Dual Component Polymeric Epoxy-Polyaminoamide Based Zinc Phosphate Anticorrosive Formulation for 15CDV6 Steel
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of 15CDV6 Steel Panels
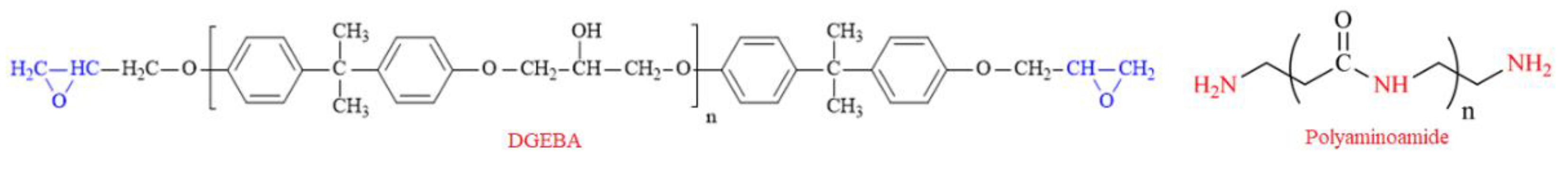
2.3. Epoxy Resin Formulation and Steel Coating
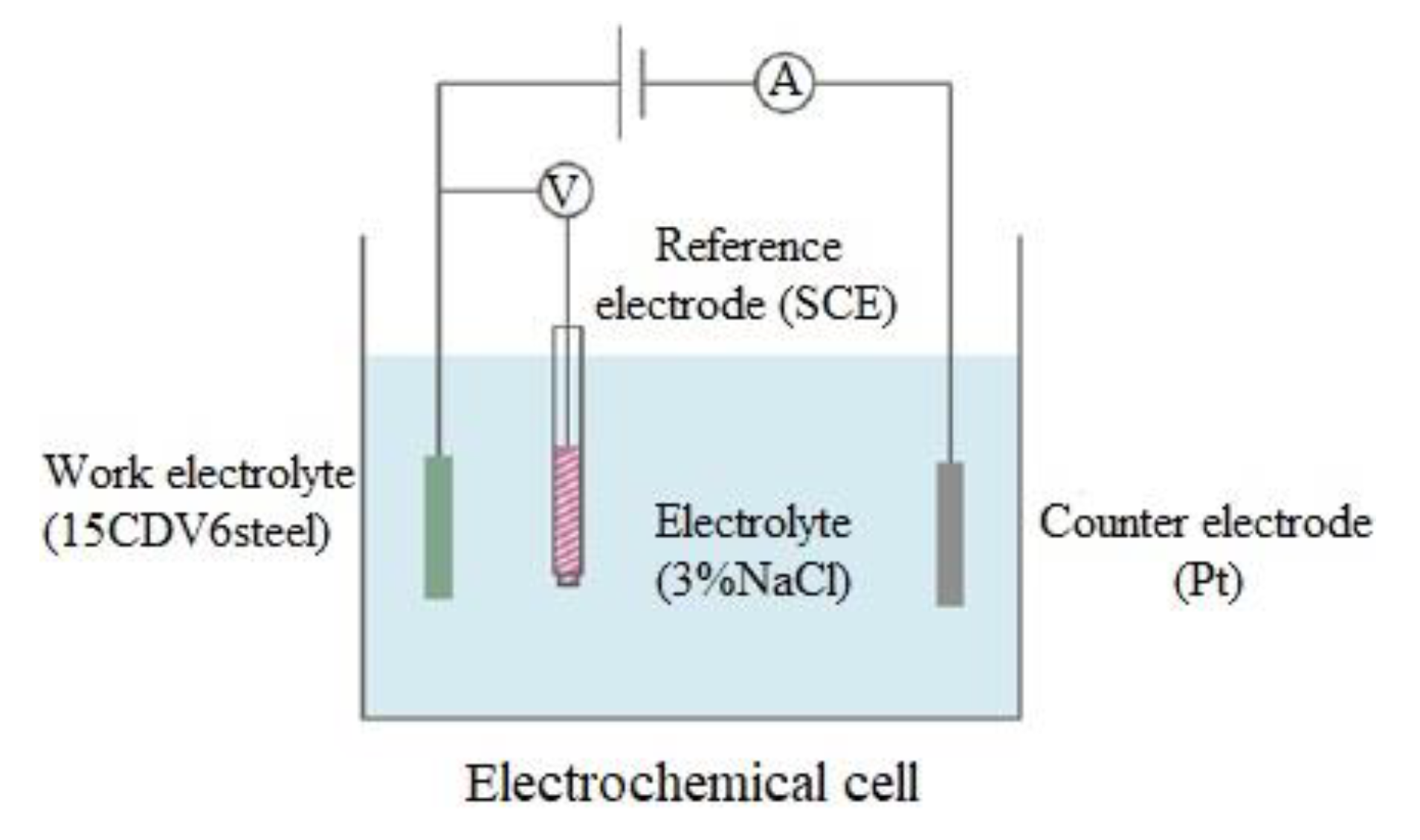
2.4. Electrochemical Corrosion Tests and Surafce Characterization of Coating
3. Results and Discussion
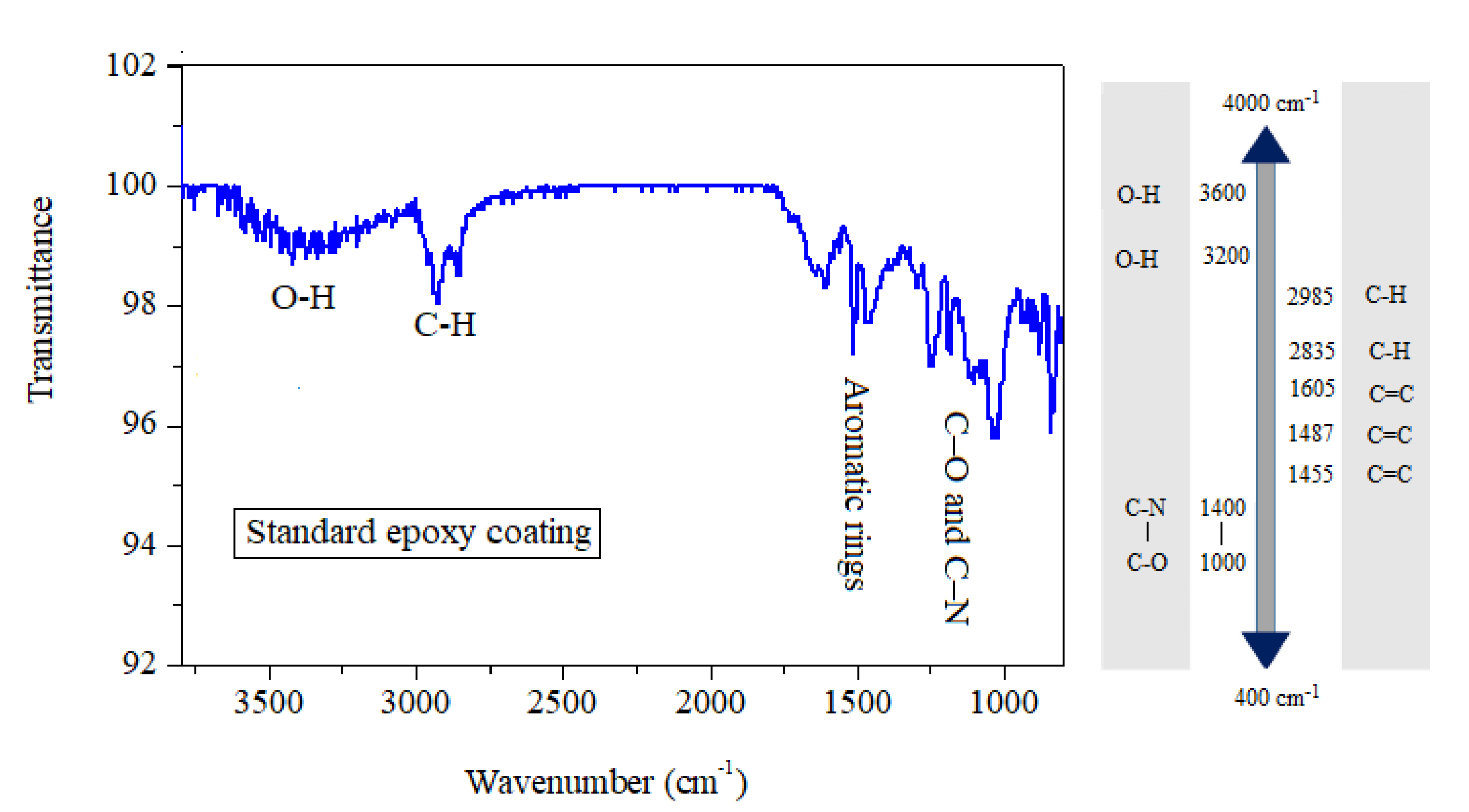
3.1. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
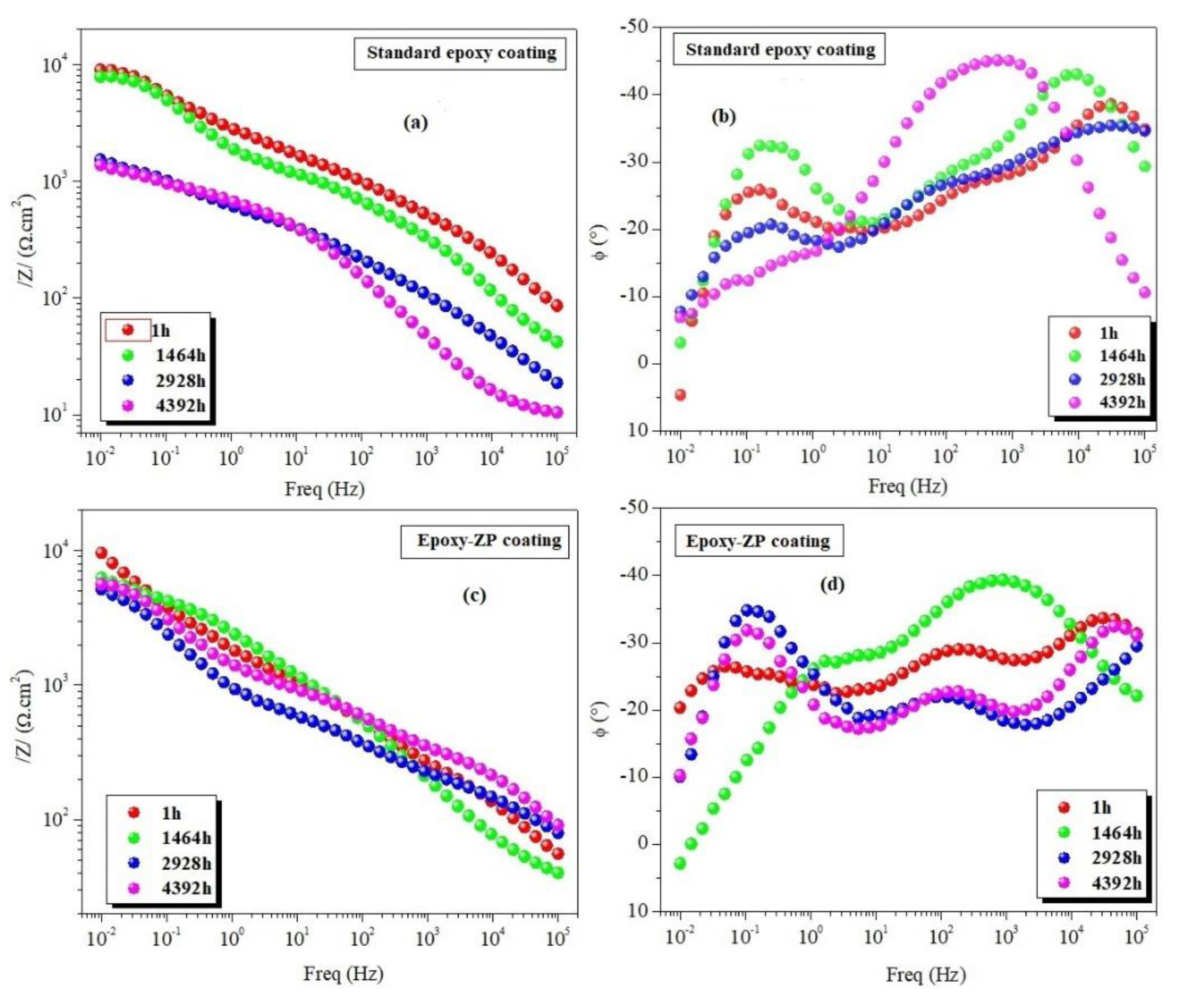
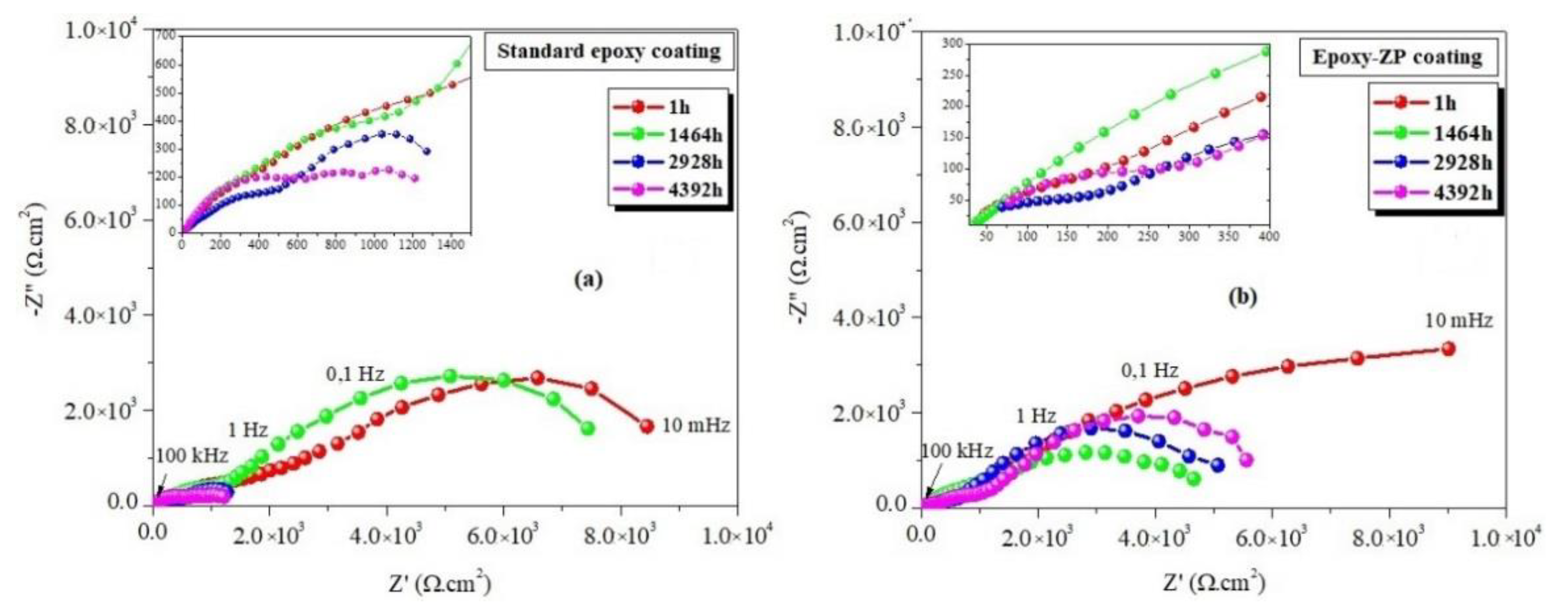
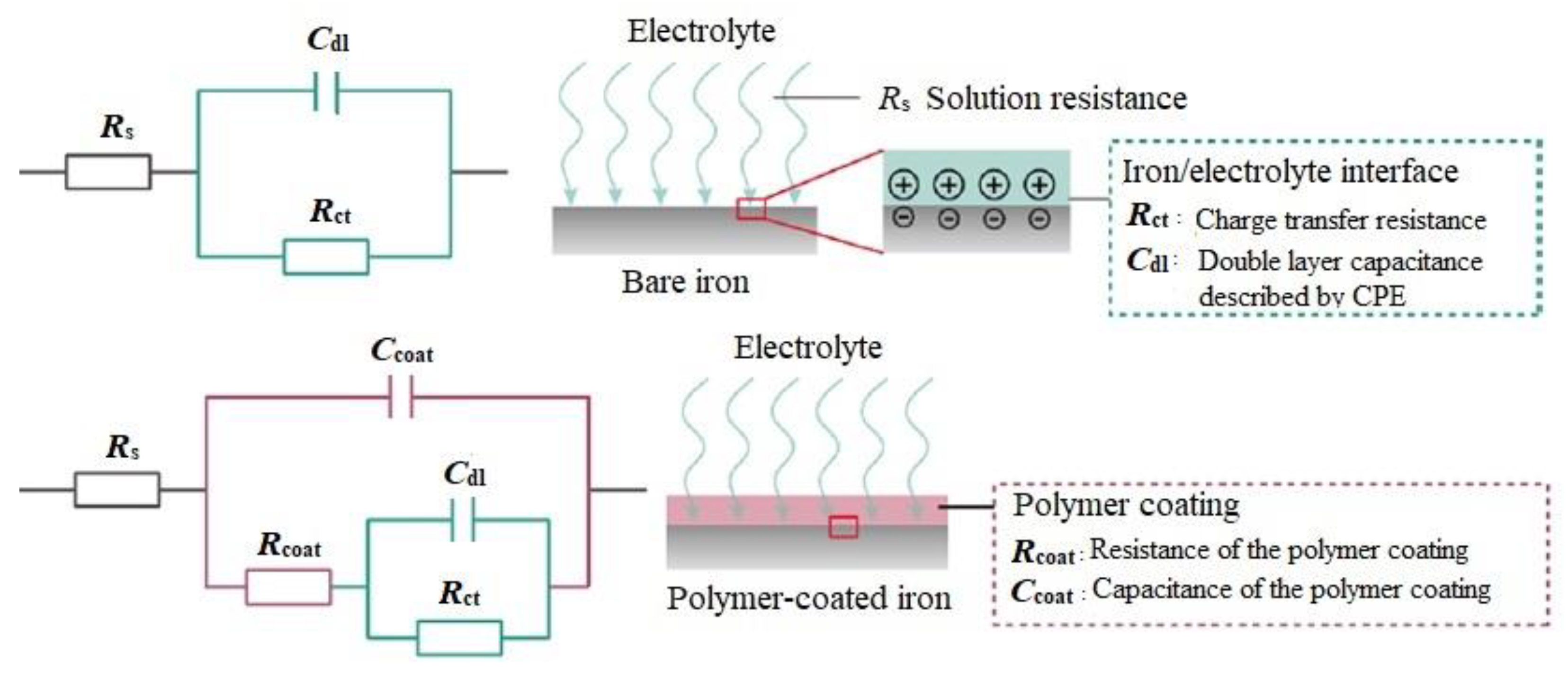
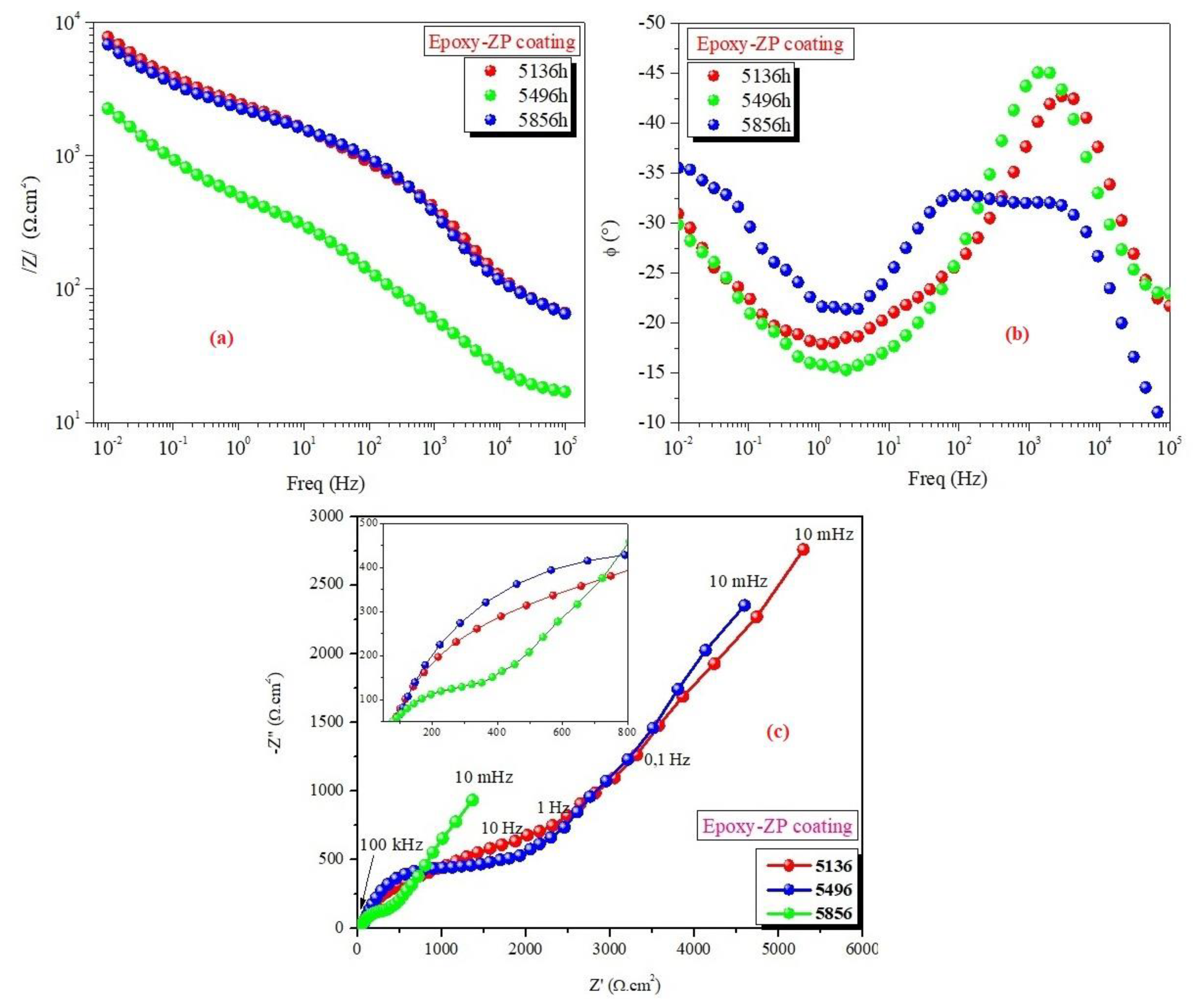
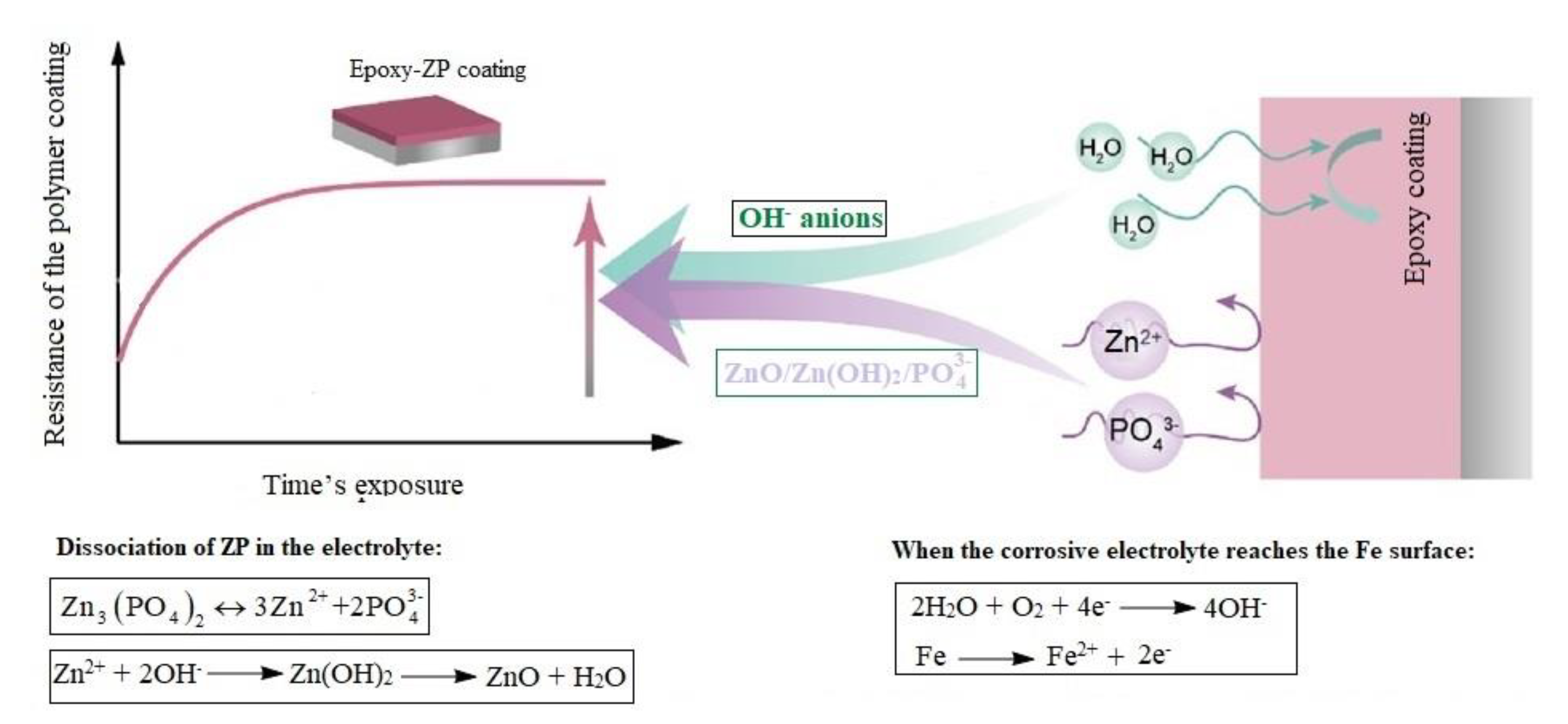
3.2. EIS Measurements
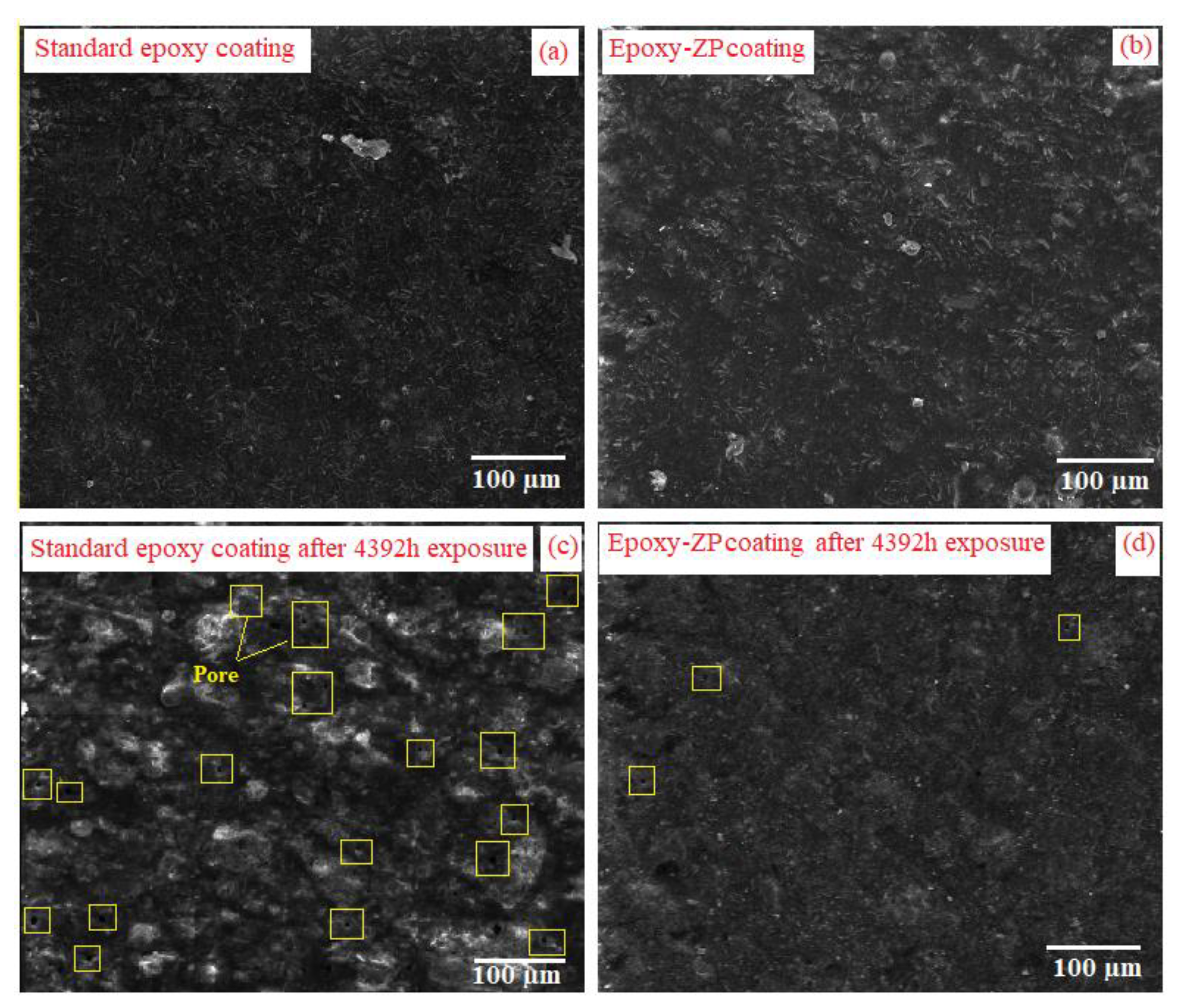
