Preparation and Formula Analysis of Anti-Biofouling Titania–Polyurea Spray Coating with Nano/Micro-Structure
Abstract
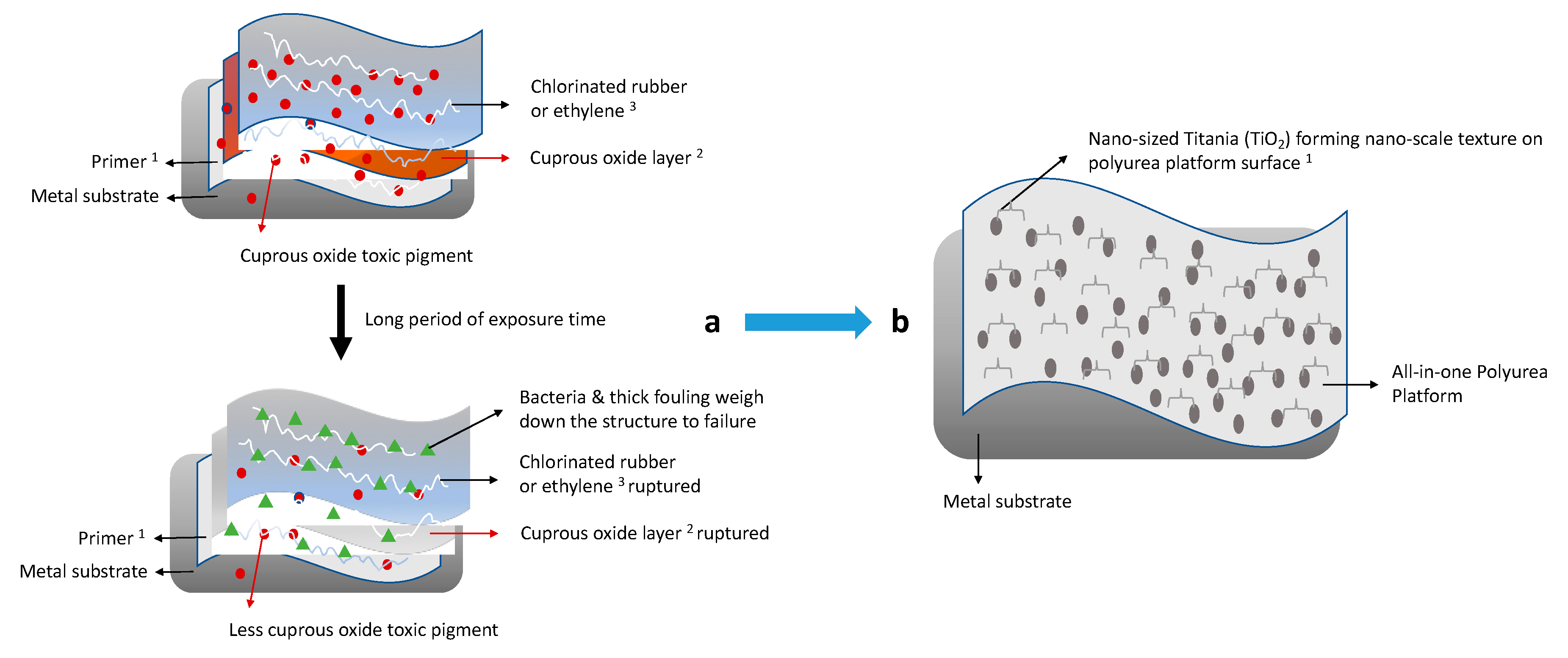
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.2.1. Formulations and Fabrication of Titania–Polyurea (TiO2–SPUA) Spray Coating
2.2.2. Surface Characterizations
2.2.3. Preparation of Bio-Medium and Bioassays
2.2.4. Surface Hardness Test
3. Result and Discussion
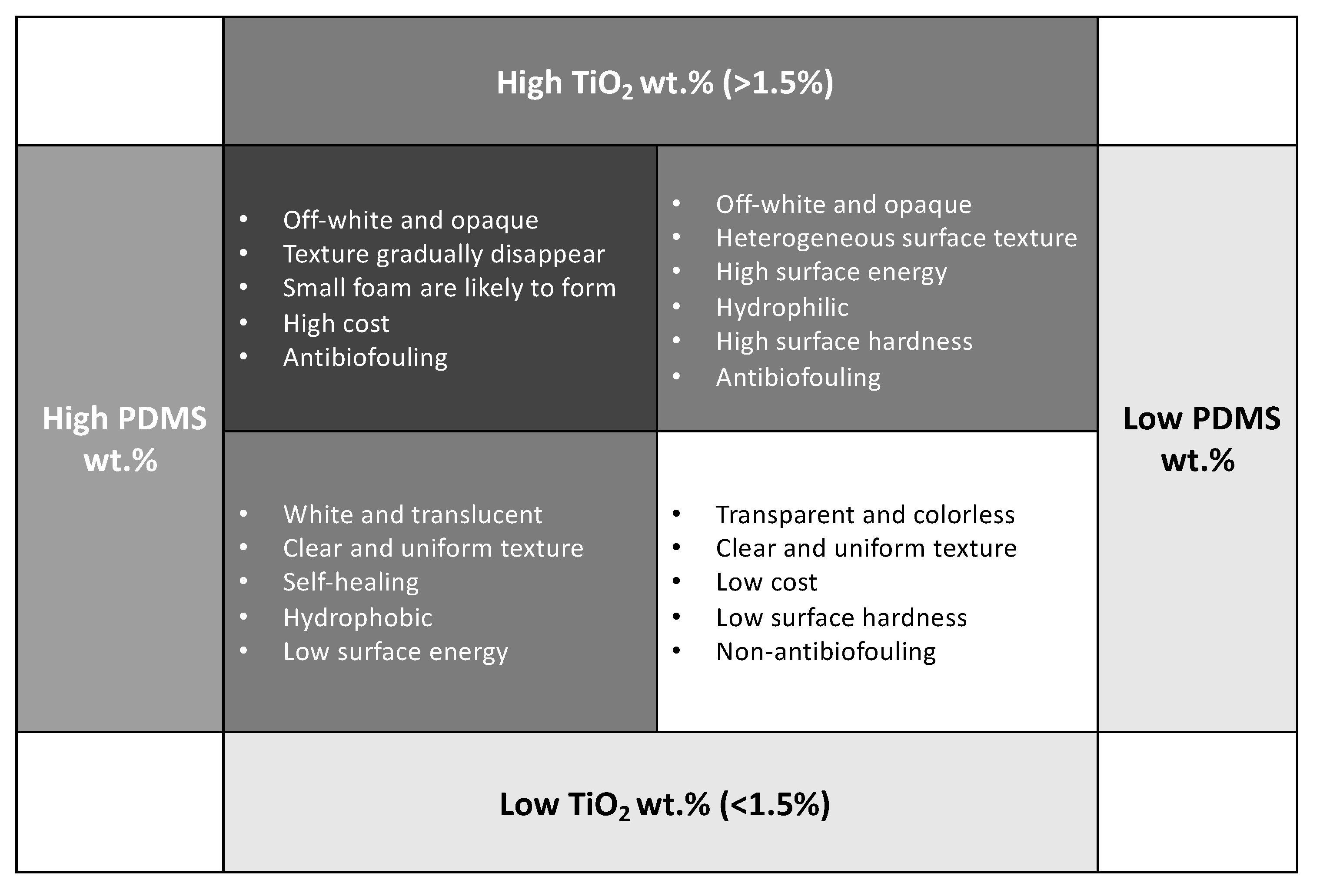
3.1. Formulating of Surface Features
