1. Introduction
Modern materials are often subject to heavy exploitation conditions and the impacts of climate change. This places high demands on their selection and corrosion protection properties. The main construction material with a wide practical application in almost all industrial areas is galvanized low-carbon steel. As is well known, zinc coatings used in that case belong to the so-called “sacrificial type”, protecting the steel substrate electrochemically and dissolving from the beginning of the corrosion attack. One possibility to improve their protective characteristics is to apply inhibitors, preferably environmentally friendly ones due to international anti-pollution regulations [
1]. Another way is to elaborate new coating types with improved properties—for example, hybrid ones.
Polyaniline (PANI) is a corrosion inhibitor which affects cathodic and anodic processes. Its strong adsorption on metal surfaces is mainly due to the presence of delocalized π-electrons in the PANI-macromolecules. The practical insolubility of this polymer limits its use as a corrosion inhibitor in aquatic media.
Polyaniline finds application as a component of various protective organic and other coatings. PANI can be effectively used as a substitute for hazardous surface treatment with phosphates or chromium [
2,
3,
4] due to its environmental friendliness and ease of synthesis. It is resistant to high temperatures [
5] and also to different chemical influences.
Involved in appropriate systems, PANI enables to minimize the risk for human health and damage to the environment [
6]. PANI layers are obtained for a short time, building a physical barrier to the corrosive agents [
7,
8]. It has been proven that polyaniline is applied for the protection of stainless steel [
9], iron [
9,
10], soft steel [
11,
12,
13], copper [
10], aluminum [
14], and zinc [
15]. The proposed reasons for the improved corrosion protection of these coatings in chloride medium [
13] can be ascribed to the presence of metal oxides or metal complexes [
16,
17,
18] on the surface. Generally, the creation of a unique mechanism for corrosion protection seems difficult to achieve since it depends on the type of the metal, coating, corrosion environment, etc.
Until now, two forms of PANI have been studied as anti-corrosion coatings: non-conductive base (emeraldine base–EB) and conducting salt (emeraldine salt–ES), respectively. The positive protection effects of the EB form are probably due to its good adhesion to the metal substrate and redox catalytic properties [
19,
20,
21,
22]. Other researchers have revealed that the ES-shape of PANI coating can also provide protection for various metals [
23,
24,
25] and its activity is strongly influenced by the type and nature of the additives [
26].
Several mechanisms of protective action of the PANI-based coatings are proposed, such as anodic protection, barrier protection, corrosion inhibitors etc. [
27]. The anodic protection of the coating refers to the observed changes in the passive region and to the increase in polarization resistance [
28]. The formation of a thin oxide layer on the surface can offer additional protection to the metal [
29]. Some studies suggest that PANI can release anions when the coating is damaged which leads to a second physical barrier that impedes the penetration of the aggressive agents. This mechanism is clearly illustrated by Wessling [
30,
31,
32], Schauer et al. [
33], Sathiyanarayanan et al. [
34], and Nguyen et al. [
35]. Some other suggestions about the corrosion protection improvement of Zn-based functional layers have been elaborated in [
36].
Our previous investigations showed that the aqueous dispersion of PANI is an effective corrosion inhibitor for metals and alloys limiting both the general and local corrosion processes in acid media [
37], and the dispersed PANI-particles can be electroplated on the metal surface [
38].
To the best of our knowledge, the data concerning the electrodeposition of hybrid zinc coatings containing PANI-particles on low-carbon steel substrates are practically missing in the literature. Therefore, the aim of the present investigation is to incorporate PANI-particles directly in the metal matrix via a one-step electrodeposition process from a simple galvanic bath obtaining in such a way a hybrid protective coating. In our opinion it could be expected that the incorporation of PANI-particles directly in the metal matrix will improve the protective characteristics of the hybrid coating due to its inhibitor activity.
2. Materials and Methods
2.1. Materials and Preparation Methods
PANI is used in the form of an aqueous dispersion as a component of the zinc electrolyte. The dispersion is obtained according to some literature sources [
39] by oxidation polymerization, in the presence of suitable stabilizers-polyvinyl pyrrolidone (PVP,
Mw = 360,000) or colloidal silicon dioxide (Ludox AS-40, Merck, Sofia, Bulgaria). The optimal reaction temperature is between −5 and 0 °C. Under these conditions, stable dispersions with in general uniform particle sizes are obtained. As is well known, in acidic media, polyaniline appears as a poly-cation which allows its electrochemical deposition on the cathode [
38].
The coating is deposited from a starting electrolytic bath for zinc consisting of 150 g/L ZnSO4·7H2O; 30 g/L NH4Cl and 30 g/L H3BO3, at a pH value of 3.3 and containing also 0.025 g/L PANI particles. For better coating quality and optimization of the electroplating process, organic additive is added to the electrolyte. The applied additive is a non-ionic surfactant block copolymer based on polyethylene and polypropylene oxides. The average molecular weight of the polymer is between 1400 and 4500, and the ratio of the masses of polypropylene oxide to polyethylene oxide blocks can range from 1:1 to 1:2.5.
Electrodeposition is carried out in experimental cell with a volume of 300 mL at a room temperature, current density value of 2 A/dm2 and application of soluble zinc anodes. The coatings with a thickness of about 10 μm are electrodeposited on low-carbon steel samples with sizes 20 × 10 × 1 mm3. In such a way by applying the appropriate electrolyte composition and electrodeposition conditions, the protective hybrid coatings are obtained in a one-step process and the dispersive organic polyaniline particles are deposited simultaneously with zinc on the substrate.