3.3. Surface Morphology of the Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dagdag, O.; El Harfi, A.; El Gouri, M.; Safi, Z.; Jalgham, R.T.; Wazzan, N.; Verma, C.; Ebenso, E.E.; Kumar, U.P. Anticorrosive properties of Hexa (3-methoxy propan-1, 2-diol) cyclotri-phosphazene compound for carbon steel in 3% NaCl medium: Gravimetric, electrochemical, DFT and Monte Carlo simulation studies. Heliyon 2019, 5, e01340. [Google Scholar] [CrossRef] [PubMed]
- Dagdag, O.; El Gana, L.; Hamed, O.; Jodeh, S.; El Harfi, A. Anticorrosive formulation based of the epoxy resin–polyaminoamide containing zinc phosphate inhibitive pigment applied on sulfo-tartaric anodized AA 7075-T6 in NaCl medium. J. Bio-and Tribo-Corros. 2019, 5, 25. [Google Scholar] [CrossRef]
- Indumathi, S.N.; Vasudevan, T.; Sundarrajan, S.; Rao, B.S.; Murthy, C.V.S.; Yadav, D.R. Cadmium-and chromate-free coating schemes for corrosion protection of 15CDV6 steel. Met. Finish. 2011, 109, 15–21. [Google Scholar] [CrossRef]
- Ramkumar, P.; Prakash, F.G.; Karthikeyan, M.K.; Gupta, R.K.; Muthupandi, V. Development of copper coating technology on high strength low alloy steel filler wire for aerospace applications. Mater. Today Proc. 2018, 5, 7296–7302. [Google Scholar] [CrossRef]
- Dagdag, O.; Essamri, A.; El Gana, L.; El Bouchti, M.; Hamed, O.; Cherkaoui, O.; Jodeh, S.; El Harfi, A. Synthesis, characterization and rheological properties of epoxy monomers derived from bifunctional aromatic amines. Polym. Bull. 2018, 1–15. [Google Scholar] [CrossRef]
