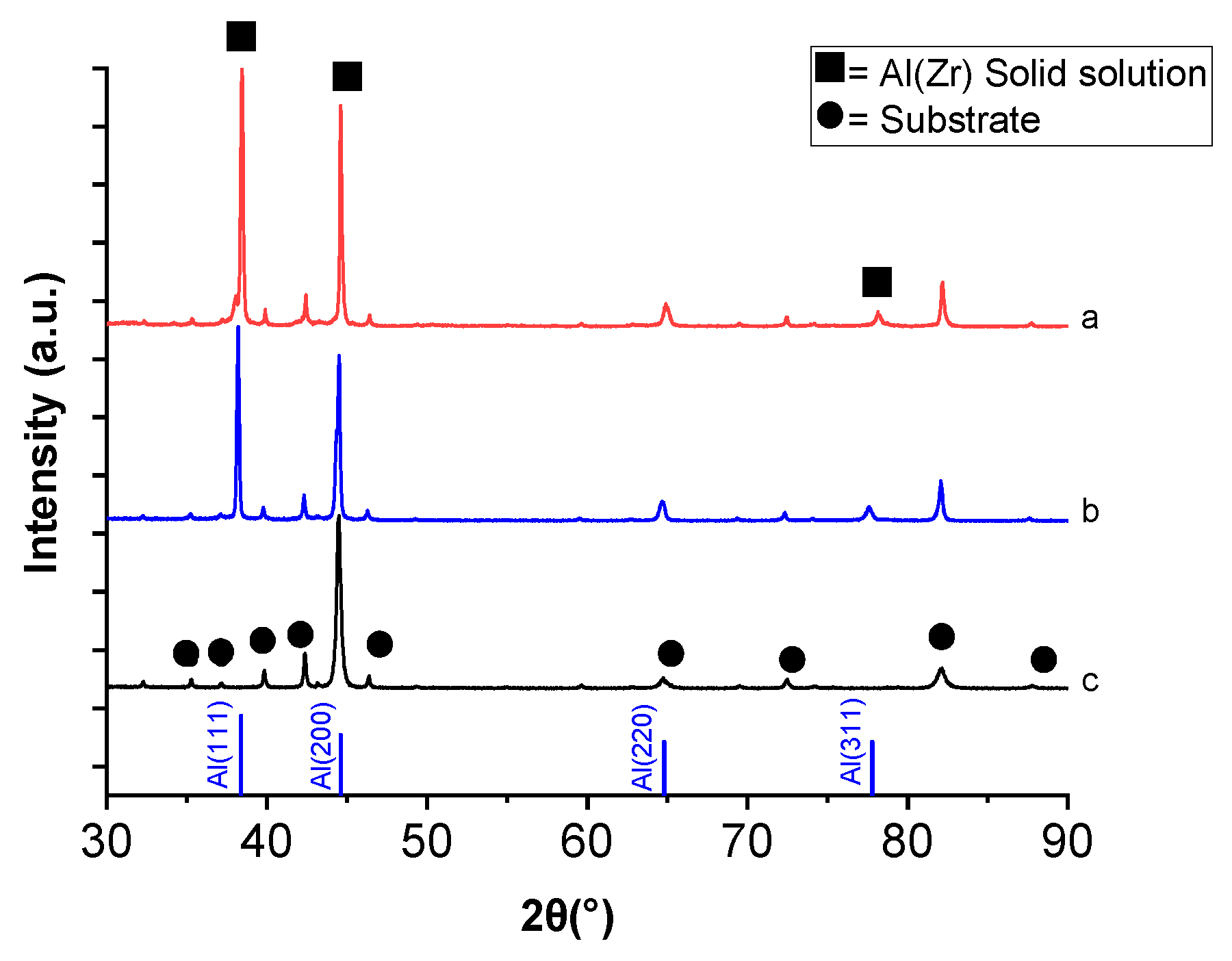
The structure of Al–Zr (4 at.% Zr) films was studied by XRD for the as deposited and also after annealing at 500 °C in air. The annealing was performed in order to check the Al–Zr stability during the TiO2 deposition by AACVD.
Figure 3 shows the XRD patterns for the HSS substrate together with the Al–Zr (4 at.% Zr) films deposited by DC magnetron sputtering in as-deposited conditions and after thermal annealing. HSS substrate is characterized by a large amount of Fe–Cr phase. The diffraction peaks in the diffractogram corresponding to as-deposited Al–Zr film can be correlated to an fcc structure indicating the growth of a supersaturated solid solution of Zr in Al without the presence of any parasitic phase.
When comparing the XRD patterns of Al–Zr film as deposited and annealed in air at 500 °C, no main changes are found; in both cases, the diffraction peaks of aluminum, with a very high intensity of (111) diffraction peak at 38° is obtained, suggesting the presence of α-Al solid solution.