3.1.1. Morphological and Chemical Composition Analysis
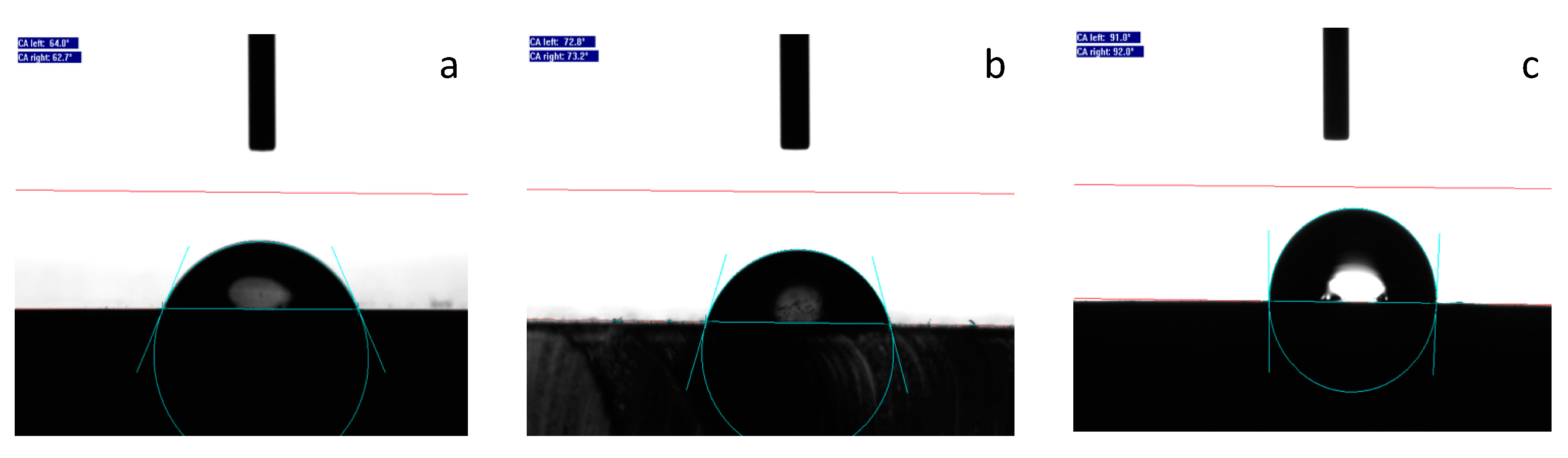
3.1.2. Surface Wettability
3.2. Formulating of Anti-Biofouling Performance
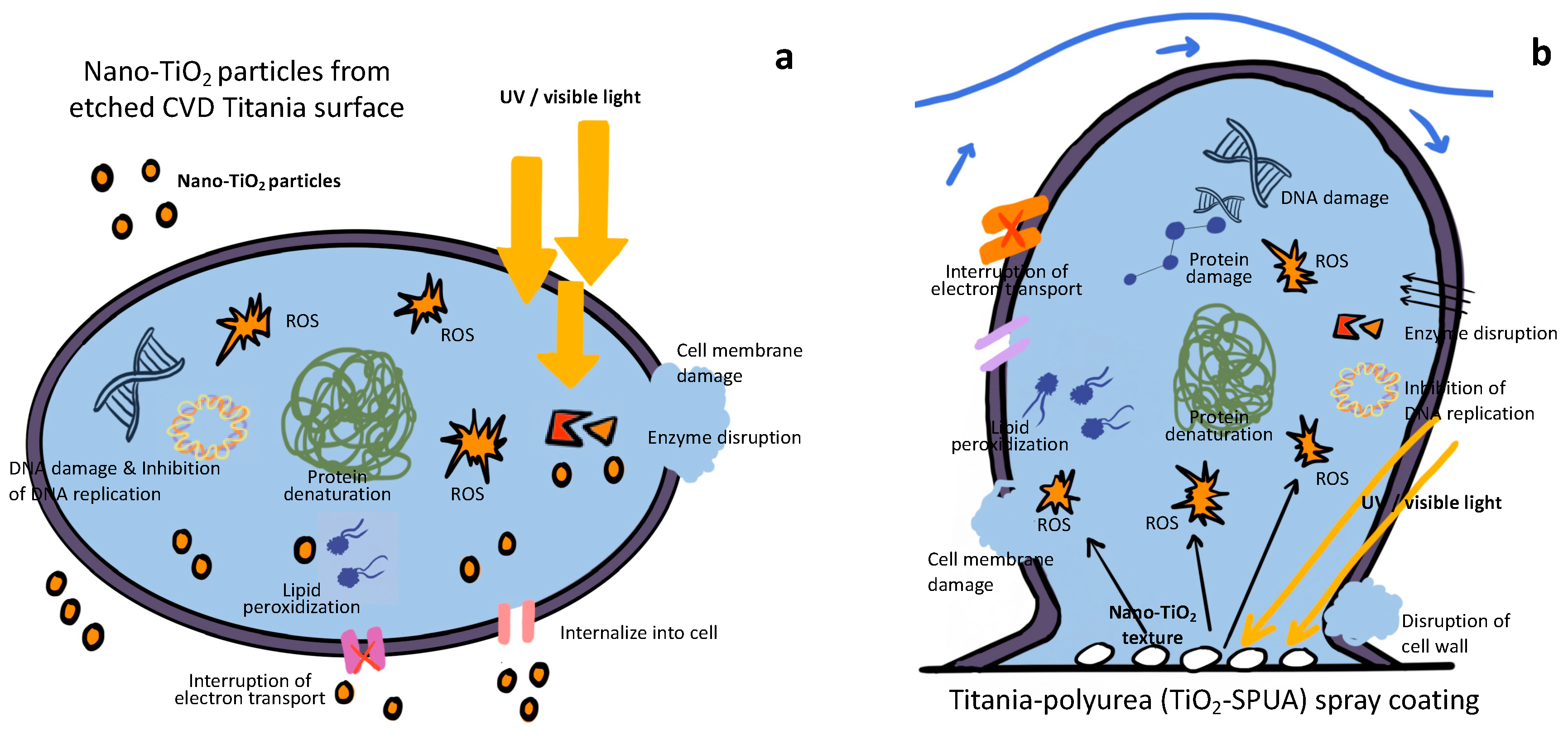
3.2.1. Nano-Titanium Dioxide vs. Photocatalytic and/or EPS Degradation & Morphology
- High weight percentage of nano-titanium dioxide (TiO2 wt.%) in the coating system may cause the photocatalytic degradation and EPS degradation to inhibit the reversible attachment of biofilm.
- Low weight percentage of nano-titanium dioxide (TiO2 wt.%) in the coating system may use photocatalytic degradation to inhibit the attachment of biofilm by damaging microbial membrane and quorum sensing.
3.2.2. Hydrophobicity/Hydrophilicity vs. Biofilm Adhesion
- The hydrophobic surface may reduce the adhesion of the biofilm more significantly than hydrophilic ones under high shear force.
- Hydrophobic and hydrophilic coating surface may both potentially reduce the adhesion of the biofilm under high shear force by convection factors.
