Characterisation of NiTi Orthodontic Archwires Surface after the Simulation of Mechanical Loading in CACO2-2 Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Experimental Setup
2.4. Surface Analysis
2.5. Measurement of Ions’ Release
3. Results and Discussion
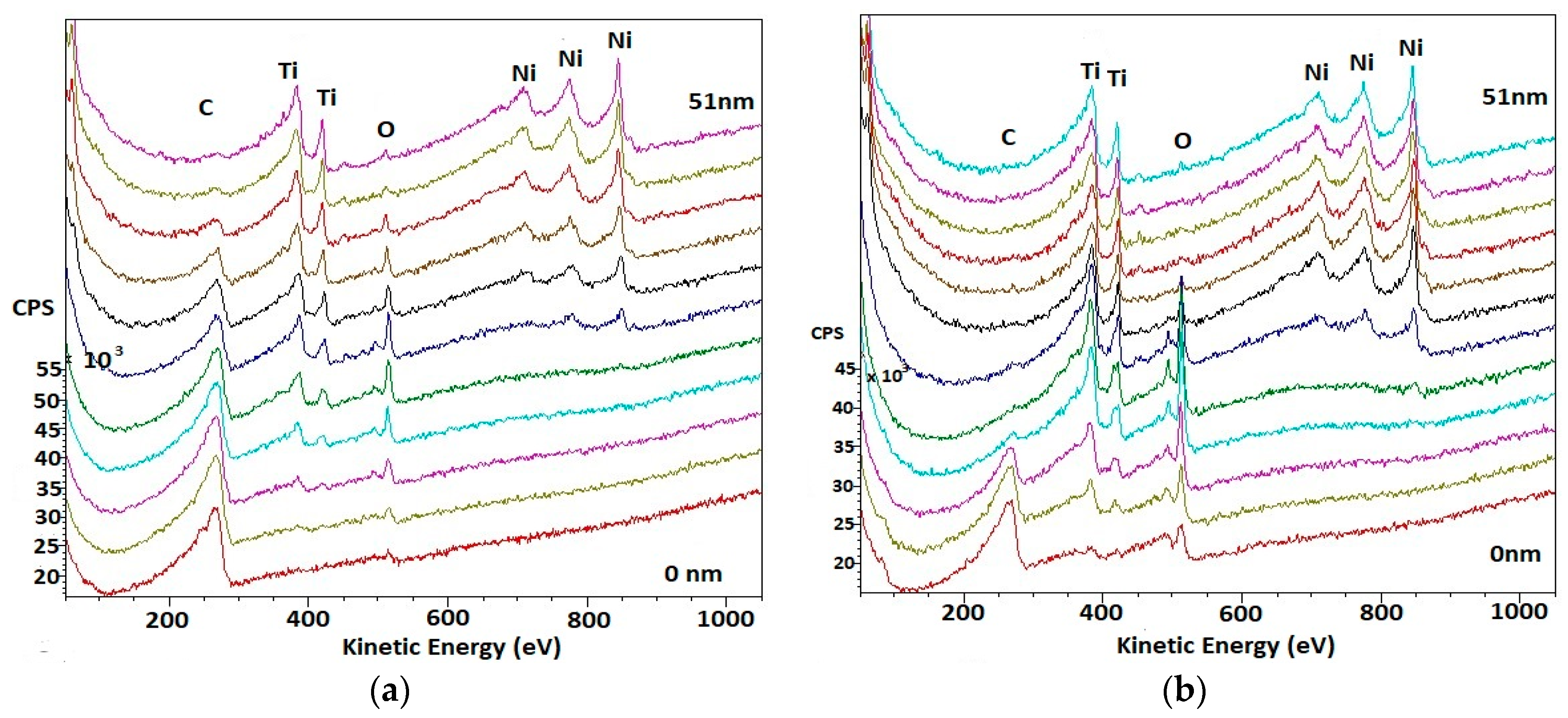
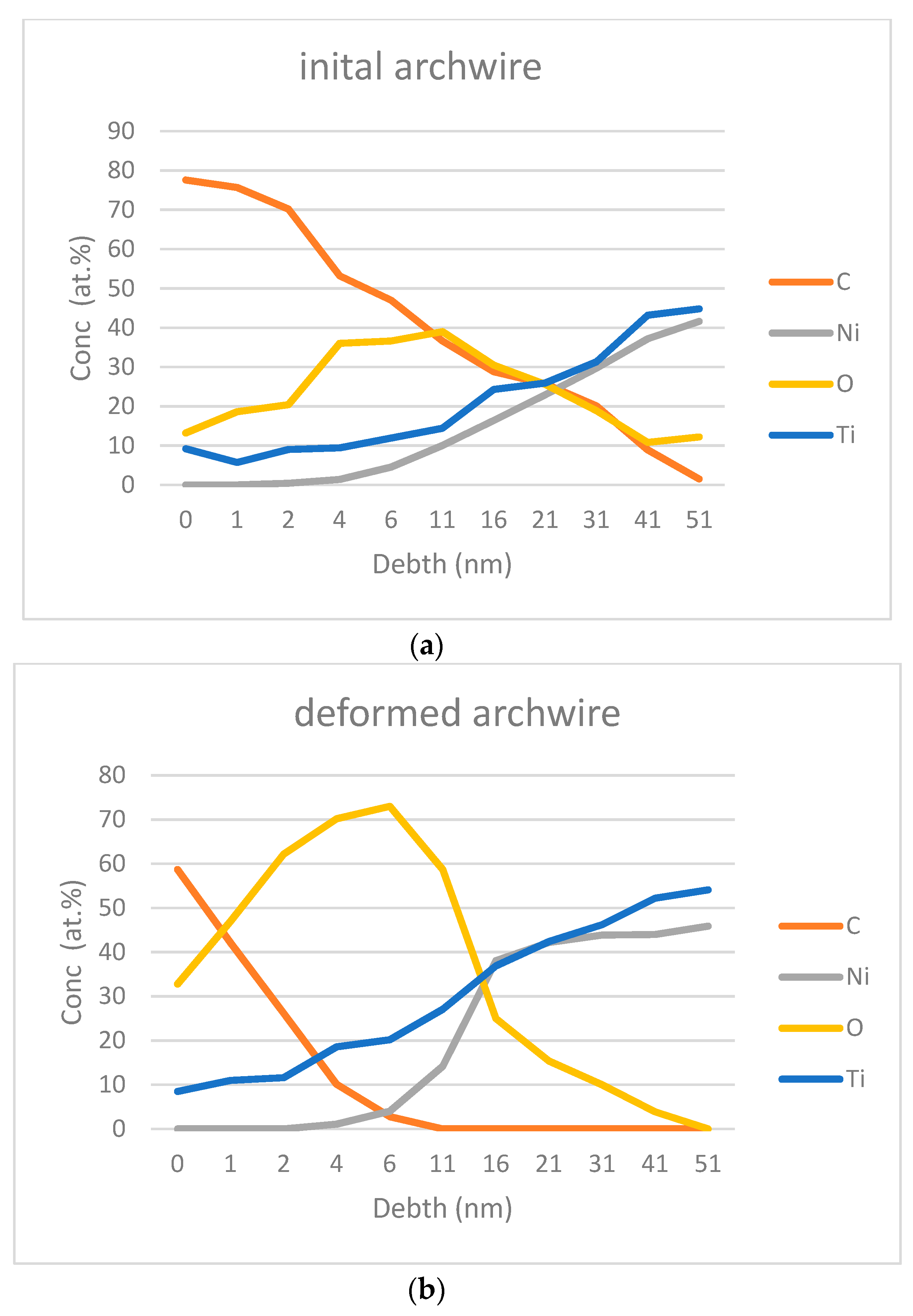
3.1. Surface Analysis
3.2. Results of Ions’ Release
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sidebottom, A.J.; Mistry, K. Prospective analysis of the incidence of metal alergy in patients listed for total replacement of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 2014, 52, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.L.; Vincent, S.K.; Barker, B.F. Allergic reaction to orthodontic wire: Report of case. J. Am. Dent. Assos. 1989, 118, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Al-Waheidi, E.M.H. Allergic reaction to nickel orthodontic wires: A case report. Quintessence Int. 1995, 26, 385–387. [Google Scholar] [PubMed]
- Bass, J.K.; Fine, H.; Cisneros, G.J. Nickel hypersensitivity in the orthodontic patient. Am. J. Orthod. Dentofacial Orthop. 1993, 103, 280–285. [Google Scholar] [CrossRef]
- Dass, K.; Buchner, V. Effects of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev. Environ. Health 2007, 22, 157–173. [Google Scholar] [CrossRef]