2.2. Characterization of PANI Dispersion and Hybrid Coating’s Surface
The dispersions obtained (PANI/PVP and PANI/SiO2) as well as the surface morphology of the hybrid zinc coatings before and after their corrosive treatment are investigated with scanning electron microscopy (SEM) using JEOL (Tokyo, Japan) JSM 6390 with an INCA Energy 350 unit.
2.3. Electrochemical Investigations
Select electrochemical methods are applied in order to obtain a realistic view on the peculiarities of the investigated hybrid coatings: polarization resistance (Rp) measurements, potentiodynamic (PD) polarization curves, open circuit potential (OCP), and cyclic voltammetry (CVA). The investigations are carried out in a three-electrode electrochemical glass cell with a volume of 300 mL. A saturated calomel electrode (SCE) is applied as a reference electrode, and a platinum plate as a counter one. The working electrode is the tested sample (hybrid coating) for Rp, OCP, and PD measurements, or a Pt-rod with area of 0.16 cm2 for the CVA investigations.
Polarization resistance (
Rp) is a very important parameter used for the evaluation of the sustainability and protective ability of the coatings since its value is inversely proportional to the corrosion current density according to the Stern-Geary equation [
40]. This characteristic has been determined by the application of “Corrovit” apparature (manufactured by the firm Tacussel, (Villeurbanne, France) a specially constructed device for studying linear polarization. All the investigated samples have been continuously immersed in the model medium during the whole period of investigation, except the time taken for the measurement itself, which is short. The procedure is carried out according to the requirements of the modified method of J. Tacussel et al. Before beginning, the potential is measured and then compensated with the same value (in mV) but with an opposite sign. Thereafter, three consecutive polarization steps are realized in the Tafel area (up to ±25 mV in the cathodic and anodic direction, respectively) near the corrosion potential. The current flowing as a result of this external polarization is recorded and the polarization resistance is calculated by using the Ohm-law. The time between the individual measurements was every three days during the first 15 days and thereafter every 5th day until the end of the experiment. Higher
Rp values are a sign for better protective ability and lower corrosion rate (lower corrosion current density), as well.
Open circuit potential (OCP) measurements are carried out simultaneously with the Rp investigations in order to estimate the potential displacement value during the immersion period of the test samples in the model medium.
PD polarization measurements are carried out at a scan rate of 1 mV/s by using of VersaStat 4 unit. Prior to the investigation the samples applied as working electrodes are immersed in the model medium during a 20 min. period of time until the achievement of a stable corrosion potential (Ecorr) value at conditions of open circuit potential (OCP). Thereafter, the polarization starts from the cathodic area (0.1 V negative from the measured corrosion potential), passing through the corrosion potential, and followed by the dissolution process in the anodic part.
The cyclic voltammetry (CVA) method is applied at a scan rate of 10 mV/s by using of computerized PAR unit-VersaStat 4–in order to evaluate the influence of PANI-particles on the cathodic and anodic processes. The electrolytic bath used with or without the presence of PANI-particles is described in
Section 2.1.
2.4. XRD Studies
An EMPYREAN X-Ray Diffractometer (Malvern-Panalytical, Almelo, The Netherlands) with Cu Kα–radiation and generator voltage 40 kV are applied in order to register the changes in the metallographic structure and composition which appeared in the tested samples as a result of the corrosion treatment in the model medium. The PANalytical program is used for data processing.
2.5. XPS Studies
The measurements are realized with an AXIS Supra electron-spectrometer (Kratos Analitycal Ltd., Manchester, UK) by using of monochromatic Al Kα radiation and photon energy of 1486.6 eV. Energy calibration is performed by normalizing the C 1s line of adsorbed adventitious hydrocarbons to 284.6 eV. The binding energy values (BE) of the registered compounds are determined with an accuracy of ±0.1 eV.
2.6. Corrosion Medium and Reproducibility
A model corrosion medium of 5% NaCl solution with pH 6.7 at room temperature is used. The reproducibility of the investigations is an average of 5 samples per sample type.
3. Experimental Results
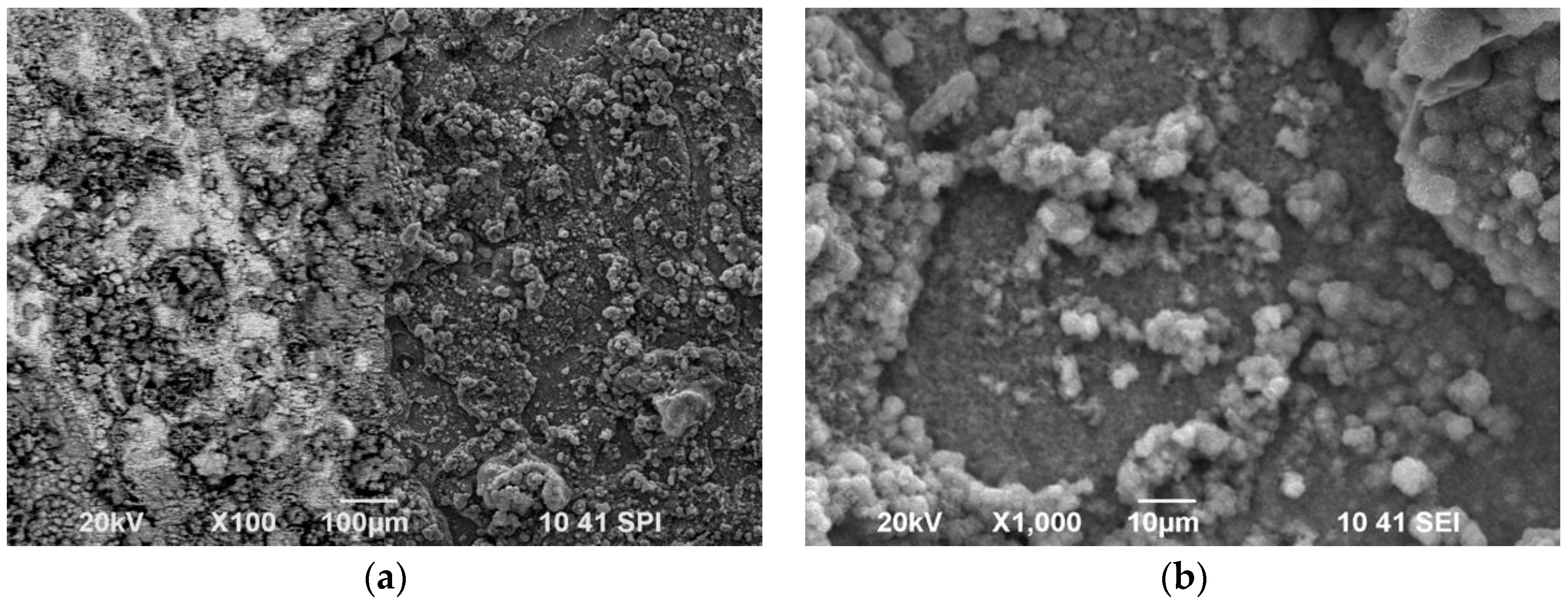
3.1. Scanning Electron Microscopy
Figure 1 presents the images of the dispersed PANI particles (at two magnifications) obtained by application of two different stabilizers–polyvinyl pyrrolidone (PVP) or colloidal silicon dioxide (SiO
2). The images are obtained after dropping the dispersion onto a glass plate and drying at air.