3.3. Formulating of Surface Hardness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fu, L.; Hamzeh, M.; Dodard, S.; Zhao, Y.H.; Sunahara, G.I. Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environ. Toxicol. Pharmacol. 2015, 39, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Fabrication and indoor air purification of composite photocatalyst spray with nano-titanium dioxide and chlorophytum leach solution. China Sci. Technol. 2014, 9, 7–8. [Google Scholar]
- Jiang, X.; Yang, L.; Liu, P.; Li, X.; Shen, J. The photocatalytic and antibacterial activities of neodymium and iodine doped TiO2 nanoparticles. Colloid Surf. B Biointerfaces 2010, 79, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Pleskova, S.N.; Golubeva, I.S.; Verevkin, Y.K.; Pershin, E.A.; Burenina, V.N.; Korolichin, V.V. Photoinduced bactericidal activity of TiO2 films. Appl. Biochem. Microbiol. 2011, 47, 23–26. [Google Scholar] [CrossRef]
- Tang, H.; Chun, Y. TiO2 photocatalytic reaction mechanism and dynamics research. Prog. Chem. 2002, 14, 662–672. [Google Scholar]
- Kim, C.-H.; Lee, E.-S.; Kang, S.-M.; de Josselin de Jong, E.; Kim, B.-I. Bactericidal effect of the photocatalystic reaction of titanium dioxide using visible wavelengths on streptococcus mutans biofilm. Photodiagnosis Photodyn. Ther. 2017, 18, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.; Rabbitt, D.; Stocca, A.; Rowan, N. Pulsed ultraviolet light inactivation of pseudomonas aeruginosa and staphylococcus aureus biofilms: Pulsed UV inactivation of microbial biofilms. Water Environ. J. 2014, 29, 36–42. [Google Scholar] [CrossRef]
- Johnston, J.A. Spray Polyurea Coating Systems. U.S. Patent US6797798, 28 September 2004. [Google Scholar]
- Broekaert, M. Polyurea spray coatings: The technology and latest developments. (International). Paint. Coat. Ind. 2002, 18, 80–93. [Google Scholar]
- Shen, C. Spray Polyurea Waterproofing Coating; Chemical Industry Press CN: Beijing, China, 2010; pp. 18–20. [Google Scholar]
- Li, Y.; Wang, Q.; Li, S.; Yang, M. Preparation of super-hydrophobic surfaces with modified polyurethane and drag reduction in microchannel. Acad. J. Nanjing Tech. Univ. 2013, 11, 19–24. [Google Scholar]
- El Saeed, A.M.; El-Fattah, M.A.; Azzam, A.M.; Dardir, M.M.; Bader, M.M. Synthesis of cuprous oxide epoxy nanocomposite as an environmentally antimicrobial coating. Int. J. Biol. Macromol. 2016, 89, 190–197. [Google Scholar] [CrossRef]
- Lin, X.; Huang, X.; Zeng, C.; Wang, W.; Ding, C.; Xu, J.; He, Q.; Guo, B. Poly(vinyl alcohol) hydrogels integrated with cuprous oxide–tannic acid submicroparticles for enhanced mechanical properties and synergetic antibiofouling. J. Colloid Interface Sci. 2019, 535, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Palraj, S.; Venkatachari, G.; Subramanian, G. Bio-fouling and corrosion characteristics of 60/40 brass in Mandapam waters. Anti-Corros. Methods Mater. 2002, 49, 194. [Google Scholar] [CrossRef]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. Antibacterial composites of cuprous oxide nanoparticles and polyethylene. Int. J. Mol. Sci. 2019, 20, 439. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep. Purif. Technol. 2012, 90, 69–82. [Google Scholar] [CrossRef]
