Abstract
Recently, edible films were shown to be an effective strategy for the delivery of functional ingredients, such as probiotics and prebiotics. With that in mind, two soluble fibres (inulin and fructooligosaccharides) were selected as prebiotic elements, in whey protein isolate (WPI) and alginate (ALG) matrices plasticized with glycerol and used for the incorporation of Bifidobacterium animalis subsp. lactis BB-12. The results obtained showed that the viability of the B. animalis subsp. lactis BB-12 probiotic strain was maintained within the minimum threshold (106 CFU/g) necessary to act as a probiotic throughout 60 days of storage at 23 °C. The incorporation of prebiotic compounds improved B. animalis subsp. lactis BB-12 viability, with inulin showing the best performance, as it maintained the viability at 7.34 log CFU/g. The compositional characteristics (biopolymer type and prebiotics addition) of the film forming solutions had no significant impact upon the viability of the probiotic strain. The incorporation of probiotics and prebiotics did not modify the infrared spectra, revealing that the molecular structure of the films was not modified. The moisture content and water solubility decreased positively in WPI- and ALG-based films with the addition of prebiotics compounds. Overall, the results obtained in this work support the use of WPI films containing inulin as a good strategy to immobilize B. animalis subsp. lactis BB-12, with potential applications in the development of functional foods.
1. Introduction
Environmental problems associated with non-natural products used in food packaging, such as synthetic plastics and other materials, and the demand for high food quality have led to the development of innovative food packaging systems. Food coatings and films have specific characteristics, such as renewability, degradability, and edibility, that make such materials suitable for food packaging applications, which are essential to preserve the physical, organoleptic, and nutritional value of food during storage, transportation, and distribution [1,2]. Furthermore, edible films incorporated with bioactive compounds promote new functionalities or extend the shelf life of food products and open new possibilities as a carrying material for functional bacteria and prebiotics beyond basic nutrition [3,4,5].
Edible films can be obtained from several materials such as lipids [6], polysaccharides [7,8], and proteins [9,10,11], or by blending of these compounds.
Among biopolymers used to produce edible films, proteins have received considerable interest since it provides a film with distinct and valuable properties. Edible films from whey protein isolate (WPI) possess relevant sensorial, optical, and mechanical barrier properties, besides its positive transparency and lack of taste and odor, which can make them a favorable carrier for functional compounds [12,13,14,15,16,17,18,19].
In addition to protein films, alginate (ALG) films are also widely used today. An ALG film can act as a semipermeable barrier to moisture, gases, and aromatics, while maintaining structural integrity and handling characteristics, and holding the capacity to retain volatile aromatic compounds from food products. Furthermore, it can be also considered a carrier for several compounds, such as anti-browning agents, colorants, flavors, nutrients, spices, and antimicrobials that can decrease the risk of pathogen growth on food surfaces and probiotics [20,21,22]. Several of these biopolymer-based films have some inherent fragility, and therefore the use of plasticizers is crucial to obtain adequate flexibility for films manufacturing. Thus, the use of glycerol as a plasticizer is widely accepted, since it is recognized by the ability in decreasing material brittleness, and in addition, is a by-product of biodiesel production [23].
According to some authors, probiotics have a large number of benefits, among which are the adjustment of the gastro-intestinal microflora, increase in immune system activity, decrease of cholesterol, prevention of cardiovascular disease, and several forms of cancer [24,25]. Probiotics can be incorporated into the food matrices through the previously described edible films [3,5,26,27,28], since it is important to protect them from the damage induced by environmental conditions generated in and outside the matrices, or by food processing and storage [29].
Since probiotics viability and microbial load are significant for their efficacy, prebiotics addition may be of key importance, since they have potential to improve probiotic cell numbers, its survival in the gastrointestinal tract, and its further attachment to the intestine [30]. The definition of prebiotics was recently reviewed and considered a substrate that is selectively utilized by host microorganisms conferring a health benefit [31]. It has been reported that the ingestion of prebiotics prevents several forms of cancer [32,33] and some intestinal disorders, such as ulcerative colitis and irritable bowel disease [34]. Furthermore, prebiotics can be added successfully as co-components for microencapsulation conferring a beneficial effect on probiotics cell viability in a dried format [35]. In addition, the symbiotic combination of prebiotics and probiotics promotes the inhibiting of human or animal pathogens and promote bifidogenicity [36].