It is well visible that the dispersion seems to be more uniform in
Figure 1B (stabilizer SiO
2). PANI-particles are almost equal in their spherical shape and size of about 350–450 nm in diameter. Several particles greater in size also appear episodically.
Contrary to this, PANI dispersion obtained in the presence of PVP (
Figure 1A) looks predominantly like an unformed mass in which some individual particles stand out. Additionally, the latter differ in their size in the range 100–250 nm. It could be supposed from these results that the non-uniformity of the dispersed particles as well as the predominantly shapeless mass of the dispersion as a whole will negatively influence the quality of the final coatings.
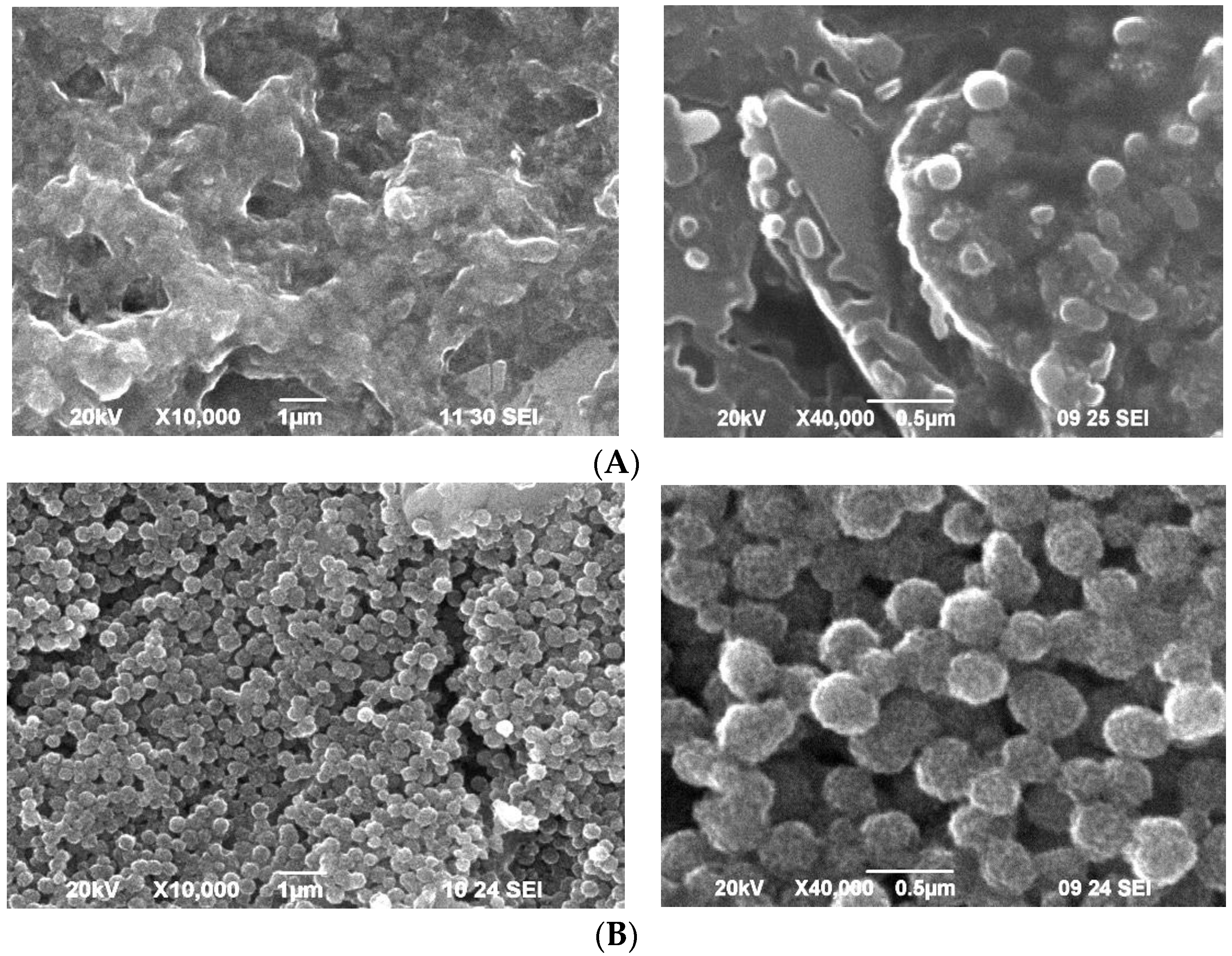
The peculiarities of the surface morphology of the hybrid zinc coatings obtained from the starting electrolytes containing PANI dispersions are reported in
Figure 2. The surface of Zn-PANI/PVP looks rougher and more uneven with convex and concaved areas, and there are several protruding sections against the backdrop of some uniform and even places. Generally, this non-homogeneity of the sample surface quantitatively corresponds to the results obtained for the PANI/PVP dispersion. Since the thickness of the electrodeposited coatings is calculated by using of the weight method it can be supposed that local differences will appear-thinner or thicker areas–which will in general negatively affect the protective ability. Taking into account the experimental data obtained by SEM, it could be expected that the application of PANI/PVP dispersion is not suitable enough from a corrosion point of view.