- Hosokawa, M.; Naito, M.; Nogi, K.; Yokoyama, T. Structural control of nanoparticles. In Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2012; Volume 49, pp. 51–112. [Google Scholar] [CrossRef]
- Broekaert, M. Polyurea spray-applied systems: For concrete protection. (International). Paint Coat. Ind. 2003, 19, 70–80. [Google Scholar]
- Gill, P.K.S. Assessment of biodegradable magnesium alloys for enhanced mechanical and biocompatible properties. Proquest 2014, 17, 201–208. [Google Scholar]
- Zhao, A.; An, J.; Yang, J.; Yang, E.-H. Microencapsulated phase change materials with composite titania-polyurea (TiO2–PUA) shell. Appl. Energy 2018, 215, 468–478. [Google Scholar] [CrossRef]
- Chen, P.; Xie, H.; Huang, F.; Huang, T.; Ding, Y. Deformation and failure of polymer bonded explosives under diametric compression test. Polym. Test. 2006, 25, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Hu, Z.; Wang, X. Evaluation of formability and material characteristics of aluminum alloy friction stir welded tube produced by a novel process. Mater. Sci. Eng. A 2012, 543, 210. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Wang, Z.; Shen, Y.; Manero, J.M.; Gil, F.J.; Rodriguez, D.; Haapasalo, M. Antibacterial coatings on titanium surfaces: A comparison study between single-species and multispecies biofilm. ACS Appl. Mater. Interfaces 2015, 7, 5992–6001. [Google Scholar] [CrossRef]
- Garvey, M.; Andrade Fernandes, J.P.; Rowan, N. Pulsed light for the inactivation of fungal biofilms of clinically important pathogenic Candida species: Inactivation of Candida biofilms using pulsed light. Yeast 2015, 32, 422. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hu, J.Y. Assessment of the extent of bacterial growth in reverse osmosis system for improving drinking water quality. J. Environ. Sci. Health Part A 2010, 296, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Fujoka, K. Toxicity test: Fluorescent silicon nanoparticles. J. Phys. Conf. Ser. 2011, 304, 1–5. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Kim, S.J.; Kim, B.H.; Yoon, H.S.; Kim, J.S.; Jung, S.C. The effect of combined processes for advanced oxidation of organic dye using CVD TiO2 film photo-catalysts. Prog. Org. Coat. 2012, 74, 758–763. [Google Scholar] [CrossRef]
- Saunders, K.J. Organic Polymer Chemistry; Springer: Berlin, Germany, 1988; pp. 358–387. [Google Scholar]
- Youssef, G.; Brinson, J.; Whitten, I. The effect of ultraviolet radiation on the hyperelastic behavior of polyurea. J. Polym. Environ. 2018, 26, 183–190. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Wang, X.; Liu, X. Investigation on the debonding failure model of anchored polyurea coating under a high-velocity water flow and its application. Sustainability 2019, 11, 1261. [Google Scholar] [CrossRef]
- Sasan, P.; Houssam, T. Monotonic and fatigue performance of RC beams strengthened with a polyurea coating system. Constr. Build. Mater. 2015, 101, 22–29. [Google Scholar]
