Preservative Effects of Gelatin Active Coating Enriched with Eugenol Emulsion on Chinese Seabass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Gelatin Active Coating Containing Eugenol/βCD Emulsions
2.2. Preparation of Seabass and Immersion Sample Treatment
2.3. pH Measurement
2.4. Water Distribution and Migration
2.5. Total Volatile Basic Nitrogen (TVB-N)
2.6. Evaluation of TBA Value
2.7. K-Values
2.8. Free Amino Acid (FAA) Analysis
2.9. Microbiological Analysis
2.10. Organoleptic Properties
2.11. Statistical Analysis
3. Results and Discussions
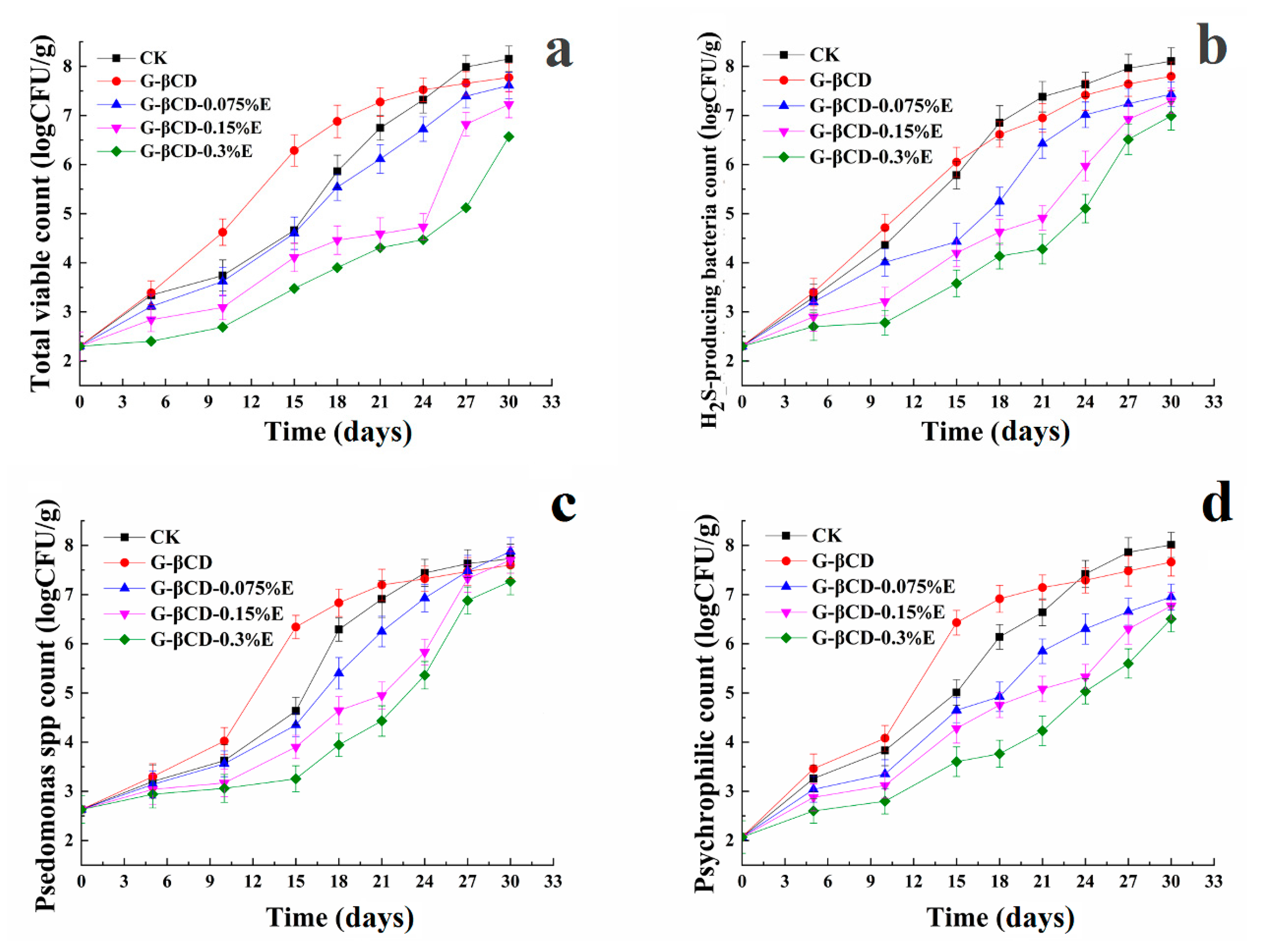
3.1. Microbiological Results
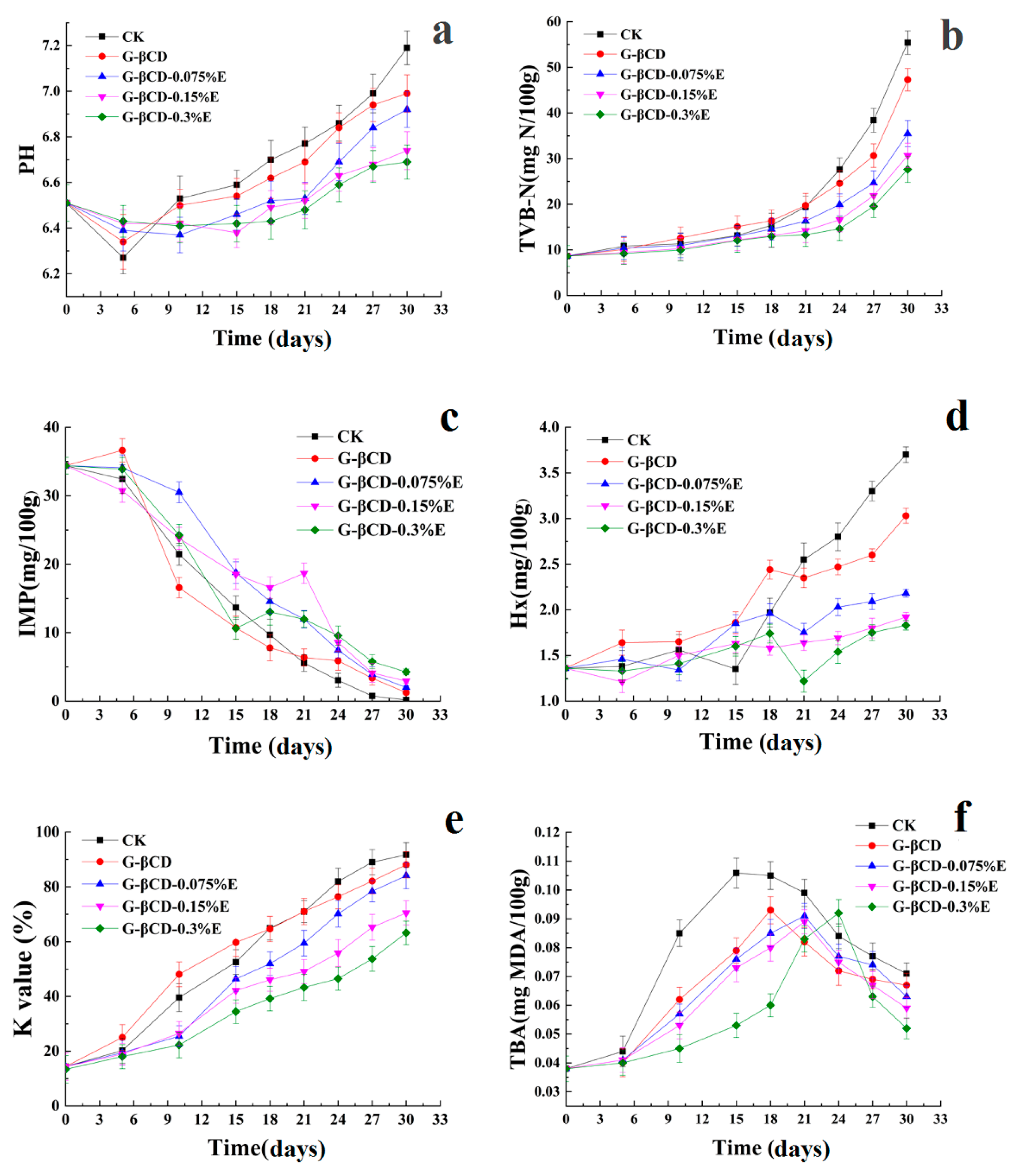
3.2. Chemical Results in Seabass Samples
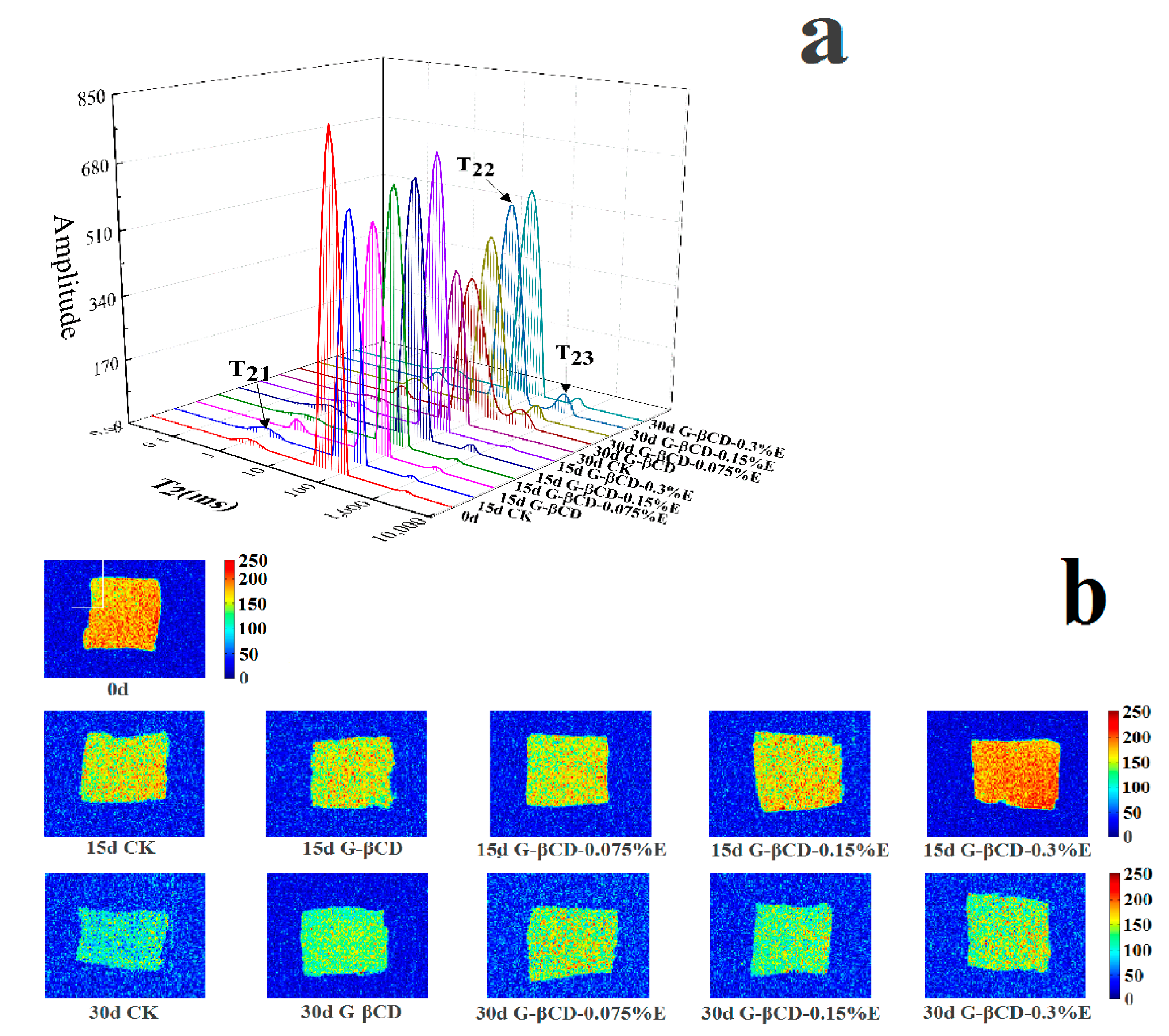
3.3. Water Distribution by LF NMR Analysis
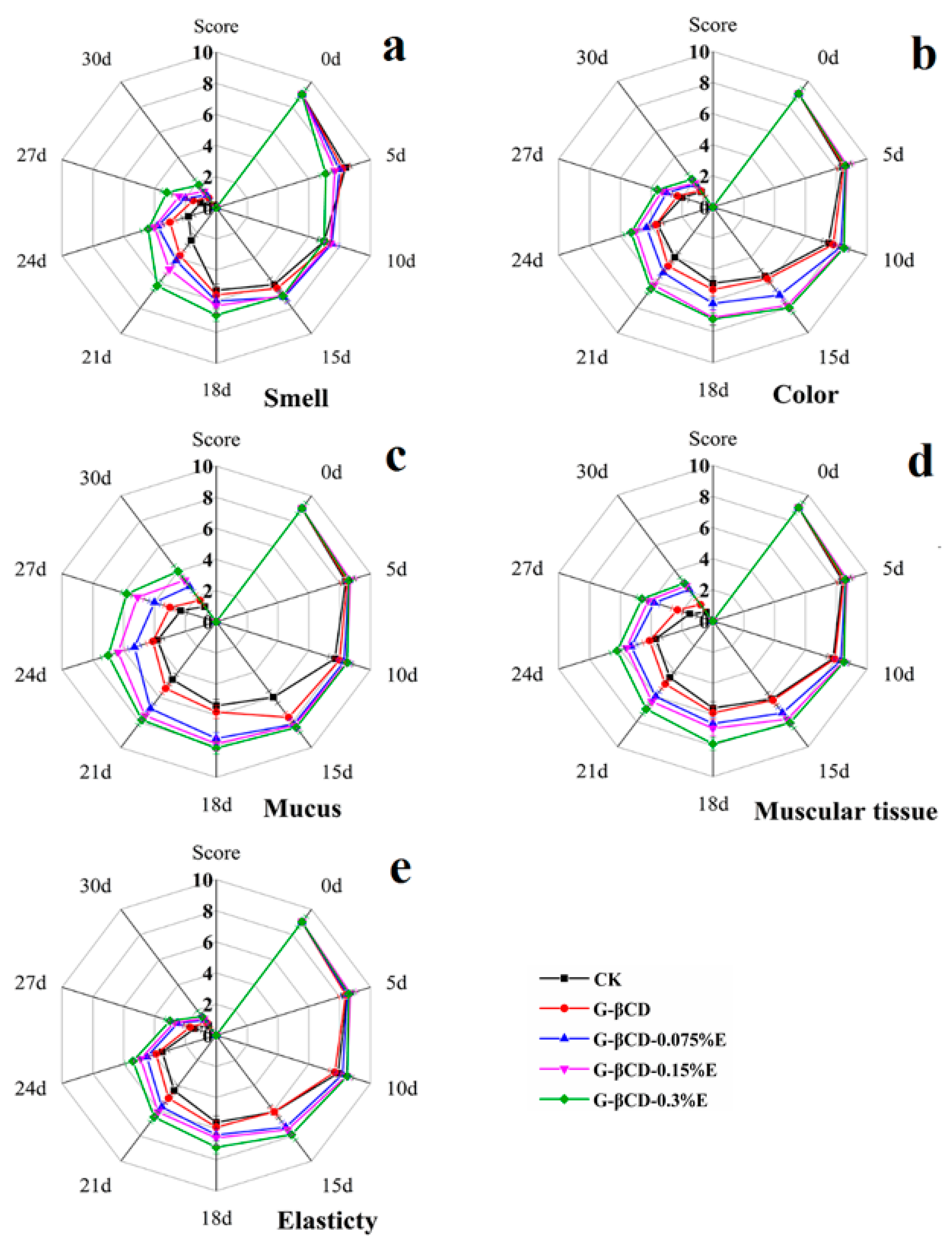
3.4. Organoleptic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Li, Y.; Peng, W.; Zhou, Z.; Shi, Y.; Pu, F.; Luo, X.; Chen, L.; Xu, P. Chromosome-level assembly of the Chinese seabass (Lateolabrax maculatus) genome. Front. Genet. 2019, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, H.; Sun, Y.; Cao, M.; Duan, Y.; Li, H.; Liu, Q.; Gu, Q.; Zhang, J. The efficacy of eugenol and tricaine methanesulphonate as anaesthetics for juvenile Chinese sea bass (Lateolabrax maculatus) during simulated transport. J. Appl. Ichthyol. 2019, 35, 551–557. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.J.E.; Boziaris, I.S. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Cao, A.; Bai, F.; Li, J. Effect of ε-polylysine in combination with alginate coating treatment on physicochemical and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) during refrigerated storage. LWT Food Sci. Technol. 2015, 62, 1053–1059. [Google Scholar] [CrossRef]
- Fukuma, Y.; Yamane, A.; Itoh, T.; Tsukamasa, Y.; Ando, M. Application of supercooling to long-term storage of fish meat. Fish. Sci. 2012, 78, 451–461. [Google Scholar] [CrossRef]
