Phragmites Communis Leaves with Anisotropy, Superhydrophobicity and Self-Cleaning Effect and Biomimetic Polydimethylsiloxane (PDMS) Replicas
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
3. Results and Discussion
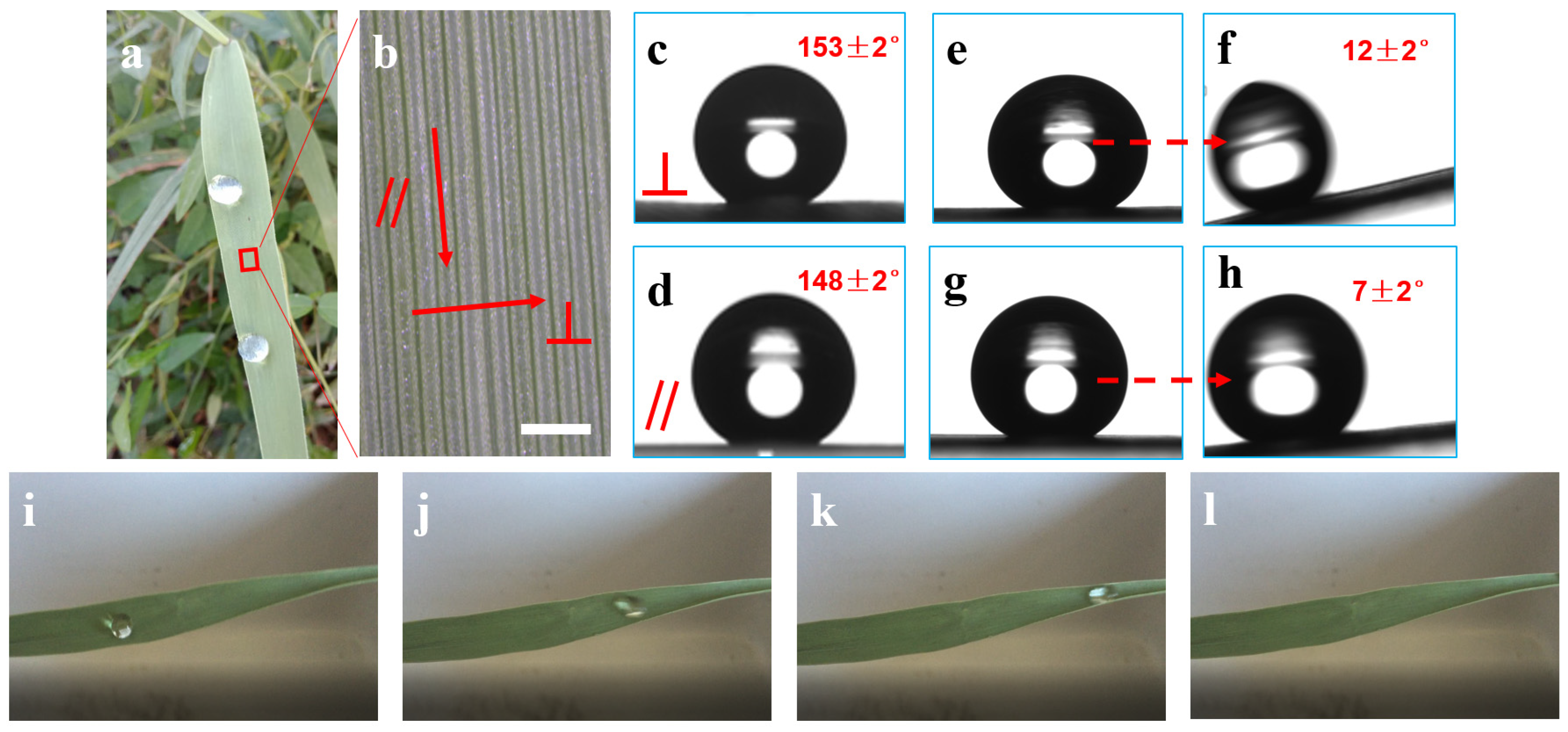
3.1. The Wettability of the PCL Surface
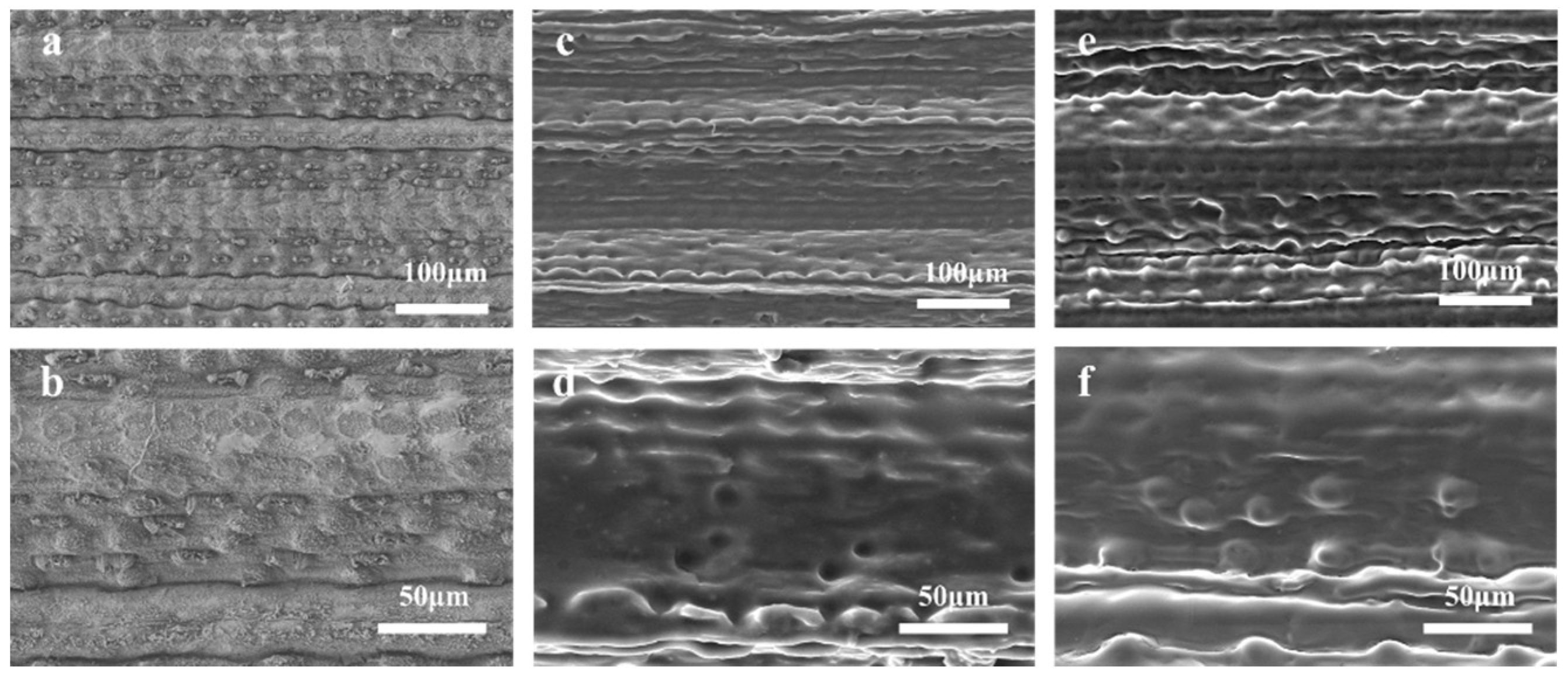
3.2. The Structures of the PCL Surface
3.3. The Analysis of the Wetting Mechanism
3.3.1. The Effect of Surface Curvature on Wettability
3.3.2. The Effect of the Sub-Millimeter Ridges Structure on Wettability
3.3.3. The Effect of the Micro-Nano Structures on Wettability
3.4. Biomimetic Materials and Their Wettability
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Jiang, L. Definition of superhydrophobic states. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Fang, R.C.; Liu, M.J.; Liu, H.L.; Jiang, L. Bioinspired interfacial materials: From binary cooperative complementary interfaces to superwettability systems. Adv. Mater. Interfaces 2018, 5, 1701176. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; Roach, P. Superhydrophilic surfaces for antifogging and antifouling microfluidic devices. Methods Mol. Biol. 2013, 949, 269–281. [Google Scholar] [PubMed]
- Ramachandran, R.; Nosonovsky, M. Coupling of surface energy with electric potential makes superhydrophobic surfaces corrosion-resistant. Phys. Chem. Chem. Phys. 2015, 17, 24988–24997. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Song, Y.; Jiang, L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Sun, R.; Zhang, X.; Feng, X.; Jiang, L. Oxygen-rich enzyme biosensor based on superhydrophobic electrode. Adv. Mater. 2016, 28, 1477–1481. [Google Scholar] [CrossRef]