- Dagdag, O.; Hamed, O.; Erramli, H.; El Harfi, A. Anticorrosive performance approach combining an epoxy polyaminoamide–zinc phosphate coatings applied on sulfo-tartaric anodized aluminum alloy 5086. J. Bio-and Tribo-Corros. 2018, 4, 52. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; Berisha, A.; Erramli, H.; Hamed, O.; Jodeh, S.; El Harfi, A. Polymeric-based epoxy cured with a polyaminoamide as an anticorrosive coating for aluminum 2024-T3 surface: experimental studies supported by computational modeling. J. Bio-and Tribo-Corros. 2019, 5, 58. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]
- Dagdag, O.; El Harfi, A.; Essamri, A.; El Bachiri, A.; Hajjaji, N.; Erramli, H.; Hamed, O.; Jodeh, S. Anticorrosive performance of new epoxy-amine coatings based on zinc phosphate tetrahydrate as a nontoxic pigment for carbon steel in NaCl medium. Arab. J. Sci. Eng. 2018, 43, 5977–5987. [Google Scholar] [CrossRef]
- Dagdag, O.; El Harfi, A.; Essamri, A.; El Gouri, M.; Chraibi, S.; Assouag, M.; Benzidia, B.; Hamed, O.; Lgaz, H.; Jodeh, S. Phosphorous-based epoxy resin composition as an effective anticorrosive coating for steel. Int. J. Ind. Chem. 2018, 9, 231–240. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Li, D.; Sun, Y.; Wang, Z.; Hou, C.; Chen, L.; Cao, Y.; Liu, Y. Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Jirasutsakul, I.; Paosawatyanyong, B.; Bhanthumnavin, W. Aromatic phosphorodiamidate curing agent for epoxy resin coating with flame-retarding properties. Prog. Org. Coat. 2013, 76, 1738–1746. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar] [CrossRef]