- Banerjee, R.; Maheswarappa, N.B. Superchilling of muscle foods: Potential alternative for chilling and freezing. Crit. Rev. Food Sci. 2017, 59, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Kolsaker, K. Superchilling of food: A review. J. Food Eng. 2011, 107, 141–146. [Google Scholar] [CrossRef]
- Stevik, A.M.; Claussen, I.C. Industrial superchilling, a practical approach. Procedia Food Sci. 2011, 1, 1265–1271. [Google Scholar] [CrossRef][Green Version]
- Magnussen, O.M.; Haugland, A.; Hemmingsen, A.K.T.; Johansen, S.; Nordtvedt, T.S. Advances in superchilling of food-process characteristics and product quality. Trends Food Sci. Technol. 2008, 19, 418–424. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Grøvlen, M.S.; Rustad, T. Superchilled, chilled and frozen storage of Atlantic mackerel (Scomber scombrus) fillets changes in texture, drip loss, protein solubility and oxidation. Int. J. Food Sci. Technol. 2019, 54, 2228–2235. [Google Scholar] [CrossRef]
- Luan, L.; Fu, S.; Yuan, C.; Ishimura, G.; Chen, S.; Chen, J.; Hu, Y. Combined effect of superchilling and tea polyphenols on the preservation quality of hairtail (Trichiurus haumela). Int. J. Food Prop. 2017, 20, S992–S1001. [Google Scholar] [CrossRef]
- Xu, Y.; Li, T.; Zhang, C.; Li, X.; Yi, S.; Li, J.; Sun, X. Protein degradation of olive flounder (Paralichthys olivaceus) muscle after postmortem superchilled and refrigerated storage. Int. J. Food Prop. 2018, 21, 1911–1922. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Taoukis, P.S. Effect of storage temperature and osmotic pre-treatment with alternative solutes on the shelf-life of gilthead seabream (Sparus aurata) fillets. Aquac. Fish. 2017, 2, 39–47. [Google Scholar] [CrossRef]
- Socaciu, M.I.; Semeniuc, C.A.; Vodnar, D.C. Edible films and coatings for fresh fish packaging: Focus on quality changes and shelf-life extension. Coatings 2018, 8, 10. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef]