The products containing both probiotics and prebiotics are commonly defined as “symbiotic”, attributed to products in which the prebiotic selectively favors the probiotics strains [37]. Recently, some authors suggested the inclusion of prebiotics was a suitable strategy to preserve probiotics in films [5,38,39].
While those studies represent innovations of great interest, studies linking the combination of probiotics with prebiotics in edible films are, to the best of our knowledge, still quite limited. Therefore, the main objective of this study was to compare the microbiological and physicochemical characteristics of ALG- and WPI-based films incorporated with Bifidobacterium animalis subsp. lactis BB-12 and with prebiotic compounds, namely, inulin and fructooligosaccharides (FOS).
2. Materials and Methods
2.1. Bacterial Strain, Media and Growth Conditions
Bifidobacterium animalis subsp. lactis BB-12 (Christian Hansen, Hørsholm, Denmark) was stored at −80 °C in de Man Rogosa and Sharpe (MRS) broth (Biokar Diagnostics, Allonne, France) supplemented with 30% (v/v) glycerol. Afterwards, the probiotic strain was reactivated in MRS broth supplemented with sterilized L-cysteine·HCl at 0.05% (w/v) (Fluka, St. Gallen, Switzerland), incubated at 37 °C throughout 24 h in an anaerobic chamber (Whitley DG250, West Yorkshire, UK), and cells harvested by centrifugation (4000 rpm for 30 min; Universal 320R, Hettich, Tuttlingen, Germany) at 4 °C. The obtained pellet was suspended in a 0.9% (w/v) NaCl solution for film solution incorporation.
2.2. Film Formulations
WPI film forming solution was prepared by dissolving WPI at 10% (w/v) (Armor Proteins, Saint Brice en Coglés, France) in deionized water, as demonstrated by Pérez-Gago and Krochta [40], glycerol (Panreac, Barcelona, Spain) was included at 5% (w/w), and solutions homogenized during 2 h at room temperature.
ALG-based film forming solutions were prepared by dissolving sodium-ALG at 2% (w/v) (FMC Biopolymer, Cork, Ireland) and glycerol at 1.2% (w/w) in deionized water and homogenized for 2 h at room temperature. Then, both solutions were heated in a water bath at 80 °C for 20 min and cooled at room temperature. The prebiotics were included in the film formulation by adding high soluble inulin at 2% (w/v) (Orafti®HIS, BENEO, Mannheim, Germany) or oligofructose at 2% (w/v) (Orafti®P95, BENEO, Mannheim, Germany), and the probiotic strain (5% v/v), was incorporated to reach a final concentration of 109 CFU/mL.
Films were prepared according to Gounga et al. [41] and Oses et al. [42]. Briefly, 300 mL of the final solution was cast in sterile Teflon plates and dried at room temperature for 24 h in a ventilated incubator. Subsequently, the films were peeled off and conditioned in a controlled storage room (Packaging Center, CBQF, Porto, Portugal) at 23 ± 2 °C and 50% ± 2% RH, for at least 72 h prior to testing [43]. Films with only probiotics were prepared as controls. All films were produced in triplicate and are described in detail in Table 1.

Table 1.
Compositional aspects of the film forming solutions.
2.3. Enumeration of Bacteria and Storage Stability
Films of circular shape (1 cm in diameter) were stored in plastic bags under vacuum conditions at 23 °C and sampled at 0, 3, 5, 10, 40, and 60 days of storage. At each sampling point, the disks were added to 2 mL of sterile peptone water (1 g/L), vortexed for 1 min and plated on MRS at 37 °C for 48 h under anaerobic conditions as described above.
2.4. Film Characterization
2.4.1. Thickness
Film thickness was measured from four independent measurements in each film, taken randomly at different locations, by using a micrometer Model m120 (from Adamel Lhomargy, Roissy en Brie, France).
2.4.2. Water Activity
The water activity (aw) was measured using a HygroLab 2 (Bassersdrof, Germany). Films (ca. 0.5 g) were placed on the sample holder and a sealed system was formed by placing the water activity probe on top of the sample holder. The probe was equipped with a small fan to circulate air inside the sample container, a thin film capacitance sensor able to measure RH from 0 to 100% ± 1.5%, and a platinum resistance temperature detector with a precision of ±0.3 °C. When aw became constant, its value was recorded. Calibration curves were drawn using six saturated solutions of known aw (viz. LiCl = 0.114, MgCl2 = 0.329, K2CO3 = 0.443, Mg(NO3)2 = 0.536, NaBr = 0.653, and KCl = 0.821). The tests were performed in quadruplicate.