- Zhang, P.S.; Wang, R.-M.; He, Y.; Song, P.; Wu, Z. Waterborne polyurethane-acrylic copolymers crosslinked core-shell nanoparticles for humidity-sensitive coatings. Prog. Org. Coat. 2013, 76, 729–735. [Google Scholar] [CrossRef]
- Pons, L.; Délia, M.-L.; Bergel, A. Effect of surface roughness, biofilm coverage and biofilm structure on the electrochemical efficiency of microbial cathodes. Bioresour. Technol. 2011, 102, 2678–2683. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, F.; Kong, Y.; Wu, Z.; Wang, R.-M.; Song, P.; He, Y. Polyurea-crosslinked cationic acrylate copolymer for antibacterial coating. Colloids Surf. A 2018, 549, 122–129. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Kong, H.; Song, J.; Jang, J. Photocatalytic antibacterial capabilities of TiO2-biocidal polymer nanocomposites synthesized by a surface-initiated photopolymerization. Environ. Sci. Technol. 2010, 44, 5672–5676. [Google Scholar] [CrossRef] [PubMed]
- Namkung, E.; Rittmann, B.E. Soluble microbial products (SMP) formation kinetics by biofilms. Water Resour. 1986, 20, 795–806. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Cui, D. Recent advances in nanotechnology applied to biosensors. Sensors 2009, 9, 1033–1053. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199. [Google Scholar] [CrossRef]
- Wandiyanto, J.V.; Linklater, D.; Tharushi Perera, P.G.; Orlowska, A.; Truong, V.K.; Thissen, H.; Ghanaati, S.; Baulin, V.; Crawford, R.J.; Juodkazis, S.; et al. Pheochromocytoma (PC12) Cell response on mechanobactericidal titanium surfaces. Materials 2018, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Shahadat, M.; Teng, T.T.; Rafatullah, M.; Arshad, M. Titanium-based nanocomposite materials: A review of recent advances and perspectives. Colloids Surf. B Biointerfaces 2015, 126, 121–137. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Jahn, A.; Palmgren, R. Conceptual model for production and composition of exopolymers in biofilms. Water Sci. Technol. 1997, 36, 9–11. [Google Scholar] [CrossRef]
- Khorshidi, B.; Biswas, I.; Ghosh, T.; Thundat, T.; Sadrzadeh, M. Robust fabrication of thin film polyamide-TiO2 nanocomposite membranes with enhanced thermal stability and anti-biofouling propensity. Sci. Rep. (Nature Publisher Group) 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Horswill, A.R.; Stoodley, P.; Stewart, P.S.; Parsek, M.R. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal. Bioanal. Chem. 2007, 387, 371–380. [Google Scholar] [CrossRef]
- Alpkvist, E.; Klapper, I. Description of mechanical response including detachment using a novel particle model of biofilm/flow interaction. Water Sci. Technol. 2007, 55, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brindle, E.R.; Miller, D.A.; Stewart, P.S. Hydrodynamic deformation and removal of Staphylococcus epidermidis biofilms treated with urea, chlorhexidine, iron chloride, or DispersinB. Biotechnol. Bioeng. 2011, 108, 2968–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, W.M.; Pitts, B.; Stewart, P.S. Spatial and temporal patterns of biocide action against Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2010, 54, 2920–2927. [Google Scholar] [CrossRef] [PubMed]