The Zn-PANI/SiO2 hybrid coatings look much more uniform and the differences in the sample topography are practically indistinguishable. Their decorative appearance is much better since they are bright and without visible defects on the surface. It can be concluded that SiO2 is more appropriate as a stabilizer for obtaining Zn-PANI hybrid coatings with good decorative appearance.
3.2. Polarization Resistance (Rp)
The results obtained by this method for both hybrid coatings and ordinary zinc (given for comparison) during 50 days of immersion in 5% NaCl solution are reported in
Figure 3.
The ordinary zinc coating (curve 1) remains generally stable and without any visible corrosion damages for a period of about 50 days receiving
Rp value of ~900 Ω·cm
2 at the end of the test period. Zn-PANI/PVP hybrid coating (curve 2) demonstrates
Rp values close to those of the ordinary Zn during the test. In the last 10 days (between 40th and 50th day) its corrosion resistance gradually, slightly increases until the end of the period and reaches approximately 1150 Ω·cm
2. The other hybrid coating—Zn-PANI/SiO
2–curve 3—shows relatively close
Rp values with Zn-PANI/PVP during the first 40 days of the investigation, but thereafter its corrosion resistance increases drastically up to about 3500 Ω·cm
2. The reason for this behavior seems to be the appearance of a mixed protective layer of corrosion products with PANI-particles, relatively randomly distributed on the sample and ensuring an additional inhibitor effect. It can be also supposed that a similar film appears on PANI/PVP but with worse protective characteristics as a result of the insufficient quality of the starting dispersion (see
Figure 1A).
Having in mind the obtained results it can be concluded that the differences in the shape and sizes of the dispersed PANI-particles obtained as well as their distribution into the metal matrix seem to be a very important parameter which determines the behavior of the investigated hybrid coatings.
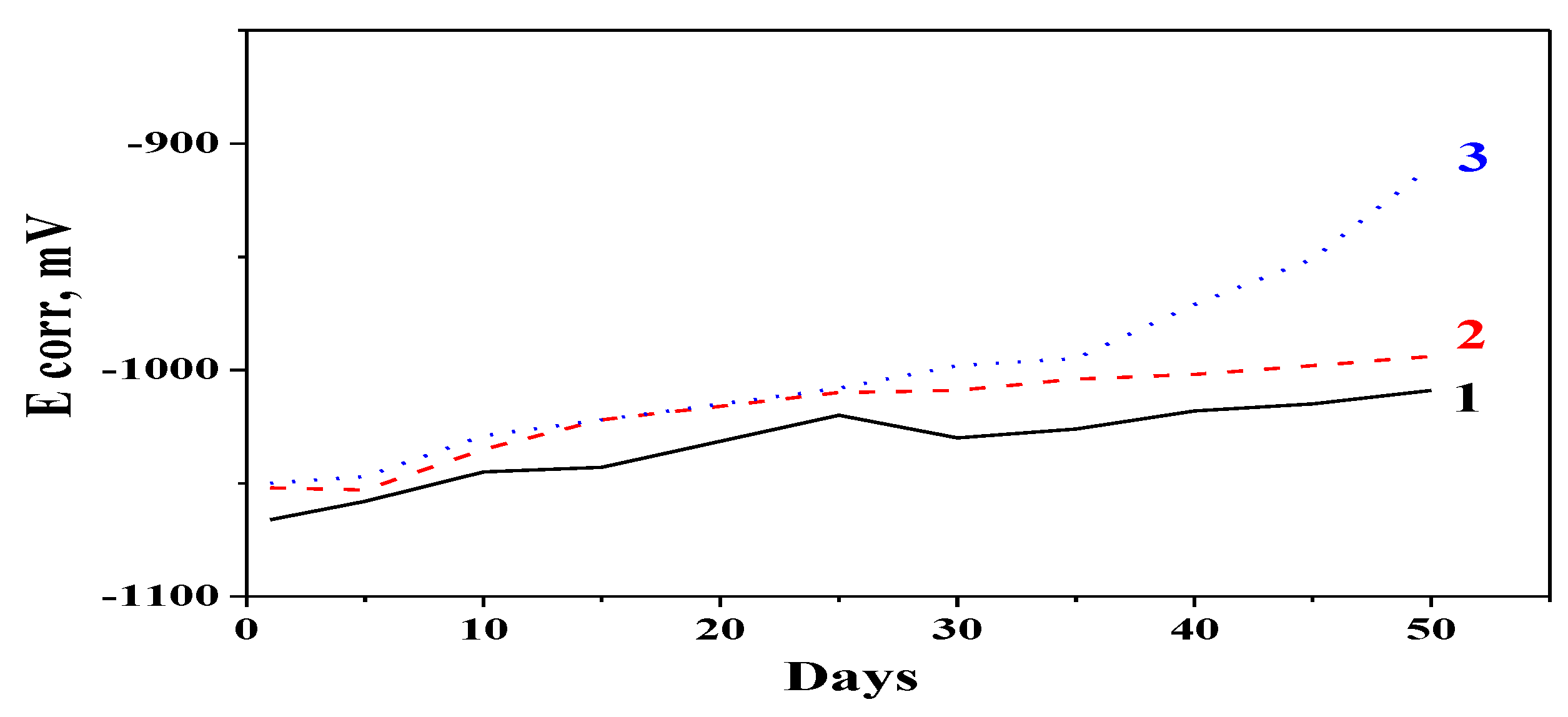
3.3. OCP Measurements
The OCP data obtained during the
Rp measurements of the test samples are presented in
Figure 4. Generally, the observed trends of changing the OCP values correspond to the polarization resistance presented in
Figure 3. It could be summarized that OCP values of both hybrid coatings are more positive than that of the ordinary zinc during the whole time of investigation. After 35–40 days of immersion in the test medium, the OCP value of the Zn-PANI/SiO
2 sample is strongly shifted in a positive direction (becomes nobler). These results together with the
Rp data obtained demonstrate the positive influence of the polyaniline on the protective ability of the hybrid coatings in an aggressive medium containing chlorine ions.