- Sababi, M.; Terryn, H.; Mol, J.M.C. The influence of a Zr-based conversion treatment on interfacial bonding strength and stability of epoxy coated carbon steel. Prog. Org. Coat. 2017, 105, 29–36. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M.M. The role of zinc aluminum phosphate anticorrosive pigment in protective performance and cathodic disbondment of epoxy coating. Corros. Sci. 2010, 52, 1291–1296. [Google Scholar] [CrossRef]
- Mousavifard, S.M.; Nouri, P.M.; Attar, M.M.; Ramezanzadeh, B. The effects of zinc aluminum phosphate (ZPA) and zinc aluminum polyphosphate (ZAPP) mixtures on corrosion inhibition performance of epoxy/polyamide coating. J. Ind. Eng. Chem. 2013, 19, 1031–1039. [Google Scholar] [CrossRef]
- Blustein, G.; Deyá, M.C.; Romagnoli, R.; Del Amo, B. Three generations of inorganic phosphates in solvent and water-borne paints: A synergism case. Appl. Surf. Sci. 2005, 252, 1386–1397. [Google Scholar] [CrossRef]
- Deyab, M.A.; Ouarsal, R.; Al-Sabagh, A.M.; Lachkar, M.; El Bali, B. Enhancement of corrosion protection performance of epoxy coating by introducing new hydrogenphosphate compound. Prog. Org. Coat. 2017, 107, 37–42. [Google Scholar] [CrossRef]
- Cotting, F.; Aoki, I.V. Smart protection provided by epoxy clear coating doped with polystyrene microcapsules containing silanol and Ce (III) ions as corrosion inhibitors. Surf. Coat. Technol. 2016, 303, 310–318. [Google Scholar] [CrossRef]
- Deyab, M.A. Effect of carbon nano-tubes on the corrosion resistance of alkyd coating immersed in sodium chloride solution. Prog. Org. Coat. 2015, 85, 146–150. [Google Scholar] [CrossRef]
- ASTM B117-18 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM: West Conshohocken, PA, USA, 2019.