- Cogan, N.G. Two-fluid model of biofilm disinfection. Bull. Math. Biol. 2008, 70, 800–819. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.C.; Webster, T.J. Decreased Pseudomonas aeruginosa biofilm formation on nanomodified endotracheal tubes: A dynamic lung model. Int. J. Nanomed. 2016, 2016, 3825–3831. [Google Scholar]
- Adán, C.; Marugán, J.; Mesones, S.; Casado, C.; van Grieken, R. Bacterial inactivation and degradation of organic molecules by titanium dioxide supported on porous stainless steel photocatalytic membranes. Chem. Eng. J. 2017, 318, 29–38. [Google Scholar] [CrossRef]
- Eberl, H.J.; Sudarsan, R. Exposure of biofilms to slow flow fields: The convective contribution to growth and disinfection. Theor. Biol. 2008, 253, 788–807. [Google Scholar] [CrossRef]
- Lappin-Scott, H.M.; Stoodley, P. The influence of fluid shear on the structure and material properties of sulphate-reducing bacterial biofilm. Ind. Microbiol. Biotechnol. 2002, 29, 347–353. [Google Scholar]
- Besemer, K.; Singer, G.; Hödl, I.; Battin, T.J. Bacterial community composition of stream biofilms in spatially variable-flow environments. Appl. Environ. Microbiol. 2009, 75, 7189–7195. [Google Scholar] [CrossRef]
- Dockery, J.; Klapper, I. Finger formation in biofilm layers. SIAM J. Appl. Math. 2001, 62, 853–869. [Google Scholar]
- Marrelli, M.; Pujia, A.; Palmieri, F.; Gatto, R.; Falisi, G.; Gargari, M.; Caruso, S.; Apicella, D.; Rastelli, C.; Nardi, G.M.; et al. Innovative approach for the in vitro research on biomedical scaffolds designed and customized with cad-cam technology. Int. J. Immunopathol. Pharmacol. 2016, 29, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Asaro, R.J.; Lattimer, B.; Mealy, C.; Steele, G. Thermo-physical performance of a fire protective coating for naval ship structures. Compos. Part A 2009, 40, 11–18. [Google Scholar] [CrossRef]
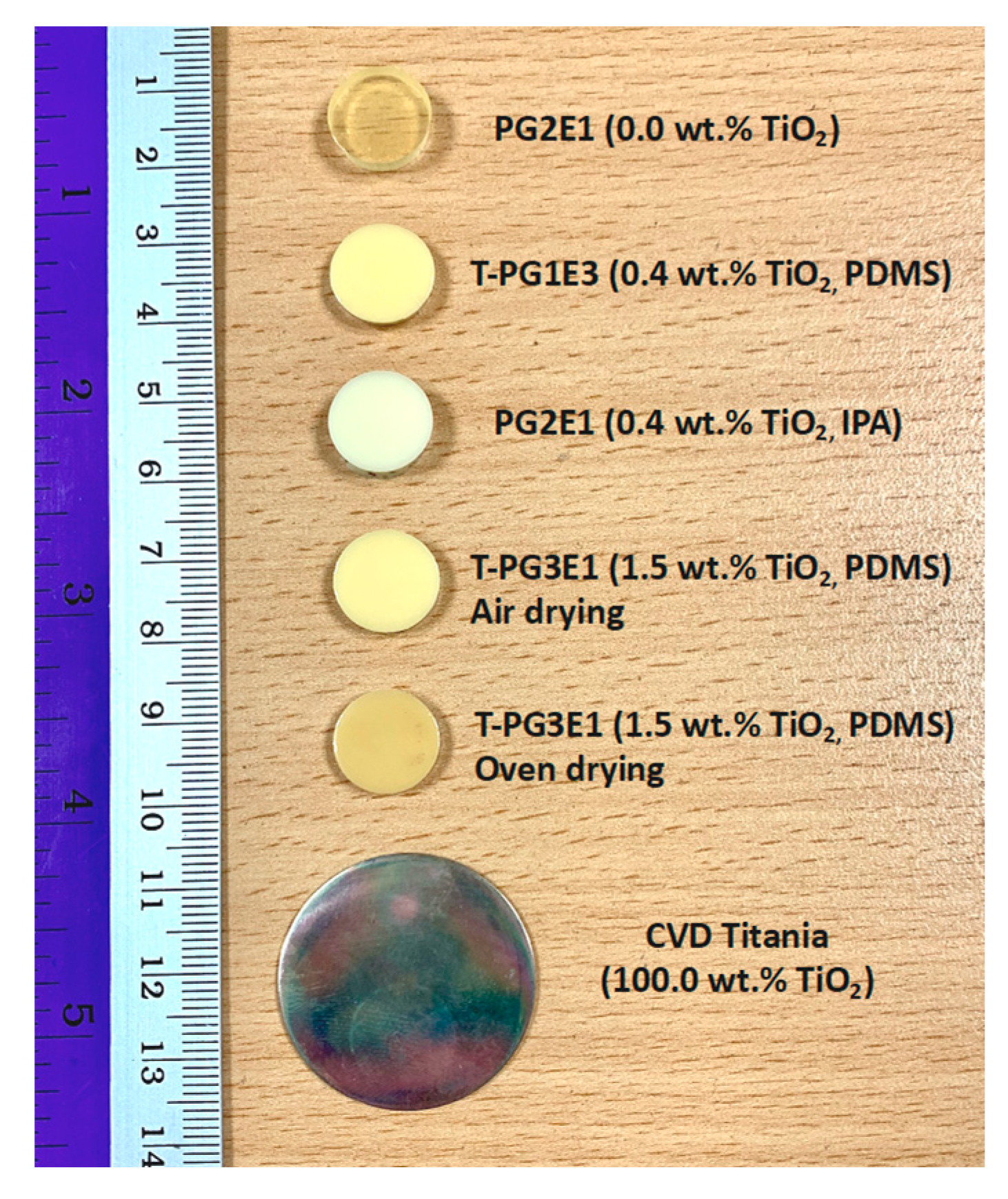
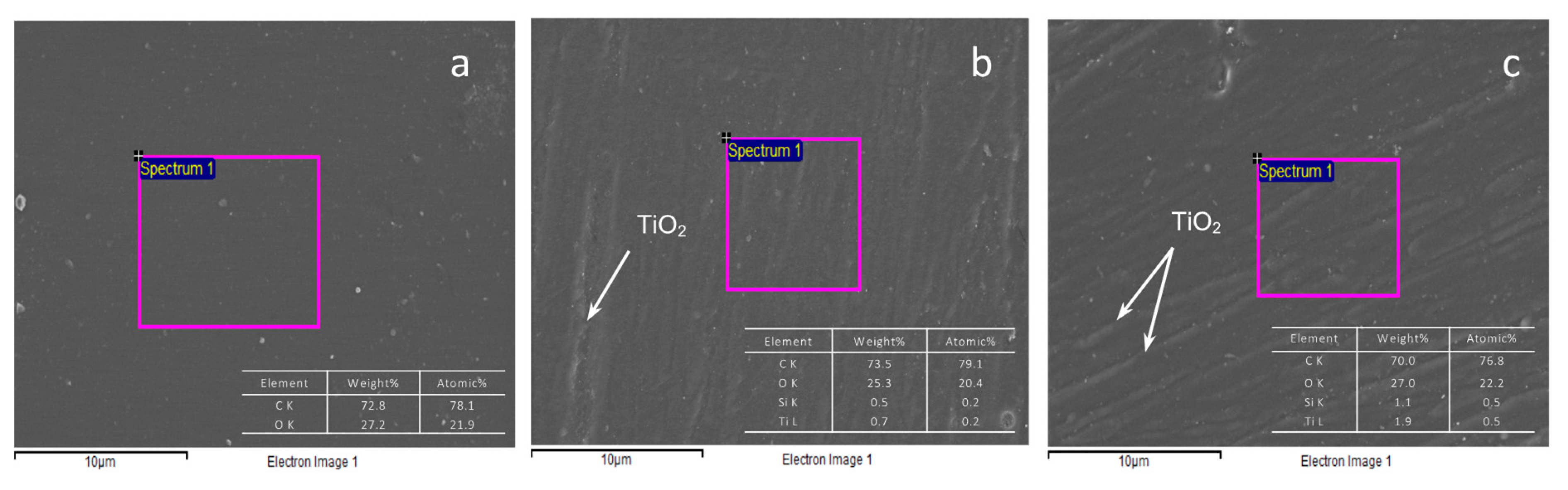
| Formulation Code Name | Part A | Part B | ||||
|---|---|---|---|---|---|---|
| Component “A” | Component “B” | P1000 | TiO2 | PDMS | IPA | |
| PG1E1 | 62.5 | 15.0 | 17.5 | – | – | – |
| T-PG1E2 | 62.5 | 14.8 | 22.3 | 0.4 | – | – |
| T-PG1E3 | 62.5 | 14.7 | 22.0 | 0.4 | 0.4 | – |
| T-PG1E4 | 62.5 | 14.7 | 22.0 | 0.4 | – | 0.4 |
| PG2E1 | 60.0 | – | 40.0 | – | – | – |
| PG2E2 | 60.0 | 8.0 | 32.0 | – | – | – |
| T-PG3E1 | 50.0 | 10.5 | 36.5 | 1.5 | 1.5 | – |
| T-PG3E2 | 50.0 | 10.5 | 36.5 | 1.5 | 1.5 | – |
| Formulations & Effect | PG1E1 | T-PG1E2 | T-PG1E3 | T-PG1E4 | PG2E1 | PG2E2 | T-PG3E1 | T-PG3E2 |
|---|---|---|---|---|---|---|---|---|
| TiO2 (wt.%) | – | 0.4 | – | 0.4 | – | – | 1.5 | 1.5 |
| PDMS (wt.%) | – | – | 0.4 | – | – | – | 1.5 | 1.5 |
| CA (°) | 61.5 ± 2.7 | 66.9 ± 2.9 | 73.0 ± 4.9 | 64.4 ± 1.4 | 68.4 ± 6.9 | 63.8 ± 1.9 | 88.5 ± 6.4 | 91.5 ± 2.6 |