- Sharifimehr, S.; Soltanizadeh, N.; Hossein Goli, S.A. Effects of edible coating containing nano-emulsion of Aloe vera and eugenol on the physicochemical properties of shrimp during cold storage. J. Sci. Food Agric. 2019, 99, 3604–3615. [Google Scholar] [CrossRef]
- Monteschio, J.O.; Vargas-Junior, F.M.; Almeida, F.L.; Pinto, L.A.D.M.; Kaneko, I.N.; Almeida, A.A.; Freitas, L.W.; Alves, S.P.; Bessa, R.J.; Prado, I.N. The effect of encapsulated active principles (eugenol, thymol and vanillin) and clove and rosemary essential oils on the structure, collagen content, chemical composition and fatty acid profile of Nellore heifers muscle. Meat Sci. 2019, 155, 27–35. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant starch-based films with encapsulated eugenol. Application to sunflower oil preservation. LWT Food Sci. Technol. 2019, 113, 108290. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan nanoemulsions by ultrasound-mediated emulsification: Formulation, characterization and antimicrobial activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hong, W.S.; Oh, S.W. Effect of layer-by-layer antimicrobial edible coating of alginate and chitosan with grapefruit seed extract for shelf-life extension of shrimp (Litopenaeus vannamei) stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef]
- Li, N.; Shen, Y.; Liu, W.; Mei, J.; Xie, J. Low-field NMR and MRI to analyze the effect of edible coating incorporated with MAP on qualities of half-smooth tongue sole (Cynoglossus Semilaevis Gunther) fillets during refrigerated storage. Appl. Sci. 2018, 8, 1391. [Google Scholar] [CrossRef]
- Neira, L.M.; Agustinelli, S.P.; Ruseckaite, R.A.; Martucci, J.F. Shelf life extension of refrigerated breaded hake medallions packed into active edible fish gelatin films. Packag. Technol. Sci. 2019, 1–10. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Pu, H.B.; Wang, Q.J.; Chen, Y.N. Suitability of hyperspectral imaging for rapid evaluation of thiobarbituric acid (TBA) value in grass carp (Ctenopharyngodon idella) fillet. Food Chem. 2015, 171, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, W.R.; Shen, Y.; Mei, J.; Xie, J. Coating effects of epsilon-polylysine and rosmarinic acid combined with chitosan on the storage quality of fresh half-smooth tongue sole (Cynoglossus semilaevis Gunther) fillets. Coatings 2019, 9, 273. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Nasri, R.; Mora, L.; Toldrá, F.; Nasri, M. Rheological and structural properties of Hemiramphus far skin gelatin: Potential use as an active fish coating agent. Food Hydrocoll. 2019, 87, 331–341. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Venkateshwarlu, G.; Sivaraman, G.K.; Ravishankar, C.N. Combined effect of O2 scavenger and antimicrobial film on shelf life of fresh cobia (Rachycentron canadum) fish steaks stored at 2 °C. Food Control 2017, 71, 71–78. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Grković, N.; Bošković, M.; Baltić, M.Ž.; Dojčinović, S.; Karabasil, N.; Vasilev, D.; Teodorović, V. Inhibition of Listeria monocytogenes growth on vacuum packaged rainbow trout (Oncorhynchus mykiss) with carvacrol and eugenol. J. Food Saf. 2019, 39, e12553. [Google Scholar] [CrossRef]
- Vatavali, K.; Karakosta, L.; Nathanailides, C.; Georgantelis, D.; Kontominas, M.G. Combined effect of chitosan and oregano essential oil dip on the microbiological, chemical, and sensory attributes of red porgy (Pagrus pagrus) stored in ice. Food Bioprocess Technol. 2013, 6, 3510–3521. [Google Scholar] [CrossRef]
- Singh, S.; Lee, M.; Gaikwad, K.K.; Lee, Y.S. Antibacterial and amine scavenging properties of silver–silica composite for post-harvest storage of fresh fish. Food Bioprod. Process. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT Food Sci. Technol. 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Wei, Q. Enhancing visible and near-infrared hyperspectral imaging prediction of TVB-N level for fish fillet freshness evaluation by filtering optimal variables. Food Anal. Method 2017, 10, 1888–1898. [Google Scholar] [CrossRef]