2.4.3. Moisture Content
Film samples (0.5 g) were dried at 105 °C in an oven for 24 h until constant weight, and the moisture content was determined as a percentage of weight loss after drying and reported on a wet basis. The analysis was performed in quadruplicate for each film.
2.4.4. Water Solubility
The previously dried film samples were immersed in 50 mL of distilled water at 25 °C during 24 h, and the samples were filtered through Whatman No. 1 filter paper and dried at 105 °C for 24 h. The water solubility (WS) was calculated using the following equation described by Norajit et al. [44]:
where W0 and Wf are initial and insoluble dry matter, respectively. All tests were carried out in quadruplicate for each film tested.
WS (%) = [(W0 – Wf)/W0] × 100
2.4.5. Film Color
Film color was performed using a portable Chroma meter CR-400 (from Minolta Chroma, Osaka, Japan) with a *C D65 illuminant, a light source of pulsed xenon lamp, an aperture size of 8 mm, a closed cone, and a standard observer of 2° closely matches CIE 1931 (λ, λ, λ). A CIELab color scale was employed to measure the degree of lightness (L), redness (+a) or greenness (−a), and yellowness (+b) or blueness (−b) of the films. Film disks were measured, on the surface of the white standard plate, with color coordinates Lstandard = 97.7, astandard = 0.04 and bstandard = 1.47. The color of the films was expressed as the total difference in color (ΔE), calculated according to the equation bellow.
ΔE = [(Lfilm – Lstandard)2 + (afilm – astandard)2 + (bfilm – bstandard)2]1/2
For each condition, four samples were measured and on each film disk, four readings were made on each side.
2.4.6. Texture Analysis
Texture analysis was performed using a texturometer (TA.XT plus Texture Analyzer, Stable Micro Systems, Cardiff, UK) with rectangular film probes (100 mm × 15 mm) following the ASTM D-882-02 standard [43] at 23 ± 2 °C and 50% ± 2% RH. Force calibration was achieved with a weight of 5 kg and height calibration was performed for Mini Tensile Grips (Stable Micro Systems). Assessed parameters were Young’s modulus (Equation. (3)), Tensile strength, and Elongation at break. Tensile strength (MPa) is the maximum tensile stress that the test sample can carry. Elongation at break (%) was obtained as the strain at the fracture point, corresponding to the ratio of the change of length of the specimen to initial length. All measurements were performed five times for each film formulation.
2.4.7. FTIR-ATR Analysis
The spectra of films were obtained with a FTIR, model ABB MB3000 (ABB, Zürich, Switzerland), with a horizontal attenuated total reflectance (ATR) accessory (PIKE Technologies, Madison, WI, USA) with a diamond/ZnSe crystal. All spectra were acquired with 32 scans and 4 cm−1 resolution, in the region of 4000–600 cm−1. Three measurements were collected for each film surface sample.
2.5. Statistical Analyses
Statistical analyses were done by the Statistical Package for Social Sciences, v. 17.0 (SPSS, IBM, Chicago, IL, USA), via two-way ANOVA at the 0.05 level of significance. To test for significant differences in microbiological and physicochemical properties, a Fischer’s least significant difference (LSD) test was used.
3. Results and Discussion
In this work, we have studied the physicochemical and microbiological properties of WPI- and ALG-based films with incorporation of two types of prebiotics to evaluate the possible synergisms of probiotics strains with those prebiotics. It has been recognized that a synergistic mixture of probiotic bacteria and prebiotics encourages intestinal colonization and has been associated with a reduction of the risk of developing several forms of cancer. The structure of some oligosaccharides makes them resilient to digestive enzymes, and thus, they can reach the large intestine where they become available to be fermented by some beneficial bacteria [45]. Thus, prebiotics addition to bioactive edible films and coatings represents an appealing technological solution for the protection of probiotic bacteria embedded with edible films. In this way, the film’s chemistry and the film forming procedure is crucial for microbial survival during the storage period and their resistance to the digestive process present imperative parameters that affect the films performance.