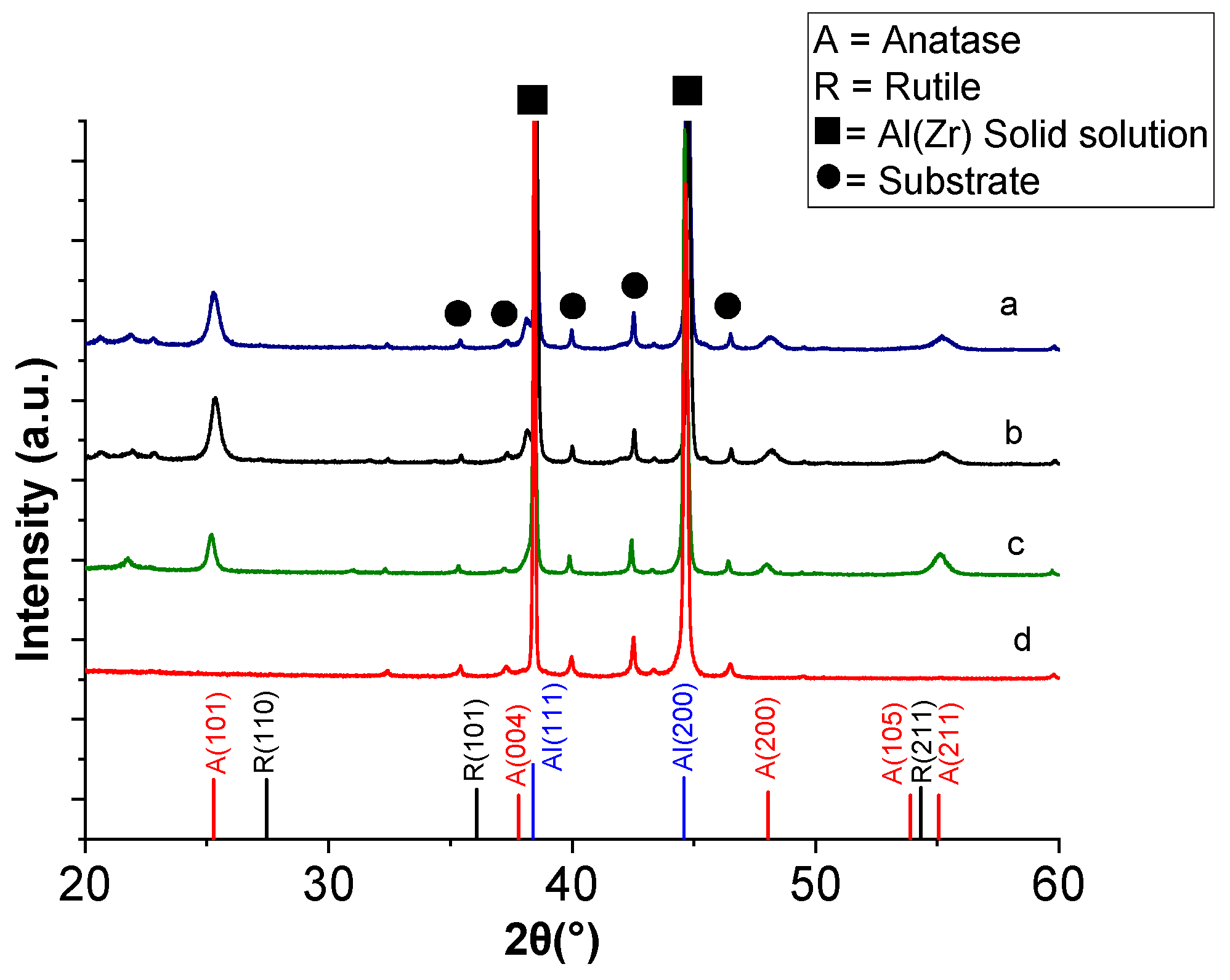
XRD characterisation was also performed after deposition of TiO
2 at 500, 540 and 550 °C on the Al–Zr coated substrates for a deposition time of 40 min. The XRD patterns of these TiO
2 coatings are shown in
Figure 4. They reveal anatase phase in all samples, mainly detected by the (101) and (200) diffraction peaks at 25° and 48°, respectively, without the presence of a rutile phase, even for the highest deposition temperature of 550 °C. This is favorable because rutile has a lower charge-transfer rate and higher recombination rate of photogenerated electron–hole pairs than anatase [
38].
Many works have been reported in the literature about the Raman signature of the different TiO
2 phases [
39]. Moreover, this technique is more sensitive to anatase, rutile, and amorphous phases of TiO
2 films than XRD [
40]. Besides, the technique is very local, allowing one to probe different microstructures at the sample surface.