- Volpe, M.G.; Siano, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varricchio, E. Active edible coating effectiveness in shelf-life enhancement of trout (Oncorhynchusmykiss) fillets. LWT Food Sci. Technol. 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Vilas, C.; Alonso, A.A.; Herrera, J.R.; Bernárdez, M.; García, M.R. A mathematical model to predict early quality attributes in hake during storage at low temperature. J. Food Eng. 2018, 222, 11–19. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Song, S.; Wang, Z.; Kong, C.; Luo, Y. The role of microorganisms in the degradation of adenosine triphosphate (ATP) in chill-stored common carp (Cyprinus carpio) fillets. Food Chem. 2017, 224, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, D.W.; Qu, J.H.; Pu, H.B.; Zhang, X.C.; Song, Z.; Chen, X.; Zhang, H. Developing a multispectral imaging for simultaneous prediction of freshness indicators during chemical spoilage of grass carp fish fillet. J. Food Eng. 2016, 182, 9–17. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery-In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Çoban, Ö.E. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Khan, M.I.; Adrees, M.N.; Arshad, M.S.; Anjum, F.M.; Jo, C.; Sameen, A. Oxidative stability and quality characteristics of whey protein coated rohu (Labeo rohita) fillets. Lipids Health Dis. 2015, 14, 58. [Google Scholar] [CrossRef][Green Version]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Qi, L.; Fan, F.; Guo, Z.; Wang, Z.; Song, W.; Du, M. Analysis of volatile compounds and nutritional properties of enzymatic hydrolysate of protein from cod bone. Food Chem. 2018, 264, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Bapary, M.A.J.; Ahasan, C.T.; Islam, M.N.; Kamal, M. Shelf life of several marine fish species of Bangladesh during ice storage. Int. J. Food Sci. Technol. 2009, 44, 1485–1494. [Google Scholar] [CrossRef]