3.1. Viability of B. animalis subsp. lactis BB-12 during the Drying Process
The changes in viable counts of B. animalis subsp. lactis BB-12 during the drying process are displayed in Figure 1. According to the ANOVA results, the type of film forming solution had no significant impact (p > 0.05) upon the inactivation of the probiotic strain. Overall, only a mean reduction of 0.40 and 0.24 log CFU/g were detected in WPI and ALG-based films, respectively. Therefore, no severe toxic effects were observed upon the survival of B. animalis subsp. lactis BB-12 in the film forming solutions. Furthermore, viability losses due to heat induced injuries should were considered as non-significant due to the low drying temperatures used [5]. In fact, during the drying process (23 °C, 50% RH, 24 h), no significant (p > 0.05) decrease was observed in B. animalis subsp. lactis BB-12 viability, with the only exception being for WCBA films in which case a significant (p < 0.05) reduction in viable counts was verifies.
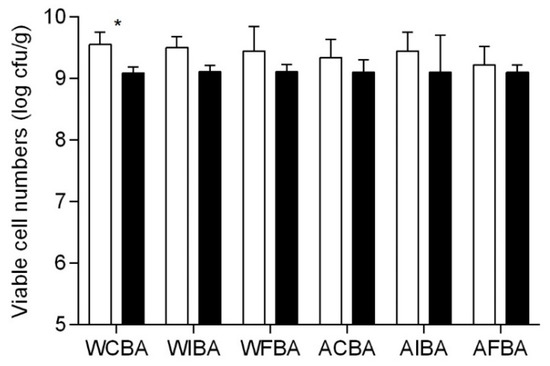
Figure 1.
Bifidobacterium animalis subsp. lactis BB-12 total viable counts during air drying for each film composition (as described in Table 1). Each bar represents the mean with standard deviation of the film samples produced (n = 3). Asterisk indicate significant difference between the drying process. *: p < 0.05; white bar = start of drying, and black bar = end of drying.
3.2. Viability of B. animalis subsp. lactis BB-12 in Films during Storage
Storage conditions are one of the most important factors when considering the stability of probiotics. Figure 2 shows the survival of the probiotic strain incorporated (stored for 60 days at 23 °C) into the biopolymer-based films with or without prebiotics addition. The viability of B. animalis subsp. lactis BB-12 demonstrated a negative correlation with the storage time (p < 0.0001), i.e., the number of viable cells of B. animalis subsp. lactis BB-12 dropped from an initial population of 109 CFU/g to 105–106 in WCBA and ACBA (controls) films, and to 106–107 CFU/g in films containing prebiotics after storage. Moreover, the difference between prebiotic containing films and the controls was statistically significant (p < 0.0001) for all biopolymer-based films assayed. Overall, the highest viability loss was observed in ACBA at the end of the 60 days storage period, with the viable cells counts reaching only 105 CFU/g film.
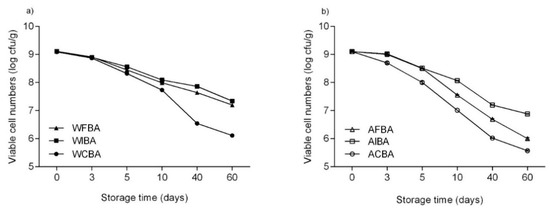
Figure 2.
Survival of B. animalis subsp. lactis BB-12 during storage (60 days) at room (23 °C) temperature in (a) whey protein isolate (WPI)-based and (b) alginate (ALG)-based films with or without prebiotic incorporation. Each time point presented corresponds to the mean with standard deviation of the film samples analyzed (n = 3).