- Xue, Z.X.; Wang, S.T.; Lin, L.; Chen, L.; Liu, M.J.; Feng, L.; Jiang, L. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 8. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.N.; Yu, Y.H.; Yang, H.; Xu, Y. Biomimetic fabrication and characterization of an artificial rice leaf surface with anisotropic wetting. Chin. Sci. Bull. 2012, 57, 2631–2634. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.A.; Xi, J.M.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Barthlott, W.; Schimmel, T.; Wiersch, S.; Koch, K.; Brede, M.; Barczewski, M.; Walheim, S.; Weis, A.; Kaltenmaier, A.; Leder, A.; et al. The Salvinia paradox: Superhydrophobic surfaces with hydrophilic pins for air retention under water. Adv. Mater. 2010, 22, 2325–2328. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Biophysics: Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef]
- Kinoshita, S.; Yoshioka, S.; Kawagoe, K. Mechanisms of structural colour in the Morpho butterfly: Cooperation of regularity and irregularity in an iridescent scale. Proc. Biol. Sci. 2002, 269, 1417–1421. [Google Scholar] [CrossRef]
- Zi, J.; Yu, X.D.; Li, Y.Z.; Hu, X.H.; Xu, C.; Wang, X.J.; Liu, X.H.; Fu, R.T. Coloration strategies in peacock feathers. Proc. Natl. Acad. Sci. USA 2003, 100, 12576–12578. [Google Scholar] [CrossRef]
- Gao, X.F.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.H.; Yang, B.; Jiang, L. The dry-style antifogging properties of Mosquito compound eyes and artificial analogues prepared by soft lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Liao, T.; Liu, K.S.; Jiang, L.; Kim, J.H.; Dou, S.X. Fly-eye inspired superhydrophobic anti-fogging inorganic nanostructures. Small 2014, 10, 3001–3006. [Google Scholar] [CrossRef]
- Parker, A.R.; Townley, H.E. Biomimetics of photonic nanostructures. Nat. Nanotech. 2007, 2, 347–353. [Google Scholar] [CrossRef]
- Gou, X.L.; Guo, Z.G. Superhydrophobic plant leaves with micro-line structures: An optimal biomimetic objective in bionic engineering. J. Bionic. Eng. 2018, 15, 851–858. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on-CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Dumanli, A.G.; Savin, T. Recent advances in the biomimicry of structural colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef]