- Albertos, I.; Martin-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Rico, D. Shelf-life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innov. Food Sci. Emerg. Technol. 2019, 53, 85–91. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, S.; Song, Y.; Xia, K.; Xu, X.; Zhu, B.W.; Tan, M. Non-destructive analysis of caviar compositions using low-field nuclear magnetic resonance technique. J. Food Meas. Charact. 2017, 11, 621–628. [Google Scholar] [CrossRef]
- Sun, X.H.; Xiao, L.; Lan, W.Q.; Liu, S.C.; Wang, Q.; Yang, X.H.; Zhang, W.J.; Xie, J. Effects of temperature fluctuation on quality changes of large yellow croaker (Pseudosciaena crocea) with ice storage during logistics process. J. Food Process. Preserv. 2017, 42, e13505. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, W.; Fan, H.; Xie, J.; Qian, Y.F. Technology, Study on the mobility of water and its correlation with the spoilage process of salmon (Salmo solar) stored at 0 and 4 °C by low-field nuclear magnetic resonance (LF NMR 1H). J. Food Sci. Technol. 2018, 55, 173–182. [Google Scholar] [CrossRef]
| Time | Groups | FAAs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asp | Thr | Ser | Glu | Gly | Ala | Val | Met | – | ||
| Day 0 | – | 2.36 ± 0.36 | 5.47 ± 0.55 | 7.28 ± 0.48 | 8.06 ± 0.43 | 55.63 ± 0.59 | 34.20 ± 0.32 | 4.21 ± 0.25 | 2.63 ± 0.17 | – |
| Day 15 | CK | 9.82 ± 0.26a | 8.86 ± 0.56c | 9.50 ± 0.34c | 12.03 ± 0.53b | 75.80 ± 0.47d | 47.11 ± 0.47c | 6.16 ± 0.22c | 3.40 ± 0.20b | – |
| – | G-βCD | 9.68 ± 0.22a | 12.22 ± 0.71a | 13.94 ± 0.38a | 18.87 ± 0.95a | 82.56 ± 1.13c | 59.61 ± 1.43a | 10.24 ± 0.16a | 6.16 ± 0.26a | – |
| – | G-βCD-0.075%E | 8.90 ± 0.41b | 8.09 ± 0.65d | 10.56 ± 0.44b | 9.28 ± 0.49c | 84.28 ± 0.64c | 47.78 ± 0.75c | 6.05 ± 0.19c | 3.04 ± 0.16c | – |