Regarding the effect of prebiotics’ incorporation, films with inulin showed a significantly higher survival rate of B. animalis subsp. lactis BB-12 in either WPI- and ALG-based coatings after 60 days of storage. Considering that, in control films (WCBA and ACBA) a 3.3 mean log reduction was observed after 60 days of storage, while in films with inulin (WIBA and AIBA) or FOS (WFBA and AFBA) a 2.0 and 2.5 mean log reduction was detected, respectively. Our results suggest that prebiotics addition may play an important role in improving the viability of B. animalis subsp. lactis BB-12 strain when incorporated into edible films or coatings. Overall, there are few studies focusing on the incorporation of prebiotics in probiotic containing edible films, in an attempt to improve the stability of the incorporated probiotic strains. Similar findings were described by Soukoulis, Behboudi-Jobbehdar, Yonekura, Parmenter, and Fisk [5] when studying the stability of L. rhamnosus GG in prebiotic containing films. They observed that the supplementation of edible films with prebiotics improved the storage stability of the probiotic strain considered with inulin being the most effective prebiotic (based on its capacity to maintain the survival of L. rhamnosus GG), followed by wheat dextrin, glucose oligosaccharides and polydextrose. Similarly, Romano, Tavera-Quiroz, Bertola, Mobili, Pinotti, and Gómez-Zavaglia [38] demonstrated that the incorporation of 3% (w/v) FOS into methylcellulose-based films also improved the viability of L. delbrueckii subsp. bulgaricus CIDCA 333 after film preparation.
Overall, WPI-based films experienced a 2.2 mean log reduction while ALG-based film registered a 3.0 mean log reduction of viable cells. These results suggest that the stability of probiotics was promoted by the addition of whey protein to the films solutions, by providing nutrients to the cells and reducing redox potential of the medium as well as through the increase of the buffering capacity of the medium [5,46,47]. Soukoulis, Yonekura, Gan, Behboudi-Jobbehdar, Parmenter, and Fisk [26] studied the development of probiotic baked cereal products (with L. rhamnosus GG) through the application of film solutions comprised of either 1% (w/w) sodium ALG or binary blends of 0.5% (w/w) sodium ALG and 2% (w/w) whey protein concentrate containing with the samples with whey protein exhibiting an improved survival of L. rhamnosus GG throughout room temperature storage.
Although, the results indicated a significant reduction in the viability of the probiotic strains, the levels of viable cells still assure the recommended viable cell counts for probiotic bacteria to be delivered to the humans [48], since the commonly accepted concentration of 106 viable CFU/g was maintained until the end of storage at room temperature [30,49].
3.3. Edible Films Physical Properties
To be used as food coating materials, edible films should be sufficiently resilient to external factors, while also being elastic and remaining strong during packaging and storage [50]. Thus, the physical properties of protein- and polysaccharide-based films (containing prebiotics as possible carriers for functional bacteria) were studied during 60 days of storage. In Table 2, the thickness, aw, moisture content, and water solubility properties of those films can be found. Thickness is a critical parameter that influences, among others, the mechanical properties of the films and also contribute to improve the mechanical integrity of food products [51]. The thickness ranged from 0.117 to 0.400 mm among protein and polysaccharide-based films. Prebiotics incorporation was not a statistically significant (p > 0.05) factor influencing films’ thickness. Similar results were previously observed by Odila Pereira, Soares, Sousa, Madureira, Gomes, and Pintado [3] during 60 days of storage (at 23 and 4 °C) of edible films incorporated with lactic acid bacteria. Soukoulis, Behboudi-Jobbehdar, Yonekura, Parmenter, and Fisk [5] also reported no significant modifications of film thickness due to the addition of prebiotic fibers to probiotic films. Furthermore, ALG-based films were significantly thinner than WPI films (p < 0.0001) with similar findings being reported by Soukoulis, Yonekura, Gan, Behboudi-Jobbehdar, Parmenter, and Fisk [26] whom showed that ALG-based films with probiotics had a significantly lower thickness than WPC-based films.

Table 2.
Physicochemical and color properties of edible films containing B. animalis subsp. lactis BB-12 and different types of prebiotics fibres. The results are displayed as mean ± standard deviation of the film samples (n = 3).
Moisture content, an important parameter for measuring mouth melting of edible films, also affects probiotics viability during long term storage [51]. Inulin or FOS incorporation was associated with a decrease (p < 0.001) in the moisture content of edible films. Therefore, the highest moisture content was exhibited in WCBA and ACBA films. According to the ANOVA results, the biopolymer type had no significant differences in moisture content (p > 0.05). Similarly, the impact of prebiotic addition or biopolymer type upon aw was also not significant (p > 0.05).
Generally, food applications may require low water solubility to improve the product integrity and water resistance but, in some cases such as food coatings, a high water solubility might be beneficial [52]. All WPI-based films dissolved in water after 24 h, whereas the solubility among ALG films was of around 70%. The incorporation of prebiotics into edible films had no significant impact upon the film’s solubility (p > 0.05).