| SFE (mJ/m2) | 57.3 | 49.4 | 45.8 | 51.5 | 47.1 | 52.3 | 37.2 | 32.5 |
| Formulation Code Name | Surface Features | Nano-TiO2 wt.% | 60 ± 5 rpm/10 days (Low Shear Force) | 240 ± 5 rpm/10 days (High Shear Force) |
|---|---|---|---|---|
| CVD TiO2 Surface (Control Group 1)-Live | Super hydrophilic (CA < 5°) | 100.0 |  |  |
| CVD TiO2 Surface (Control Group 1)-Dead | Super hydrophilic (CA < 5°) | 100.0 | 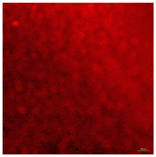 |  |
| Formulation Code Name | Surface Features | Nano-TiO2 wt.% | 60 ± 5 rpm/10 days (Low Shear Force) | 240 ± 5 rpm/10 days (High Shear Force) |
|---|---|---|---|---|
| T-PG1E2 | Hydrophilic (5° < CA < 90°) | 0.4 | 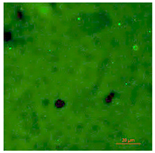 | 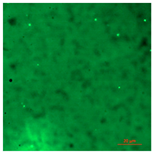 |
| T-PG1E2 | Hydrophilic (5° < CA < 90°) | 0.4 |  | 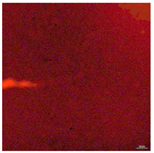 |
| T-PG3E2 | Hydrophobic (90° < CA < 150°) | 1.5 |  | 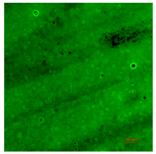 |
| T-PG3E2 | Hydrophobic (90° < CA < 150°) | 1.5 | 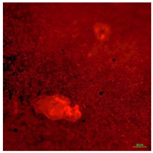 | 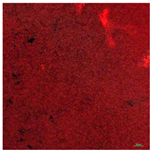 |
| Formulation Code Name | Surface Features | Nano-TiO2 wt.% | 60 ± 5 rpm/10 days (Low Shear Force) | 240 ± 5 rpm/10 days (High Shear Force) |
|---|---|---|---|---|
| Concrete coupon of CDC biofilm reactor-Live | Super hydrophilic (CA < 5°) | 0.0 | 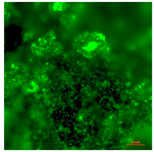 | 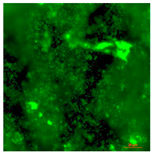 |
| Concrete coupon of CDC biofilm reactor-Dead | Super hydrophilic (CA < 5°) | 0.0 | 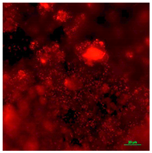 | 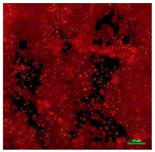 |
| Fluoro-modified elastomeric polyurethane (Control Group 2)-Live | Superhydrophobic (CA > 150°) | 0.0 | 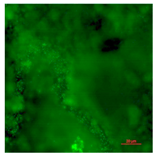 | 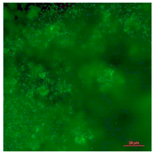 |
| Fluoro-modified elastomeric polyurethane (Control Group 2)-Dead | Superhydrophobic (CA > 150 °) | 0.0 |  |  |
| Formulations & Effect | PG1E1 | T-PG1E2 | T-PG1E3 | T-PG1E4 | PG2E1 | PG2E2 | T-PG3E1 | T-PG3E2 |
|---|---|---|---|---|---|---|---|---|
| Component “B”: P1000 (wt.%) | 1.5 | 1.5 | 1.5 | 1.5 | 0.0 | 4.0 | 3.5 | 3.5 |
| PDMS (wt.%) | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 1.5 | 1.5 |
| TiO2 (wt.%) | 0.0 | 0.4 | 0.0 | 0.4 | 0.0 | 0.0 | 1.5 | 1.5 |
| SH (A) | 77 | 98 | 48 | 75 | 50 | 92 | 72 | 74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Luo, B.; Guet, C.; Narasimalu, S.; Dong, Z. Preparation and Formula Analysis of Anti-Biofouling Titania–Polyurea Spray Coating with Nano/Micro-Structure. Coatings 2019, 9, 560. https://doi.org/10.3390/coatings9090560
Li Y, Luo B, Guet C, Narasimalu S, Dong Z. Preparation and Formula Analysis of Anti-Biofouling Titania–Polyurea Spray Coating with Nano/Micro-Structure. Coatings. 2019; 9(9):560. https://doi.org/10.3390/coatings9090560
Chicago/Turabian StyleLi, Yuanzhe, Boyang Luo, Claude Guet, Srikanth Narasimalu, and Zhili Dong. 2019. "Preparation and Formula Analysis of Anti-Biofouling Titania–Polyurea Spray Coating with Nano/Micro-Structure" Coatings 9, no. 9: 560. https://doi.org/10.3390/coatings9090560