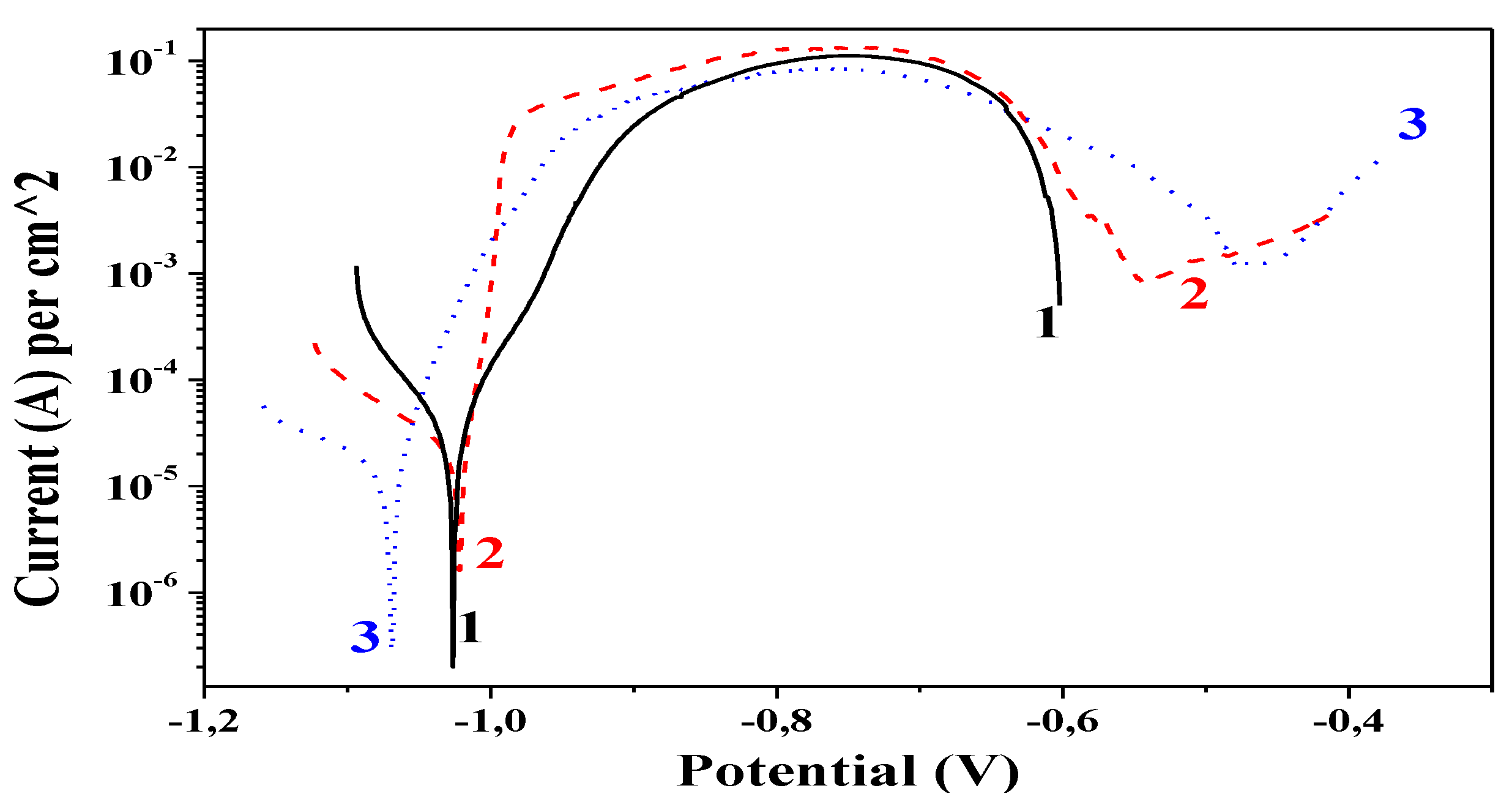
3.4. Potentiodynamic (PD) Polarization Curves
Potentiodynamic curves for the three coating types are reported in
Figure 5. The ordinary Zn and the hybrid Zn-PANI/PVP coating show very close (almost equal) values of their corrosion potentials (
Ecorr)–about −1025 mV. For comparison, the corrosion potential of Zn-PANI/SiO
2 is more negative-about −1070 mV. Generally, this difference could be adopted as a more pronounced ability for this hybrid coating to protect the steel substrate electrochemically. Theoretically, in the case of a corrosion attack Zn-PANI/SiO
2 will dissolve earlier (sacrificial effect) leading to the appearance of an additional layer of corrosion products, which will impede the penetration of the chloride ions deeply inside.