To ensure the acceptability of food coatings, color is a crucial parameter not only from the consumer’s standpoint but also for the packaging of light-sensitive materials [53]. Table 2 displays the color characteristics of the edible films containing B. animalis and different types of prebiotics.
Both biopolymer-based films showed high brightness values (L* ≥ 93.13), demonstrating that films appeared clear and transparent. The color values for WPI films are in agreement with those reported by Odila Pereira, Soares, Sousa, Madureira, Gomes, and Pintado [3], and color values for ALG films were similar to the values obtained by Moreira, Gullón, Gullón, Gomes, and Tavaria [52]. The ALG-based films exhibited higher luminosity (L*) values than WPI- based films, which could be attributed to their lower solid contents and subsequent lower thickness (Table 2). In addition, the incorporation of prebiotics was not associated with differences (p > 0.05) in L* values of the WPI and ALG films.
According to ANOVA results, film type (WPI vs ALG) had a significant effect (p < 0.0001) upon a* and b* values. With WPI-based films exhibiting the highest (p < 0.0001) scores for green and yellow hue color components, which confirms previous findings [54]. In terms of color differences (ΔE*), ALG-based films had the lowest and WPI films, particularly WFBA, had the highest color divergence from films without prebiotics, respectively. Nevertheless, ΔE* values were lower than three which have been reported as the threshold of human perceivable color differences.
3.3.1. Mechanical Properties of Films
Typically, edible films must have good mechanical properties in order to resist the external factors involved in processing, management, and storage of the food products [47]. The mechanical aspects of the different types of films can be found in Table 3. To best of our knowledge, this is the first report evaluating the combined effect of prebiotics and probiotics upon texture parameters of WPY- and ALG-based films. The addition of the plasticizer (glycerol) facilitated the development of flexible and extensible films. The polysaccharide-based films exhibited similar mechanical profiles i.e., higher stiffness and tensile strength and lower elongation properties compared to protein-based films. Films containing WPI were more extensible, which may be due to its protein network. Regarding the addition of prebiotic compounds to WPI- and ALG-based films, significant differences were found when comparing the tensile strength of the films (p < 0.0001) while no effects on elongation at break (p > 0.05) or on Young’s modulus (p > 0.05) were observed.

Table 3.
Mechanical properties of edible films containing B. animalis subsp. lactis BB-12 and different types of prebiotics fibres. The data is displayed as mean ± standard deviation of the film samples (n = 3).
3.3.2. Fourier Transform Infrared Spectroscopy (FTIR) Measurements
FTIR was performed in order to consider potential changes in the molecular structure of biopolymer-based (WPI or ALG) films incorporated, or not, with Inulin or FOS. FTIR spectra of the different films can be observed in Figure 3. The most relevant peaks were at 3600–3000 cm−1, 3000–2800 cm−1, and 1700 and 800 cm−1. The broad band 3600–3000 cm−1 was attributed to a stretching vibration of –OH and –NH groups [55]. The range between 3000 and 2800 cm−1 was assigned to the C–H stretching vibrations of the carbonyl groups of triglycerides. The peaks observed at 2924 and 2854 cm−1 were identified as related to the fat present in dairy products [56]. The area of these peaks is greater in WPI-based films, which might be due to the larger amounts of fat found in WPI-based films. Between 1700 and 1500 cm−1, highest peaks were observed, with significant differences being found among the samples. Two major peaks are clearly evidenced in WPI-based-films (amide I (1640 cm−1) and amide II (1550 cm−1) which are related to peptide bonds (CO–NH). These peaks are closely associated with the sample’s protein concentration. As expected, WPI-based films exhibited the highest spectral intensity height due to the higher amount of protein. Consequently, in Figure 3, the decrease in intensity of the amide I peak, and lack of amide II was observed in ALG-based films. Finally, the region between 1150 and 800 cm−1, the absorption bands observed were attributed to glycerol [3]. This spectrum region remained practically unchanged with prebiotics incorporation. However, a slight decrease in band intensity was observed when comparing WPI- and ALG-based films, which could be related to the migration of glycerol [57].

Figure 3.
Fourier transform infrared spectroscopy (FTIR) spectra of (a) whey protein isolate (WPI)-based and (b) alginate (ALG)-based films containing B. animalis subsp. lactis BB-12 with or without prebiotic incorporation at 0 days of storage.