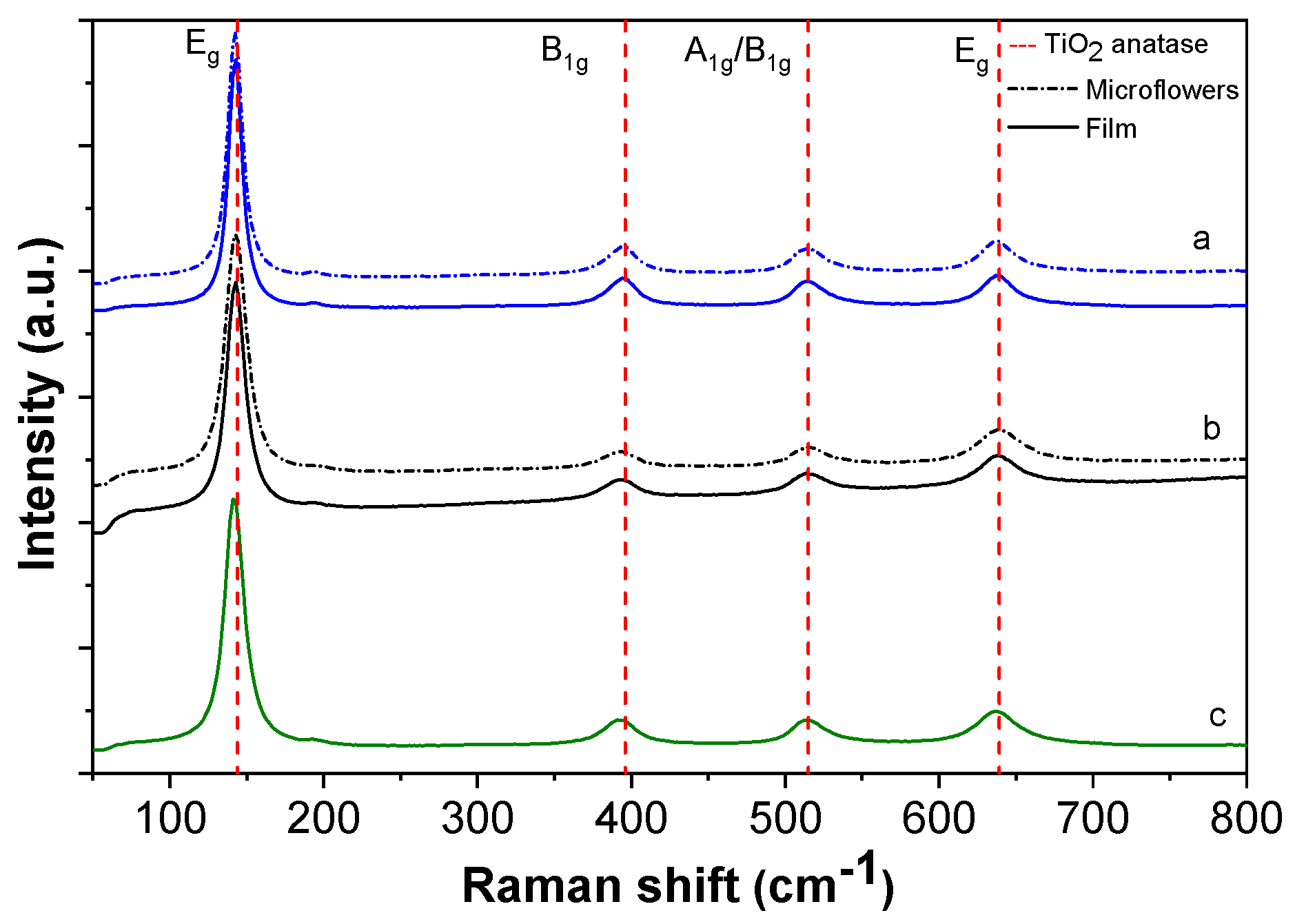
Figure 5 shows the Raman spectra of TiO
2/Al–Zr multilayers for films deposited at different temperatures ranging between 500 and 550 °C. The Raman spectra of the film containing microflowers on the surface shows characteristic modes of the TiO
2 anatase phase [
41]: two
Eg modes at 141 and 635 cm
−1, one A
1g mode at 513 cm
−1 and one B
1g mode at 397.5 cm
−1. The sharp peak at 144 cm
−1 clearly identifies the anatase phase of TiO
2. Smaller peaks at 398 and 639 cm
−1 can also be assigned to the anatase phase. No band corresponding to the rutile phase of TiO
2 was observed. Moreover, the absence of a broadband background in the Raman spectra allows one to discard the growth of amorphous TiO
2.
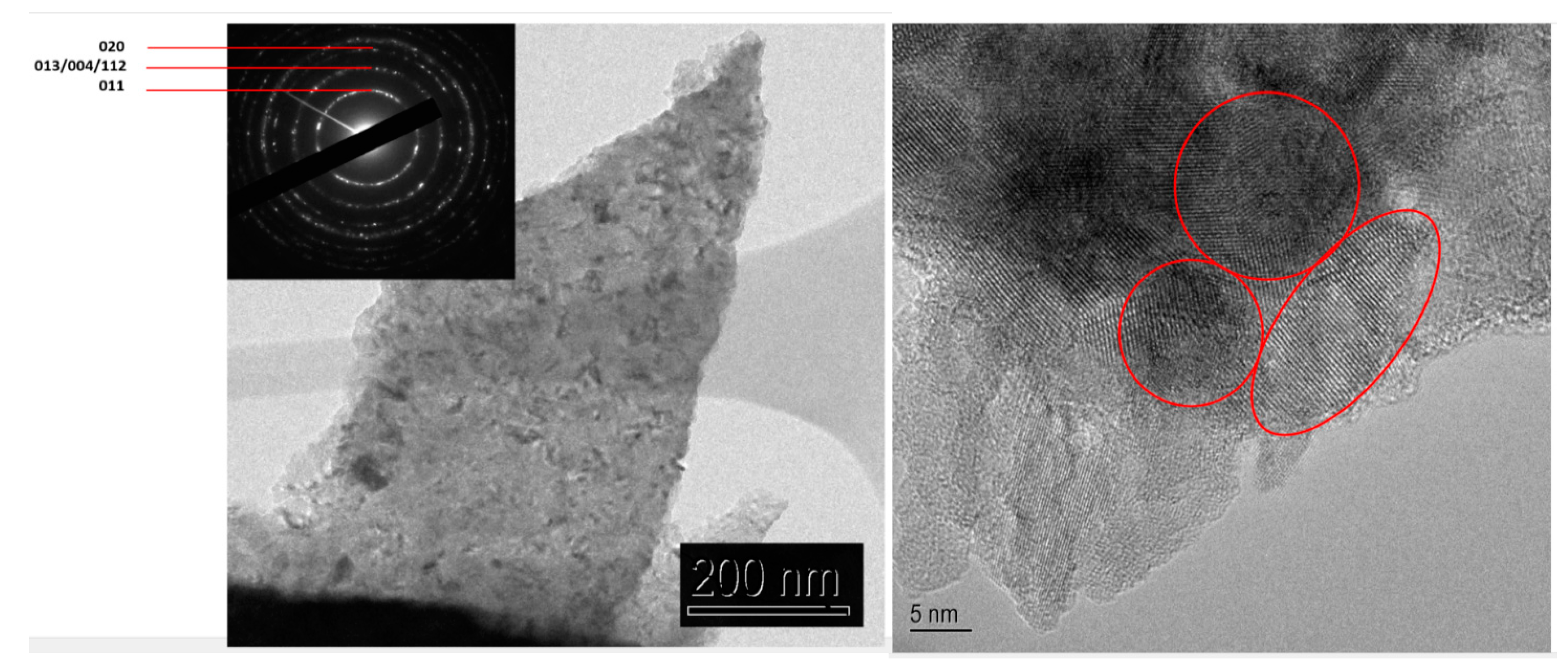
TEM observations show that the coatings are composed of anatase TiO
2 nanograins as deduced from the diffraction pattern (
Figure 6) with a grain size about 10–20 nm.