The corrosion current densities (Icorr) of the samples also differ–for ordinary Zn-3.4 × 10−5 A/cm2; for Zn-PANI/PVP-2.4 × 10−5 A/cm2; for Zn-PANI/SiO2-1.5 × 10−5 A/cm2. In addition, the length of the individual curves (until appearance of the steel substrate) lead to the conclusion that at conditions of external anodic polarization Zn-PANI/SiO2 hybrid lasts longer in that test medium (until −370 mV) compared to Zn-PANI/PVP–until −400 mV and ordinary Zn-until −600 mV.
When comparing the results from
Section 3.2,
Section 3.3 and
Section 3.4. It should be kept in mind that PD and
Rp methods differ—PD curves are carried out for a relatively short time under external polarization (~30 min) while
Rp measurements are pursued for a prolonged time (50 days). In the latter case, the samples are continuously under OCP conditions except for the time of the measurement itself.
3.5. XRD Investigations
Having in mind the experimental data from the electrochemical tests presented above, the XRD method is applied only for the Zn-PANI/SiO2 hybrid coating since the latter seems to be more promising from a corrosion point of view.
Generally, it well known from some previous investigations that the main reason for the improved corrosion resistance of the Zn-based coatings in such medium is the appearance of corrosion products with a low product of solubility value–mainly zinc hydroxide chloride (ZHC) [
41,
42,
43,
44]. The XRD patterns of the corrosive untreated and corrosive treated samples are reported in
Figure 6.
The corrosive untreated sample is characterized with the presence of the main peaks of the Zn and of the steel substrate (iron). This result shows that the application of PANI do not significantly affect the metallographic structure of the metal matrix. The XRD pattern of the corrosive treated sample shows numerous series of peaks, the latter corresponding to the presence of compounds like zinc hydroxide chloride monohydrate (Zn5(OH)8Cl2·H2O), zinc hydroxide-Zn(OH)2, and zinc oxide-ZnO. They appear as a result of the corrosion processes occurring on the sample and contribute to the formation of a protective surface film with barrier properties.
Compared to the case of the ordinary zinc coating where predominantly zinc hydroxide chloride (ZHC) appears [
41,
42,
43,
44] in the hybrid one additional compounds are also available. According to the experimental data obtained this fact could be taken as a reason for the better corrosion resistance of the hybrid coating since these compounds will improve the barrier properties of the protective layer.
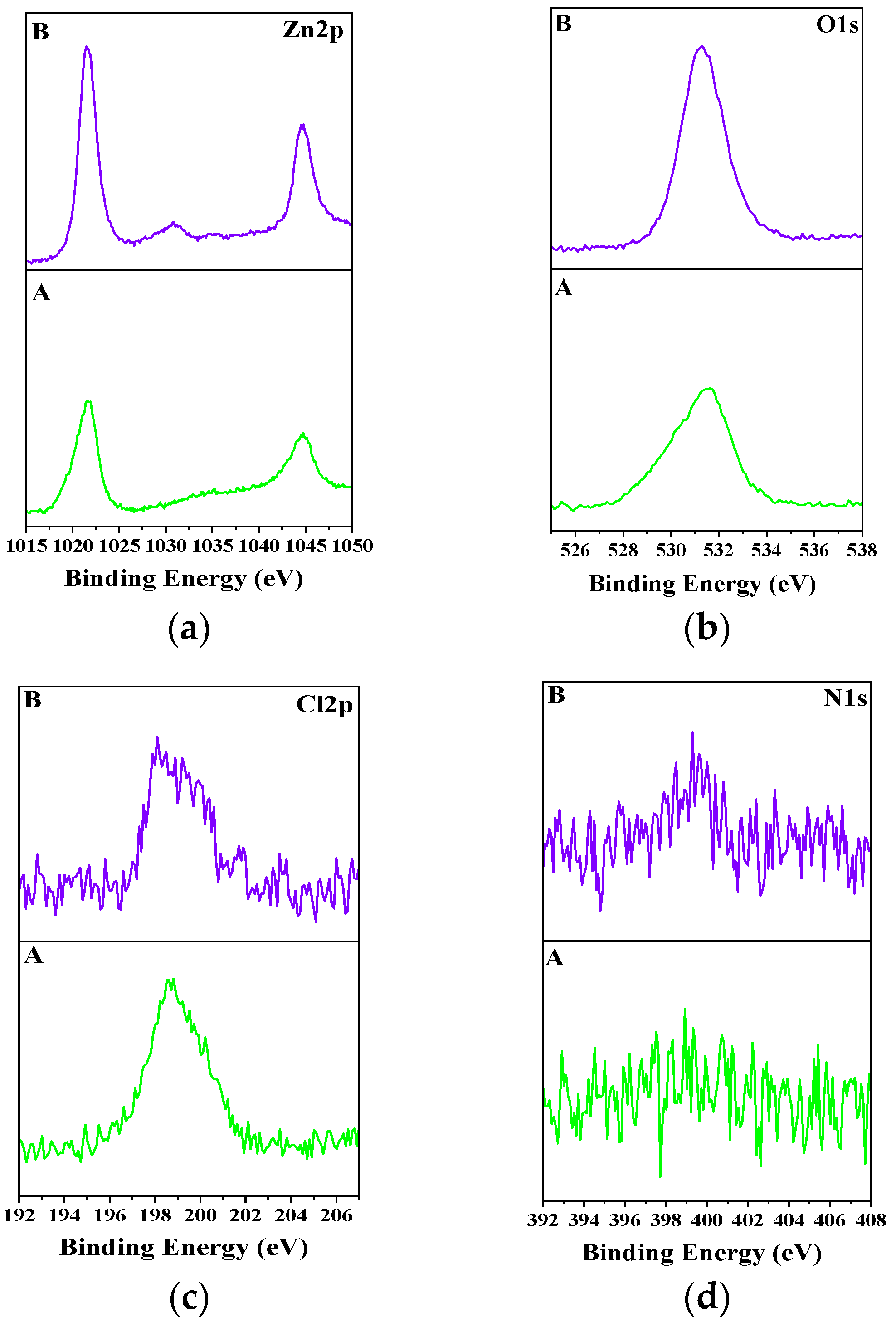
3.6. XPS Investigations
The experimental data for Zn-PANI/SiO
2 hybrid coating are presented in
Figure 7. Similar investigations concerning the ordinary zinc have been previously realized elsewhere [
41,
42,
45] and the results obtained unambiguously confirmed the presence of the compound ZHC on the sample. Having in mind these results it can be concluded that ZHC appears also on the hybrid coating (see Zn 2
p, O 1
s and Cl 2
p spectra).