- Deflorian, F.; Rossi, S.; Fedel, M.; Motte, C. Electrochemical investigation of high-performance silane sol–gel films containing clay nanoparticles. Prog. Org. Coat. 2010, 69, 158–166. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Amelioration of anticorrosion and hydrophobic properties of epoxy/PDMS composite coatings containing nano ZnO particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Yu, D.; Wen, S.; Yang, J.; Wang, J.; Chen, Y.; Luo, J.; Wu, Y. RGO modified ZnAl-LDH as epoxy nanostructure filler: A novel synthetic approach to anticorrosive waterborne coating. Surf. Coat. Technol. 2017, 326, 207–215. [Google Scholar] [CrossRef]
- Liu, X.; Gu, C.; Wen, Z.; Hou, B. Improvement of active corrosion protection of carbon steel by water-based epoxy coating with smart CeO2 nanocontainers. Prog. Org. Coat. 2018, 115, 195–204. [Google Scholar] [CrossRef]
- Roselli, S.N.; Romagnoli, R.; Deyá, C. The anti-corrosion performance of water-borne paints in long term tests. Prog. Org. Coat. 2017, 109, 172–178. [Google Scholar] [CrossRef]
| Component (Liquid Epoxy) | Weight Percent (wt %) | Supplier |
|---|---|---|
| DGEBA (average molecular weight < 700) | 25 ≤ wt % < 50 | MAPAERO-Aerospace Coatings and Finishes, Pamiers CEDEX, France |
| Nitroethane | 25 ≤ wt % < 50 | MAPAERO-Aerospace Coatings and Finishes, Pamiers CEDEX, France |
| Triglycidyl ether of trimethylolpropane | 10 ≤ wt % < 25 | MAPAERO-Aerospace Coatings and Finishes, Pamiers CEDEX, France |
| Alkoxysilane | 0 ≤ wt % < 2.5 | MAPAERO-Aerospace Coatings and Finishes, Pamiers CEDEX, France |
| Component (Liquid Hardener) | Weight Percent (wt %) | Supplier |
| Polyaminoamide | 10 ≤ wt % < 25 | MAPAERO-Aerospace Coatings and Finishes, Pamiers CEDEX, France |
| Butane-2-ol | 25 ≤ wt % < 50 | MAPAERO-Aerospace Coatings and Finishes, Pamiers CEDEX, France |
| Zinc oxide | 0 ≤ wt % < 2.5 | MAPAERO-Aerospace Coatings and Finishes, Pamiers CEDEX, France |
| Demineralized water (ρ > 1 Ω·cm and σ < 1 µS/cm) | – | – |
| Methyl ethyl ketone (MEK) (≥99%) | – | Sigma Aldrich, Saint Louis, MO, USA |
| Zinc phosphate (ZP) (99.99%) | 0 ≤ wt % < 5 | Sigma Aldrich, Saint Louis, MO, USA |
| Elements | wt % Composition |
|---|---|
| C | 0.12–0.18 |
| Si | ≤0.2 |
| Mn | 0.8–1.1 |
| S | ≤0.015 |
| P | ≤0.02 |
| Cr | 1.25–1.5 |
| Mo | 0.8–1.0 |
| V | 0.2–0.3 |
| Fe | Balance |
| Sample | Time (h) | Magnitude |Z|0.01 Hz (kΩ·cm²) | Phase −θ10 kHz (deg) | Rs (Ω·cm²) | CPEcoat | Rcoat (kΩ·cm²) | CPEdl | Rct (kΩ·cm²) | χ2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ccoat (µF/cm²) | ncoat | Cdl (µF/cm²) | ndl | ||||||||
| Standard epoxy coating | 1 1464 2928 4392 | 8.90 7.64 1.60 1.40 | 31 33 33 30 | 25.01 11.54 03.39 09.37 | 65.6 30.3 35.1 48.8 | 0.36 0.51 0.36 0.68 | 6.90 2.13 0.73 0.03 | 15.0 20.0 10.4 51.2 | 0.74 0.73 0.66 0.19 | 6.62 6.60 1.07 2.00 | 0.21 0.18 0.03 0.07 |
| Epoxy-ZP coating | 1 1464 2928 4392 5136 5496 5856 | 9.50 6.65 5.10 5.90 7.60 6.90 2.30 | 36 43 36 33 38 33 27 | 16.7 24.6 6.11 68.9 45.8 51.0 12.7 | 24.0 53.3 33.9 29.7 10.1 9.55 22.0 | 0.30 0.52 0.26 0.22 0.62 0.64 0.51 | 6.16 3.42 2.25 2.50 2.93 2.89 1.93 | 89.2 10.4 19.3 10.0 38.6 51.4 20.5 | 1.00 0.66 0.77 1.00 0.33 0.38 0.48 | 16.60 4.21 7.53 8.50 13.6 6.46 7.94 | 0.11 0.11 0.09 0.17 0.52 0.82 0.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagdag, O.; Hanbali, G.; Khalaf, B.; Jodeh, S.; El Harfi, A.; Deghles, A. Dual Component Polymeric Epoxy-Polyaminoamide Based Zinc Phosphate Anticorrosive Formulation for 15CDV6 Steel. Coatings 2019, 9, 463. https://doi.org/10.3390/coatings9080463
Dagdag O, Hanbali G, Khalaf B, Jodeh S, El Harfi A, Deghles A. Dual Component Polymeric Epoxy-Polyaminoamide Based Zinc Phosphate Anticorrosive Formulation for 15CDV6 Steel. Coatings. 2019; 9(8):463. https://doi.org/10.3390/coatings9080463
Chicago/Turabian StyleDagdag, Omar, Ghadir Hanbali, Bayan Khalaf, Shehdeh Jodeh, Ahmed El Harfi, and Abdelhadi Deghles. 2019. "Dual Component Polymeric Epoxy-Polyaminoamide Based Zinc Phosphate Anticorrosive Formulation for 15CDV6 Steel" Coatings 9, no. 8: 463. https://doi.org/10.3390/coatings9080463
APA StyleDagdag, O., Hanbali, G., Khalaf, B., Jodeh, S., El Harfi, A., & Deghles, A. (2019). Dual Component Polymeric Epoxy-Polyaminoamide Based Zinc Phosphate Anticorrosive Formulation for 15CDV6 Steel. Coatings, 9(8), 463. https://doi.org/10.3390/coatings9080463