3.3. Photocatalytic Properties
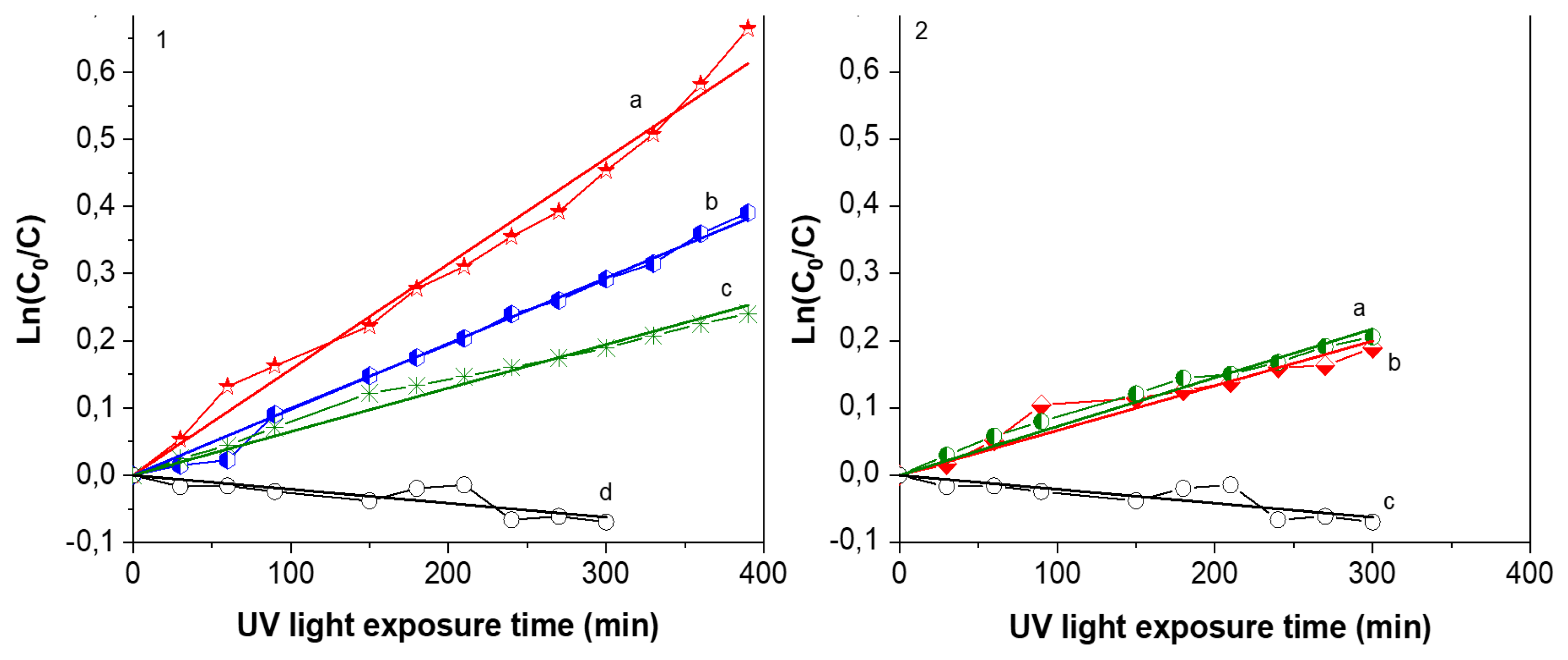
The evolution of the absorption band at 480 nm corresponding to Orange G allows one to quantify the concentration variation with UV exposure time.
Figure 7 shows plots of Ln(
C/
C0) as a function of UV light irradiation time. Al–Zr coating was tested for verification and, as expected, this layer doesn’t present any photocatalytic activity as indicated by an almost constant slope. On the contrary, the concentration of orange G decreases when exposed to TiO
2 films. Two kinds of TiO
2 films were deposited to highlight the influence of microflowers in photocatalytic properties. Using the same deposition temperature, the precursor flowrate was slightly decreased to deposit TiO
2 films without flowers. Theses layer present the same morphology and crystallized also in anatase phase (SEM and XRD are shown in the
Supplementary Materials). The photocatalytic activity of these 2 series are represented in
Figure 7. For films containing TiO
2 microflowers, the deposition temperature has an influence on the degradation rate, with a maximum value in the case of the film deposited at 550 °C, which shows 50% of mineralization after 400 min of UV light exposure. For the film deposited at 540 °C, the value is 30% of mineralization for the same exposition time and only 20% for the film deposited at 500 °C. It is important to notice that microflowers structure were mainly present in samples deposited ant 540 and 550 °C, creating a higher roughness and a larger surface for chemical exchange or reaction. The k constant for Orange G degradation calculated from the slope with Formula (3) is resumed in
Table 1. For films without microflowers deposited at 500 and 550 °C, as represented in
Figure 7-2, the k constant obtained by a linear regression is one order of magnitude lower than films containing microflowers deposited at the same temperature.
Eufinger et al. demonstrated that low grain diameter (<50 nm) increases the active surface area [
42]. Other papers show that TiO
2 photoactivity is related to grain size [
43]. In our case, films are formed of a double morphology that evolves in a different way. To determine the crystallite size evolution with temperature on the dense TiO
2 film without flowers, we performed XRD diffraction on TiO
2/Al–Zr films deposited on polished HSS substrate. In this case, the grain size obtained by Scherer’s method given in Formula (1) are 44 nm for film deposited at 500 °C and 53 nm for films deposited at 550 °C. However, if we calculate crystallite size from the samples containing flowers, the value are 18 nm and 20 nm for 500 and 550 °C, respectively. As the mean value includes the crystallites size from the film that should be equivalent to previous values in TiO
2 films, we can infer that crystallites size contained in the microflowers are rather smaller to 18 nm, in agreement with TEM observations. The crystal values for TiO
2/Al–Zr coatings deposited at 500 and 550 °C are resumed in
Table 2.
The decomposition rate (DR) is calculated by multiplying the initial concentration of Orange G
C0 = 5 × 10
−5 M by the constant k. These values are also resumed in
Table 2 for the TiO
2/Al–Zr coatings deposited at 500 and 550 °C.
Maury et al. [
44] reported a maximum DR of Orange G solution (10 ppm) of about 280 × 10
−10 mol L
−1 min
−1 for TiO
2 films deposited at 400 °C by AAMOCVD containing pure anatase phase.