It is observed that the binding energy (BE) values of the Zn 2
p3/2 peak of the corrosive untreated and corrosive treated hybrid samples are very close-1021.5 and 1021.9 eV, respectively. These values correspond mainly to the Zn-O bond and indicate the presence of ZnO. In addition, the presence of Zn(OH)
2 and ZnC1
2 is also possible according to [
46,
47]. Considering this and also BE values of O 1
s (531.3 eV) and Cl 2
p (198.6 eV) peaks the appearance of the compound Zn
5(OH)
8Cl
2·H
2O can be supposed. Quantitatively, Zn 2
p3/2 and O 1
s peaks of the corrosive treated sample are greater which could be assumed as a possible sign for greater amounts of ZHC according to the data presented elsewhere [
41].
Concerning the Cl 2
p peak of the corrosive untreated Zn-PANI sample, it seems to be a residue from the organic additive applied in the starting electrolyte. As already commented [
41,
42,
43,
44,
45] ZHC increases the protective characteristics of the coating due to the higher diffusion limitations and barrier effects as a result of its low product of solubility value.
The presence of N 1s peak for both samples can be assumed as an evidence for the availability of PANI in the coating. This peak is better expressed for the corrosive treated coating most probably due to the accelerated dissolution of the metallic zinc areas leaving more particles near the corroded surface.
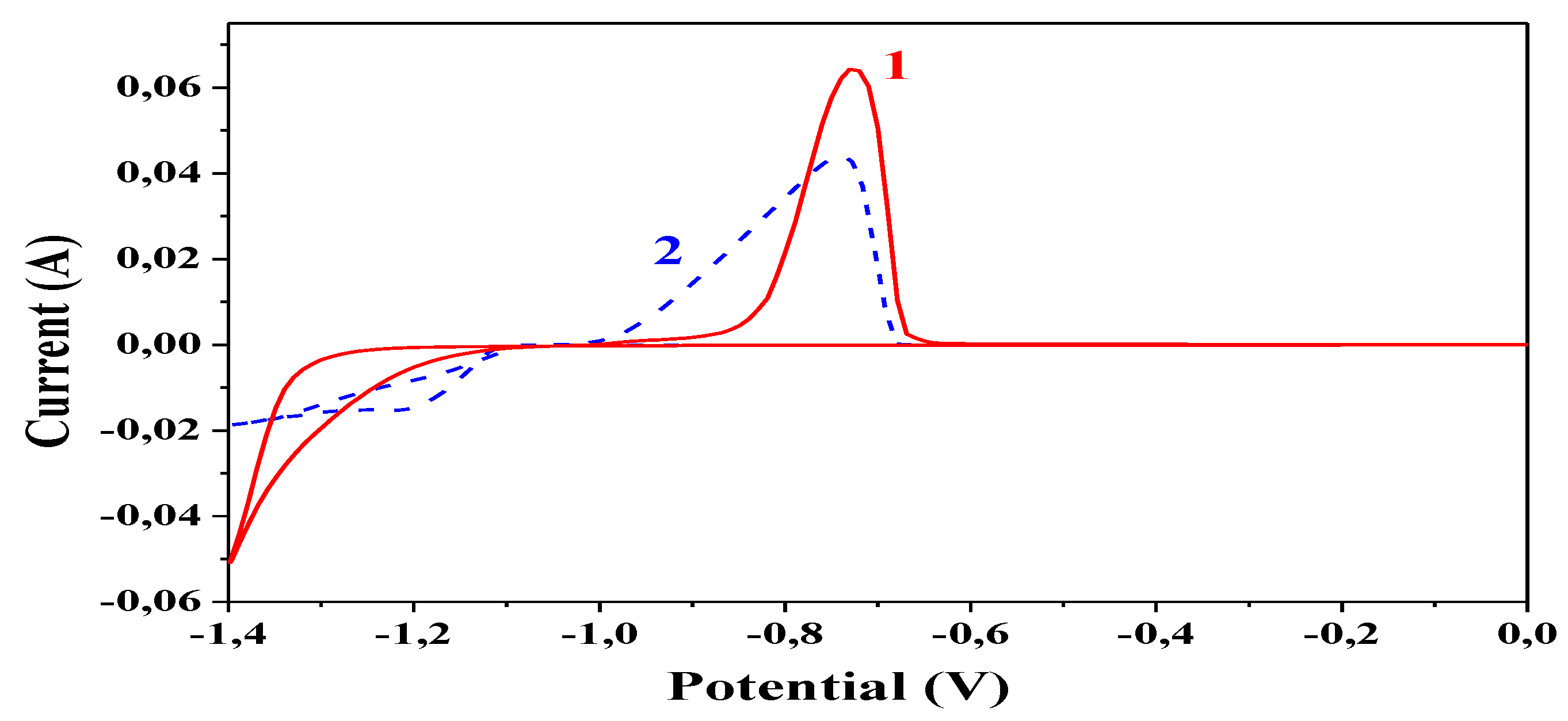
3.7. Cyclic Voltammetry
The experiments realized via this method are reported in
Figure 8. They are carried out in the starting electrolytes for the electrodeposition of ordinary Zn (curve 1) and Zn-PANI/SiO
2 (curve 2) hybrid coating. The presence of PANI particles (dashed curve) strongly affects both the cathodic and anodic processes. The electrodeposition of the hybrid coating starts at a more positive potential value (−1100 V compared to about −1250 V for the ordinary zinc coating, i.e., a depolarization effect is registered in that case). This leads to the conclusion that the availability of PANI particles in the starting electrolyte will particularly ease the processes in the cathodic region.