| – | G-βCD-0.15%E | 8.49 ± 0.19c | 9.10 ± 0.53c | 9.15 ± 0.37c | 7.37 ± 0.38d | 96.87 ± 0.68b | 48.97 ± 0.83c | 7.43 ± 0.14b | 3.66 ± 0.43b | – |
| G-βCD-0.3%E | 7.75 ± 0.28d | 9.64 ± 0.42b | 13.86 ± 0.27a | 7.13 ± 0.33d | 104.98 ± 0.49a | 52.24 ± 0.28b | 7.00 ± 0.27bc | 3.45 ± 0.32b | – | |
| Day 30 | CK | 2.64 ± 0.14a | 8.69 ± 0.43c | 10.17 ± 0.52c | 14.07 ± 0.47bc | 68.56 ± 0.63c | 41.42 ± 0.43d | 7.88 ± 0.57d | 4.55 ± 0.32c | – |
| – | G-βCD | 2.54 ± 0.75a | 17.28 ± 0.58a | 12.96 ± 0.59a | 27.50 ± 0.48a | 63.37 ± 0.67d | 58.44 ± 52a | 19.93 ± 0.73a | 11.25 ± 0.48a | – |
| – | G-βCD-0.075%E | 2.36 ± 0.35a | 10.47 ± 0.35b | 12.72 ± 0.0.93ab | 15.26 ± 0.45b | 81.34 ± 0.76bc | 54.75 ± 0.48bc | 10.06 ± 0.63b | 5.78 ± 0.37b | – |
| – | G-βCD-0.15%0E | 2.39 ± 0.18a | 11.06 ± 0.84b | 11.83 ± 0.62ab | 12.30 ± 0.44d | 82.22 ± 0.68b | 47.49 ± 0.42cd | 9.07 ± 0.59 | 5.51 ± 0.31b | – |
| – | G-βCD-0.3%E | 2.27 ± 0.32a | 10.19 ± 0.41bc | 11.69 ± 0.47b | 13.37 ± 0.46c | 86.06 ± 0.62a | 52.85 ± 0.48b | 10.25 ± 0.68b | 5.16 ± 0.35bc | – |
| – | – | Ile | Leu | Tyr | Phe | Lys | His | Arg | Pro | Total |
| Day 0 | – | 2.60 ± 0.18 | 3.83 ± 0.29 | 1.20 ± 0.21 | 2.82 ± 0.28 | 10.34 ± 0.34 | 17.81 ± 0.46 | 2.76 ± 0.22 | 3.37 ± 0.27 | 164.62 ± 4.37 |
| Day 15 | CK | 3.81 ± 0.24c | 5.98 ± 0.32c | 2.13 ± 0.18b | 3.76 ± 0.23b | 9.59 ± 0.38c | 27.28 ± 0.77a | 3.80 ± 0.25d | 3.76 ± 0.28c | 232.81 ± 4.76c |
| – | G-βCD | 6.73 ± 0.75a | 11.64 ± 0.43a | 6.10 ± 0.36a | 7.33 ± 0.46a | 22.18 ± 0.44a | 26.94 ± 0.32a | 8.80 ± 0.63a | 7.28 ± 0.36a | 310.31 ± 5.84a |
| – | G-βCD-0.075%E | 3.63 ± 0.29c | 5.57 ± 0.28c | 1.94 ± 0.17b | 3.70 ± 0.26b | 18.24 ± 0.48b | 26.42 ± 0.53a | 4.62 ± 0.18c | 5.29 ± 0.23b | 247.45 ± 4.28c |
| – | G-βCD-0.15%E | 5.00 ± 0.88b | 7.66 ± 0.25b | 2.60 ± 0.23b | 4.21 ± 0.31b | 18.79 ± 0.37b | 21.50 ± 0.38b | 5.81 ± 0.35b | 5.10 ± 0.34b | 261.75 ± 4.98b |
| – | G-βCD-0.3%E | 4.53 ± 0.27b | 6.97 ± 0.31b | 2.30 ± 0.25b | 4.11 ± 0.27b | 22.37 ± 0.36a | 19.52 ± 0.53c | 6.01 ± 0.23b | 7.29 ± 0.26a | 279.20 ± 5.22b |