In our work, the increase in decomposition rate from 332 × 10−10 mol L−1 min−1 for the TiO2/Al–Zr film deposited at 500 °C to 780 × 10−10 mol L−1 min−1 for the sample deposited at 550° is observed only in samples containing flowers. For samples without flowers de DR is similar or even lower than reported values.
The presence of microflowers with nanometric crystallite size contributes to a large specific surface, which can enhance the photocatalytic activity and improves the degradation rate of organic pollutants by multiplying the active sites for absorption in TiO
2 [
45,
46]. By increasing the surface area, adsorption of the reactants upon the photocatalyst and absorption of the light will increase. This is the twofold interest of the microflower activity.
3.4. Hydrophilicity of TiO2/Al–Zr Bilayer Films
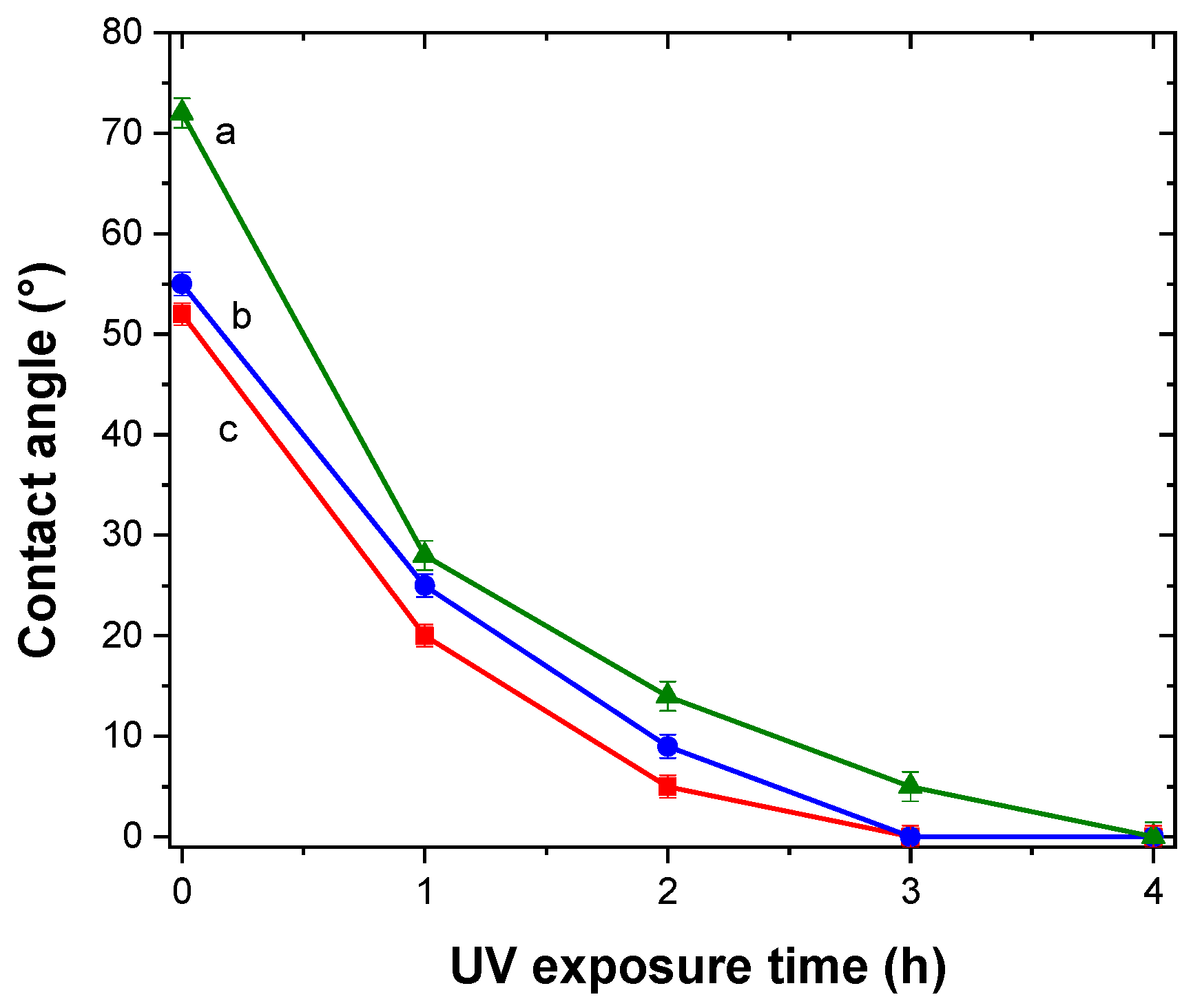
Hydrophilicity is usually present in materials with photocatalitic activity [
46]. This property can be enhanced, and even restored, by UV exposure. The self-cleaning property is directly related to this material feature [
46]. TiO
2/Al–Zr thin films were irradiated with UV light during 4 h and the water contact angle was measured at different exposure times. A total of 10 measurements were made for each sample and results are presented in
Figure 8. For all the samples, the contact angle decreases sharply with the time from values around 60° and reaching 0° after 4 h of UV exposure indicating a photoinduced superhydrophilicity. After six months in the absence of UV irradiation, the samples remained superhydrophilic presenting a contact angle of about 5°.
In the water contact angle measurements, high photoactivity means that the photogenerated holes attract hydroxyl groups from water and produce hydroxyl radicals (•OH). The higher the number of hydroxyl molecules on the surface, the higher is its hydrophilicity. This is presented as a small contact angle between the water droplet and the TiO2 surface.
3.5. Electrochemical Behavior
Creus et al. presented the classification of aluminum based alloys in saline solution where the addition of Zr as a transition metal induces the ennoblement of the corrosion potential compared to pure aluminum without losing the sacrificial character [
25]. In our work, Al–Zr films containing 4 at.% Zr were deposited on HSS substrate because this composition is the best compromise between mechanical properties, electrochemical behavior, and sacrificial character.