Overall, the hereby described results are in accordance with that of Odila Pereira, Soares, Sousa, Madureira, Gomes, and Pintado [3], in terms of FTIR spectra in regards to the spectra of WPI-based film formulations. Augusto et al. [58], who made ALG edible films with Codium tomentosum seaweed extract, also reported no significant differences between the FTIR spectra of an ALG film with and without seaweed extract in terms of wavenumber absorbance.
4. Conclusions
Overall, WPI films were more effective in preserving B. animalis subsp. lactis BB-12 viability compared to ALG films. Both prebiotic compounds added effectively protected the functional bacteria throughout the storage period, however, the most effective results were obtained with inulin incorporation.
Alginate-based films were thinner and presented a lower water solubility. FTIR spectroscopy provided structural information about edible films loaded with prebiotics, and no structural changes were founded after addiction of probiotics and prebiotics. Our results suggest that edible films containing inulin or FOS and developed in this work can be considered as a good carrier for functional bacteria to be ingested together with food and simultaneously exert specific biological activities upon the human organism.
Author Contributions
Conceptualization, J.O.P., A.G. and M.P.; Methodology, J.O.P.; Software, J.O.P.; Validation, A.G. and M.P.; Investigation, J.O.P.; Data Curation, J.O.P.; Writing—Original Draft Preparation, J.O.P., S.S., E.C. and J.S.; Writing—Review and Editing, J.S., A.G. and M.P.; Supervision, A.G. and M.P.
Funding
This research received no external funding.
Acknowledgments
The authors gratefully acknowledged the Fundação para a Ciência e Tecnologia (FCT), Portugal for providing Ph.D. scholarship (No. SFRH/BD/88383/2012) to Joana Odila Pereira. We would also like to thank the scientific collaboration of CBQF under the FCT project UID/Multi/50016/2013.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Espitia, P.J.P.; Du, W.X.; de Jesús Avena-Bustillos, R.; Soares, N.d.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Odila Pereira, J.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT Food Sci. Technol. 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Grau, M.A.; Rodriguez, F.J.; Ramirez, J.; Carmona, A.; Martin-Belloso, O. Alginate- and gellan-based edible films for probiotic coatings on fresh-cut fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, A.; Debeaufort, F.; Bonnotte, A.; Voilley, A. Influence of alginate emulsion-based films structure on its barrier properties and on the protection of microencapsulated aroma compound. Food Hydrocoll. 2009, 23, 2116–2124. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Physical properties and antioxidant capacity of starch–sodium caseinate films containing lipids. J. Food Eng. 2013, 116, 695–702. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin-chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Crit. Rev. Food Sci. Nutr. 2011, 52, 533–552. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Silva, S.I.; Soares, J.C.; Fernandes, J.C.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Features and performance of edible films, obtained from whey protein isolate formulated with antimicrobial compounds. Food Res. Int. 2012, 45, 351–361. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Edible films and coatings as active layers. In Active Food Packaging; Springer: Boston, MA, USA, 1995; pp. 111–142. [Google Scholar] [CrossRef]
- Wittaya, T. Protein-based edible films: Characteristics and improvement of properties. In Structure and Function of Food Engineering; Eissa, A.A., Ed.; IntechOpen Limited: London, UK, 2012; pp. 43–70. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L. Edible films and coatings from wheat and corn proteins. Food Technol. 1990, 44, 63–69. [Google Scholar]
- Fang, Y.; Tung, M.; Britt, I.; Yada, S.; Dalgleish, D. Tensile and barrier properties of edible films made from whey proteins. J. Food Sci. 2002, 67, 188–193. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.; Krochta, J. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, R30–R39. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.; Krochta, J. Thermal transitions and heat-sealing of glycerol-plasticized whey protein films. Packag. Technol. Sci. 2009, 22, 255–260. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Pereira, J.; Silva, S.I.; Fernandes, J.C.; Franco, M.; Lopes-da-Silva, J.; Pintado, M.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Rhim, J.W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings to incorporate active ingredients to fresh-cut fruits: A review. Trends Food Sci. Technol. 2009, 20, 438–447. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.d.C.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef]