- Liu, X.J.; Xu, Y.; Ben, K.Y.; Chen, Z.; Wang, Y.; Guan, Z.S. Transparent, durable and thermally stable PDMS-derived superhydrophobic surfaces. Appl. Surf. Sci. 2015, 339, 94–101. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, J.H.; Lee, H.M.; Chae, H.Y.; Kim, C.K. Superhydrophobic Si surfaces having microscale rod structures prepared in a plasma etching system. Surf. Coat. Tech. 2016, 306, 82–86. [Google Scholar] [CrossRef]
- Li, L.J.; Huang, T.; Lei, J.L.; He, J.X.; Qu, L.F.; Huang, P.L.; Zhou, W.; Li, N.B.; Pan, F.S. Robust biomimetic-structural superhydrophobic surface on aluminum alloy. ACS Appl. Mater. Inter. 2015, 7, 1449–1457. [Google Scholar] [CrossRef]
- Xu, L.Y.; Tong, F.Q.; Lu, X.M.; Lu, K. Multifunctional polypyrene/silica hybrid coatings with stable excimer fluorescence and robust superhydrophobicity derived from electrodeposited polypyrene films. J. Mater. Chem. C 2015, 3, 2086–2092. [Google Scholar] [CrossRef]
- Qinga, Y.Q.; Yanga, C.N.; Hub, C.B.; Zheng, Y.S.; Liua, C.S. A facile method to prepare superhydrophobic fluorinatedpolysiloxane/ZnO nanocomposite coatings with corrosion resistance. Appl. Surf. Sci. 2015, 326, 48–54. [Google Scholar] [CrossRef]
- Huang, W.H.; Lin, C.S. Robust superhydrophobic transparent coatings fabricated by a low-temperature sol-gel process. Appl. Surf. Sci. 2014, 305, 702–709. [Google Scholar] [CrossRef]
- Shang, Q.; Zhou, Y. Fabrication of transparent superhydrophobic porous silica coating for self-cleaning and anti-fogging. Ceram. Int. 2016, 42, 8706–8712. [Google Scholar] [CrossRef]
- Wei, W.; Lü, X.M.; Jiang, D.L.; Yan, Z.X.; Chen, M.; Xie, J.M. A novel route for synthesis of UV-resistant hydrophobic titania-containing silica aerogels by using potassiumtitanate as precursor. Dalton. Trans. 2014, 43, 9456–9467. [Google Scholar] [CrossRef]