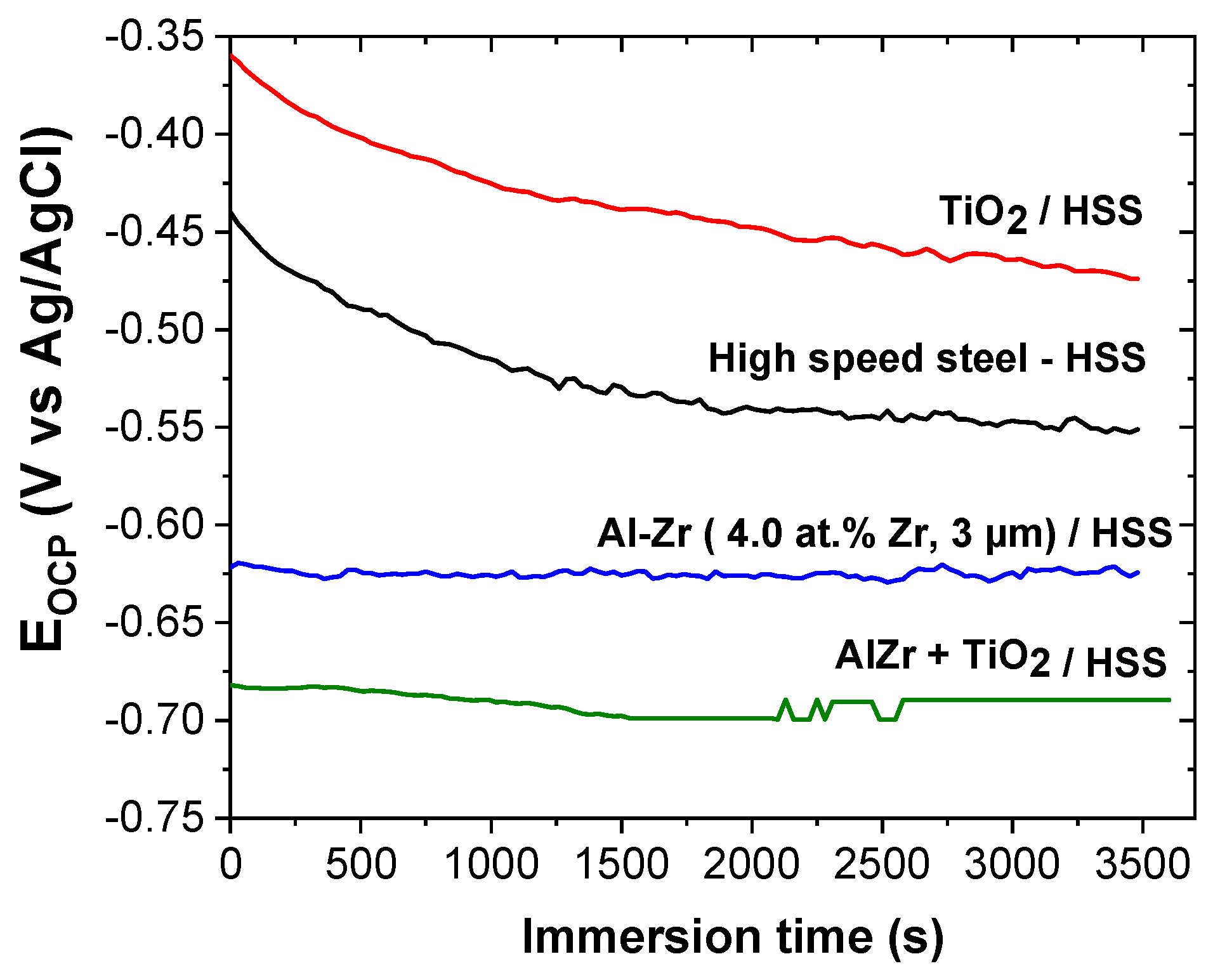
Figure 9 shows the evolution of the open circuit potential (OCP) versus time during 1 h of immersion in 5 wt.% NaCl for different coatings: Al–Zr, TiO
2, and the bilayer TiO
2/Al–Zr. The steel OCP is also reported as a reference. The steel OCP rapidly decreases and then stabilizes at around −0.55 V/Ag-AgCl. This evolution is typical of the progressive formation of iron-based corrosion products on the steel surface [
47]. The TiO
2 coated steel presents a quite similar OCP evolution during the immersion suggesting that this evolution is directly linked to the steel degradation through the open defects of the oxide film. However, the OCP values are nobler in the presence of the titanium oxide layer. Ramaprakash et al. [
48] also observed a slight ennoblement of the steel corrosion potential when is covered with TiO
2 in 3.5% NaCl solution. This ennoblement was attributed to the modification of the electronic charge transfer during the steel dissolution due to the presence of the oxide film.
The OCP of the Al–Zr coating is quite constant during the immersion at around −0.62 V/Ag-AgCl, which seems to be closed to the pitting potential as mentioned in [
25] for similar Al-based coatings in saline solution. The OCP of the bilayer TiO
2/Al–Zr coating is also quite constant during the immersion at around at −0.69 V/Ag-AgCl, which is slightly more negative compared with the Al–Zr coating. The shift of the OCP towards more negative values was also reported by Dias et al. on TiO
2 thin films deposited by atomic layer deposition on Al–Mn alloys [
49].
The OCP measurements prove that Al–Zr and TiO2/Al–Zr bilayer coatings keep the sacrificial character. It seems that the presence of the TiO2 coatings acts differently when is deposited onto steel or aluminum based materials, this could be due to the intrinsic properties of the titanium oxide film.