- Enujiugha, V.N.; Badejo, A.A. Probiotic potentials of cereal-based beverages. Crit. Rev. Food Sci. Nutr. 2017, 57, 790–804. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- Soukoulis, C.; Yonekura, L.; Gan, H.H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef]
- Rößle, C.; Brunton, N.; Gormley, R.T.; Wouters, R.; Butler, F. Alginate coating as carrier of oligofructose and inulin and to maintain the quality of fresh-cut apples. J. Food Sci. 2011, 76, H19–H29. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT Food Sci. Technol. 2014, 55, 559–564. [Google Scholar] [CrossRef]
- Fu, N.; Chen, X.D. Towards a maximal cell survival in convective thermal drying processes. Food Res. Int. 2011, 44, 1127–1149. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Inulin-type fructans: Functional food ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [CrossRef]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Bosscher, D.; Loo, J.V.; Franck, A. Inulin and oligofructose as prebiotics in the prevention of intestinal infections and diseases. Nutr. Res. Rev. 2006, 19, 216–226. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Mugambi, M.N.; Musekiwa, A.; Lombard, M.; Young, T.; Blaauw, R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: A systematic review. Nutr. J. 2012, 11, 81. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [CrossRef]
- Romano, N.; Tavera-Quiroz, M.J.; Bertola, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A. Edible methylcellulose-based films containing fructo-oligosaccharides as vehicles for lactic acid bacteria. Food Res. Int. 2014, 64, 560–566. [Google Scholar] [CrossRef]
- Tavera-Quiroz, M.J.; Romano, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A.; Bertola, N. Green apple baked snacks functionalized with edible coatings of methylcellulose containing Lactobacillus plantarum. J. Funct. Foods 2015, 16, 164–173. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Formation and properties of whey protein films and coatings. In Protein-Based Films and Coatings; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 159–180. [Google Scholar]
- Gounga, M.E.; Xu, S.Y.; Wang, Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J. Food Eng. 2007, 83, 521–530. [Google Scholar] [CrossRef]
- Oses, J.; Fernandez-Pan, I.; Mendoza, M.; Maté, J.I. Stability of the mechanical properties of edible films based on whey protein isolate during storage at different relative humidity. Food Hydrocoll. 2009, 23, 125–131. [Google Scholar] [CrossRef]
- ASTM D882-02 Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: Philadelphia, PA, USA, 2002.
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.J.E.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation improved probiotics survival during gastric transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Mohammadi, R.; Mortazavian, A.; Khosrokhavar, R.; da Cruz, A. Probiotic ice cream: Viability of probiotic bacteria and sensory properties. Ann. Microbiol. 2011, 61, 411–424. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT Food Sci. Technol. 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049. [Google Scholar] [CrossRef]
- Moreira, D.; Gullón, B.; Gullón, P.; Gomes, A.; Tavaria, F. Bioactive packaging using antioxidant extracts for the prevention of microbial food-spoilage. Food Funct. 2016, 7, 3273–3282. [Google Scholar] [CrossRef]
- Goksu, E.I.; Karamanlioglu, M.; Bakir, U.; Yilmaz, L.; Yilmazer, U. Production and characterization of films from cotton stalk xylan. J. Agric. Food Chem. 2007, 55, 10685–10691. [Google Scholar] [CrossRef]
- Soukoulis, C.; Singh, P.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocoll. 2016, 52, 876–887. [Google Scholar] [CrossRef]
- Tonyali, B.; Cikrikci, S.; Oztop, M.H. Physicochemical and microstructural characterization of gum tragacanth added whey protein based films. Food Res. Int. 2018, 105, 1–9. [Google Scholar] [CrossRef]
- Botelho, B.G.; Reis, N.; Oliveira, L.S.; Sena, M.M. Development and analytical validation of a screening method for simultaneous detection of five adulterants in raw milk using mid-infrared spectroscopy and PLS-DA. Food Chem. 2015, 181, 31–37. [Google Scholar] [CrossRef]
- Piccirilli, G.N.; Soazo, M.; Pérez, L.M.; Delorenzi, N.J.; Verdini, R.A. Effect of storage conditions on the physicochemical characteristics of edible films based on whey protein concentrate and liquid smoke. Food Hydrocoll. 2019, 87, 221–228. [Google Scholar] [CrossRef]
- Augusto, A.; Dias, J.; Campos, M.; Alves, N.; Pedrosa, R.; Silva, S. Influence of Codium tomentosum Extract in the Properties of Alginate and Chitosan Edible Films. Foods 2018, 7, 53. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).