- Liu, M.; Qing, Y.; Wu, Y.Q.; Liang, J.; Luo, S. Facile fabrication of superhydrophobic surfaces on wood substratesvia a one-step hydrothermal process. Appl. Surf. Sci. 2015, 330, 332–338. [Google Scholar] [CrossRef]
- Allen, M.; Prusnk, I.P.; Dejon, T. Using L-systems for modeling source–sink interactions, architecture and physiology of growing trees: the L-PEACH model. New Phytol. 2005, 166, 870–880. [Google Scholar] [CrossRef]
- Butt, H.J.; Roisman, I.V.; Brinkmann, M.; Papadopoulos, P.; Vollmer, D.; Semprebon, C. Characterization of super liquid-repellent surfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 343–354. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Botan. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, M.; Li, J.; Liu, H.X.; Yuan, R.; Yang, H.F.; Li, B.J.; Cai, L. Microstructure of superhydrophobic surfaces from natural to artificial. NPE 2009, 7, 381–386. (In Chinese) [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, H.; Feng, X.; Zhang, J.; Niu, S.; Han, Z. Phragmites Communis Leaves with Anisotropy, Superhydrophobicity and Self-Cleaning Effect and Biomimetic Polydimethylsiloxane (PDMS) Replicas. Coatings 2019, 9, 541. https://doi.org/10.3390/coatings9090541
Guan H, Feng X, Zhang J, Niu S, Han Z. Phragmites Communis Leaves with Anisotropy, Superhydrophobicity and Self-Cleaning Effect and Biomimetic Polydimethylsiloxane (PDMS) Replicas. Coatings. 2019; 9(9):541. https://doi.org/10.3390/coatings9090541
Chicago/Turabian StyleGuan, Huiying, Xiaoming Feng, Junqiu Zhang, Shichao Niu, and Zhiwu Han. 2019. "Phragmites Communis Leaves with Anisotropy, Superhydrophobicity and Self-Cleaning Effect and Biomimetic Polydimethylsiloxane (PDMS) Replicas" Coatings 9, no. 9: 541. https://doi.org/10.3390/coatings9090541
APA StyleGuan, H., Feng, X., Zhang, J., Niu, S., & Han, Z. (2019). Phragmites Communis Leaves with Anisotropy, Superhydrophobicity and Self-Cleaning Effect and Biomimetic Polydimethylsiloxane (PDMS) Replicas. Coatings, 9(9), 541. https://doi.org/10.3390/coatings9090541