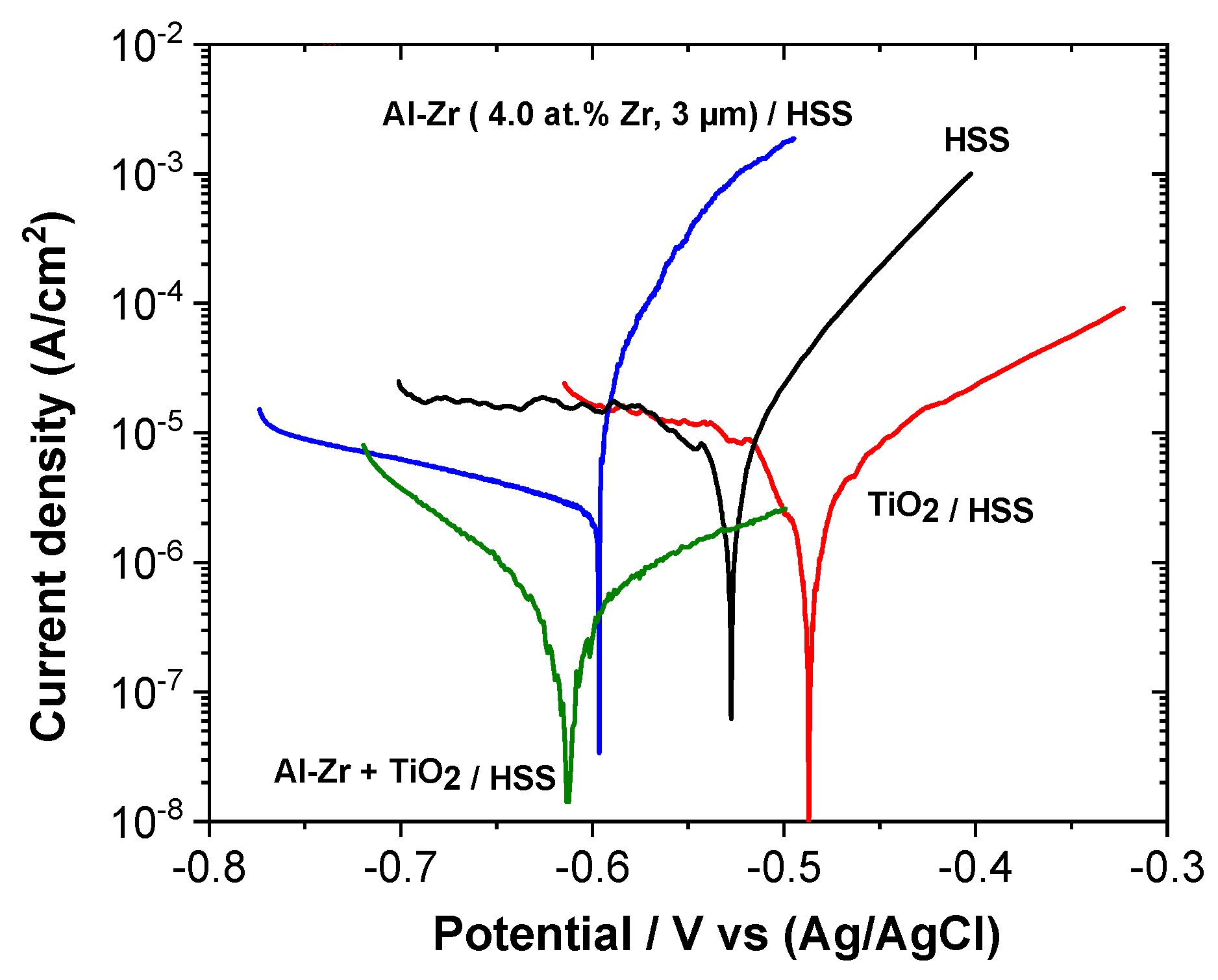
The polarization curves of the coated steels: Al–Zr, TiO
2, and the bilayer TiO
2/Al–Zr are presented in
Figure 10 and compared with the bare steel. Corrosion potential (
Ecorr) and corrosion current density (
Icorr) were determined from the polarization curves by imposing a straight line along the linear portion of the anodic and cathodic curve and extrapolate it through
Ecorr.
Table 3 gathers the corrosion current density and corrosion potential values extracted from the potentiodynamic test for all the samples.
The corrosion potential of the bare steel after one hour of immersion in stirred saline solution is around −0.52 V vs. Ag/AgCl. A diffusional plateau is observed in the cathodic domain corresponding to the dioxygen reduction and a uniform corrosion is observed in the anodic domain. The TiO
2 film induces a shift of the corrosion potential towards more positive values as observed by Ramaprakash et al. [
48]. The cathodic branch is not affected by the presence of the titanium oxide film, whereas the steel dissolution mechanism is modified. The anodic slope is decreased suggesting that the oxide film affects the charge transfer reactions during the steel dissolution.
Al–Zr coating presents a corrosion potential at around −0.59 V vs. Ag/AgCl which is slightly more negative compared to the HSS substrate suggesting that the Al-based coatings present a sacrificial behavior compared to steel. The anodic polarization curve presents a sudden rise in current close to the corrosion potential corresponding to the initiation of pitting corrosion as presented by Creus et al. [
25]. We can notice that the initiation of the pitting corrosion occurs at a potential value that is close to the corrosion potential.
The polarization curve for the bilayer coated sample (TiO2/Al–Zr) is quite different from the other configurations. The influence of the TiO2 film on the corrosion behavior is different when deposited on HSS steel or on Al–Zr coating, probably due to the fact that steel suffers from a uniform corrosion whereas pitting corrosion is the main degradation observed on Al–Zr coating. The TiO2 oxide film affects the cathodic domain with an increase of the cathodic reaction slope associated with the dioxygen reduction, suggesting that the charge transfer step in the dioxygen reduction reaction is slow down. The TiO2 film permits to reduce the contribution of the cathodic reactions leading to the decrease of the corrosion current density, down to 4.1 × 10−7 A/cm2. As observed for the Al–Zr coating, the pitting corrosion occurs at potential values very close to the corrosion potential, but in the bilayer coating, we can suppose that the pit initiation is mainly localized near the oxide film defects. An important decrease of the anodic reaction kinetic probably due to the increase of the surface impedance associated to the TiO2 layer is observed. So the pit propagation seems to be reduced in the presence of the oxide film.