| Day 30 | CK | 4.35 ± 0.87d | 7.81 ± 0.34d | 4.79 ± 0.69b | 5.22 ± 0.48c | 22.81 ± 0.74c | 45.64 ± 0.83a | 6.40 ± 0.63b | 4.69 ± 0.74c | 259.69 ± 4.28c |
| – | G-βCD | 12.00 ± 1.32a | 22.40 ± 0.75a | 14.71 ± 1.06a | 12.72 ± 0.74a | 32.77 ± 0.97a | 35.62 ± 0.59b | 12.56 ± 01.22a | 11.49 ± 0.63a | 367.57 ± 5.74a |
| – | G-βCD-0.075%E | 5.90 ± 0.59bc | 9.64 ± 0.44c | 4.74 ± 0.64b | 6.52 ± 0.48b | 20.43 ± 0.56d | 36.64 ± 0.48b | 5.95 ± 0.49b | 6.78 ± 0.72b | 289.36 ± 4.92b |
| – | G-βCD-0.15%E | 5.28 ± 0.99c | 9.21 ± 0.63bc | 4.86 ± 0.65b | 5.75 ± 0.39bc | 26.42 ± 0.64b | 33.88 ± 1.30b | 5.82 ± 0.69b | 6.38 ± 0.83b | 279.51 ± 4.73bc |
| – | G-βCD-0.3%E | 6.46 ± 0.76b | 11.07 ± 0.48b | 5.16 ± 1.59b | 6.12 ± 0.57b | 27.08 ± 0.73b | 33.47 ± 0.88b | 6.17 ± 0.53b | 6.23 ± 0.77b | 293.62 ± 5.07b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative Effects of Gelatin Active Coating Enriched with Eugenol Emulsion on Chinese Seabass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage. Coatings 2019, 9, 489. https://doi.org/10.3390/coatings9080489
Zhou Q, Li P, Fang S, Liu W, Mei J, Xie J. Preservative Effects of Gelatin Active Coating Enriched with Eugenol Emulsion on Chinese Seabass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage. Coatings. 2019; 9(8):489. https://doi.org/10.3390/coatings9080489
Chicago/Turabian StyleZhou, Qianqian, Peiyun Li, Shiyuan Fang, Wenru Liu, Jun Mei, and Jing Xie. 2019. "Preservative Effects of Gelatin Active Coating Enriched with Eugenol Emulsion on Chinese Seabass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage" Coatings 9, no. 8: 489. https://doi.org/10.3390/coatings9080489
APA StyleZhou, Q., Li, P., Fang, S., Liu, W., Mei, J., & Xie, J. (2019). Preservative Effects of Gelatin Active Coating Enriched with Eugenol Emulsion on Chinese Seabass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage. Coatings, 9(8), 489. https://doi.org/10.3390/coatings9080489