Most probably, this phenomenon follows from the fact that the conductive PANI particles which are greater in size compared to the zinc ions will cover a larger area on the cathodic surface, mitigating in such a way the zinc electrodeposition. Contrary to this, in the case of the ordinary zinc coating an overvoltage effect can be observed. However, the presence of PANI-particles leads to a delayed electrodeposition process thereafter, and the cathodic current of the hybrid coating at the vertex potential (−1.4 V) is about 2.5 lower compared to the ordinary zinc.
The anodic branches of both curves correspond to the cathodic ones having in mind the current values and the amount of the electrodeposited masses.
3.8. Scanning Electron Microscopy after Corrosion Treatment
The surface morphology of Zn-PANI/SiO
2 hybrid coating (at two magnifications) after corrosion treatment in the model medium is presented in
Figure 9. It is well visible that the immersion into the test solution has changed the coating surface leading to greater non-homogeneity and revealing some of the incorporated PANI-particles. As abovementioned in
Section 3.6, their presence is confirmed by N 1
s spectra before and after (more pronounced) the immersion in the model medium. It can be supposed that the availability of PANI-particles will improve the protective characteristics of the coating due to their inhibitor effect.
4. Summary
According to the literature sources it is well known that in recent years, several methodologies have been proposed for the application of PANI-based coatings [
48]:
as a primer alone;
as a primer coating with conventional topcoats or with other films;
blended with conventional polymer coatings, such as epoxy or polyurethane;
as an anticorrosive additive to the paint formulation, and
as a matrix where nanoparticles or nanostructures materials are incorporated.
The electrochemically deposited PANI exhibits a significant resistivity and has good anticorrosion properties, also in controlling pitting corrosion resulting from the permeation and breakdown of the protective coating [
48].
The presented investigations are carried in order to obtain corrosion resistant hybrid zinc coatings with dispersed PANI-particles via electrodeposition on low-carbon steel substrate. The main object of the experiment is the attempt to incorporate PANI-particles into the zinc coating matrix increasing in such a way its corrosion resistance. An important advantage is the fact that the coating is easily obtained without need of additional operations or equipment—that is, zinc and inhibitor particles are deposited simultaneously in a one-step process from one electrolyte, whereby the resulting coatings are reproducible and of good decorative quality.
The applied simple electrolyte is similar in composition to the weakly acidic electrolytes currently applied for the galvanizing of steel. Polyaniline in an aqueous dispersion is a suitable component of the hybrid coating not only because of its high effectiveness as an inhibitor of the corrosion of metals and alloys, but also because it is resistant to chemical and temperature effects. The dispersion is applied in low concentrations and is obtained from available raw materials.
The main advantage of the Zn-PANI/SiO2 hybrid coating is its definitely higher corrosion resistance in chloride containing medium compared to the ordinary Zn which is proved with selected electrochemical and physical methods. This will allow an extension of the service life of the products or parts leading to a decrease of the corrosion damages in an aggressive medium with chloride ions. The increased protective properties of the hybrid coating seem to be a result of the apparent mixed layer of corrosion products and PANI-particles, which improve the barrier characteristics due to their inhibitory properties.
5. Conclusions
Two types of protective Zn-PANI hybrid coatings have been electrodeposited at equal electrodeposition conditions from a starting electrolyte for ordinary zinc, the latter containing a small number of dispersed PANI-particles.
The Zn-PANI/SiO2 coating seems to be more even and uniform, containing particles with almost equal shapes and sizes. Contrary to this, the hybrid Zn-PANI/PVP coating distinguishes, with greater inhomogeneity, protruded and concaved zones with thinner or thicker thicknesses, which can be a prerequisite for a relatively more pronounced corrosion process in the model medium with chloride ions.
Since the inhibitor activity of the PANI dispersion does not depend on the type of stabilizer—PVP or silicon dioxide [
49]—the main reasons for the improved protective ability of the Zn-PANI/SiO
2 hybrid coating seem to be as follows:
uniform thickness and better surface morphology, and
the appearance of a mixed layer of corrosion products (ZHC etc.) containing more uniformly distributed PANI-particles, which ensures a better barrier and inhibitor effect.


 —Zn(OH)2; ☐—ZnO.
—Zn(OH)2; ☐—ZnO.
 —Zn(OH)2; ☐—ZnO.
—Zn(OH)2; ☐—ZnO.