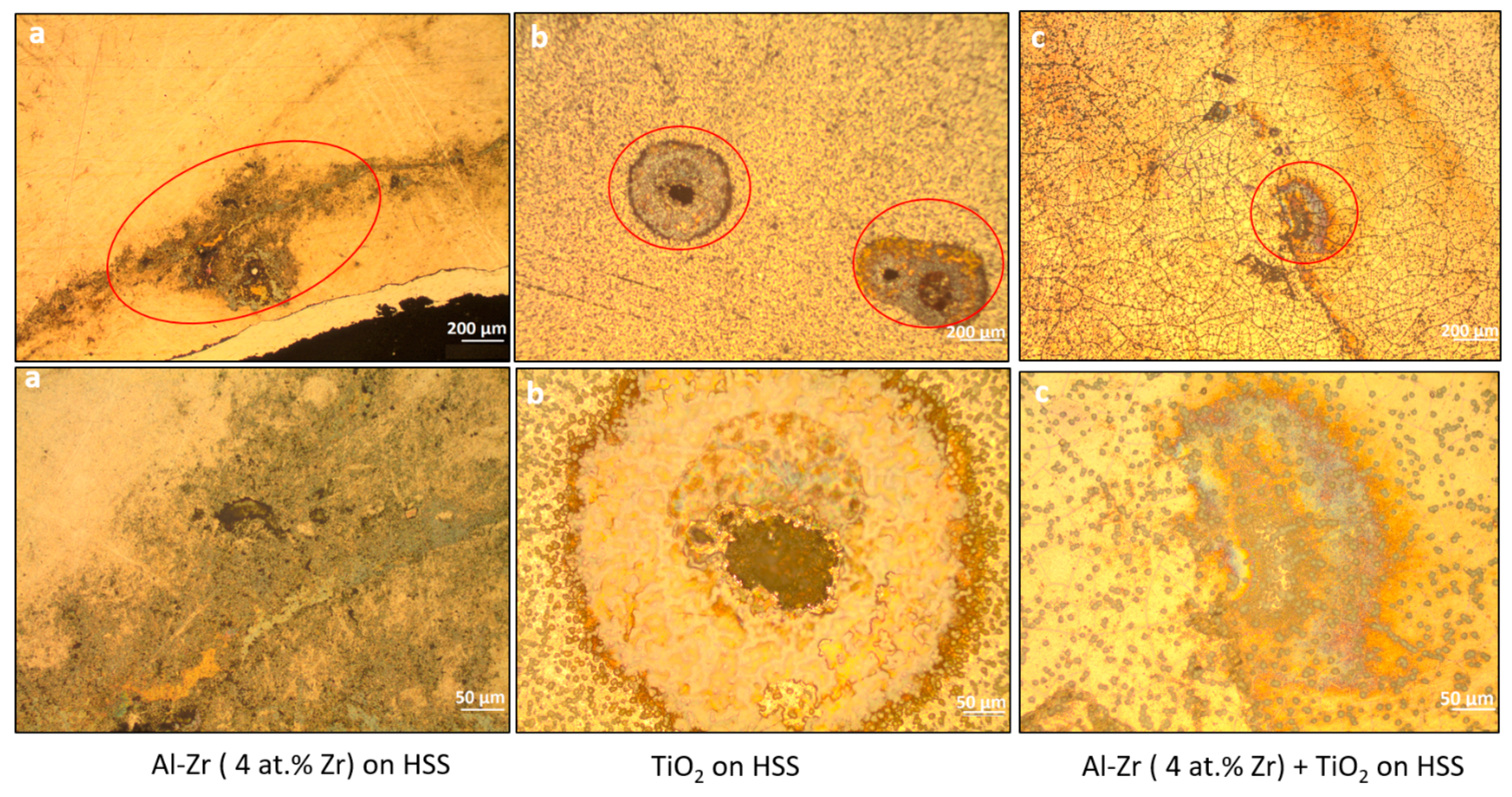
The optical observations of the corroded surface of the coated samples after the potentiodynamic polarization are presented in
Figure 11. It shows that the mean pit diameter decreases when TiO
2 is added as a second layer. It suggests that the TiO
2 coating impede the growth of pits. We can infer that the TiO
2 layer is able to increase the pitting resistance due to the fact that the cathodic reaction kinetic is lower.
4. Conclusions
The potential functionality of a TiO
2/Al–Zr bilayer coating combining anticorrosion and photocatalysis properties has been tested using high-speed steel (HSS) as substrates. The Al–Zr thin films containing a supersaturated solid solution with 4 at.% Zr were deposited on HSS substrates by DC magnetron sputtering. Then, TiO
2 coatings were deposited by aerosol-assisted CVD in optimized conditions to grow a microstructure characterized by the presence of TiO
2 microflowers physically attached to the TiO
2 thin film. Optimal growth conditions were obtained by the combination of deposition temperature, precursor concentration, and precursor feeding rate [
18]. XRD and Raman spectroscopy confirm that TiO
2 microflowers and thin film consist of TiO
2 in anatase phase. The presence of TiO
2 microflowers having a diameter and height of about 7 µm and a crystallite size of 20 nm plays a crucial role in photocatalysis of Orange G compound. This behavior is enhanced on TiO
2 samples deposited at 550 °C, showing a higher decomposition rate of Orange G (780 × 10
−10 mol L
−1) under UV light irradiation when compared to others films deposited at lower deposition temperatures (500 and 540 °C).
Finally, Al–Zr/TiO2 bilayer coatings were found to be an interesting alternative to preserve sacrificial character for the protection of steel structures in saline environments, showing a corrosion potential of −0.61 V vs. Ag/AgCl. TiO2/Al–Zr bilayer coatings deposited on steel substrates offer good protection through the preferential oxidation of the bilayer presenting a lower corrosion current density of 4.01 × 10−7 A/cm2. TiO2 as a second layer reduces the electrochemical activity of the films through the formation of a dense passive film and also increases the pitting resistance since the cathodic reaction kinetic is lower.
This study confirms the fundamental role of roughness and superhydrophilicity on the photocatalysis process and encourage the application of TiO2 nano-coatings for the fabrication of components to avoid algal adhesion on underwater structures. Biofouling field tests should be carried out in order to evaluate the performance of coatings in real conditions.