- Valko, M.; Morriss, H.; Cronin, M.T. Metals toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- M’Bemba-Meka, P.; Lemieux, N.; Chakrabarti, S.K. Role of oxidative stress, mitochondrial membrane potential and calcium homeostasis in human lymphocyte death induced by nickel carbonate hydroxide in vivo. Arch. Toxicol. 2006, 80, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Dieter, M.P.; Jameson, C.W.; Tucker, A.N.; Luster, M.I.; French, J.E.; Hong, H.L.; Boorman, G.A. Evaluation of tissue deposition myelopoetic and immunologic responses in mice after long term exposure to nickel sulfare in drinking water. J. Toxicol. Environ. Health 1988, 24, 357–372. [Google Scholar] [CrossRef]
- Hostynek, J.J. Sensitization to nickel: etiology, epidemiology, immune reactions, prevention and therapy. Rev. Environ. Health 2006, 21, 252–280. [Google Scholar] [CrossRef]
- Liang, R.; Senturker, S.; Shi, X.; Bal, W.; Dizdaro-gluand, M.; Kasprzak, K.S. Effects of Ni (II) and Cu (II) on DNA interactions with the N-terminal sequence of human protamine P2: Enhancement of binding and mediation of oxidative DNA strand scission and base damage. Carcinogenesis 1999, 20, 893–898. [Google Scholar] [CrossRef]
- Kasprzak, K.S.; Sunderman, F.W.J.; Salnikow, K. Nickel carcinogenesis. Mutat. Res. 2003, 533, 67–97. [Google Scholar] [CrossRef] [PubMed]
- Rahilly, G. Nickel allergy and orthodontics. J. Orthod. 2003, 30, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H.; Hsu, C.C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Ryhanen, J.; Niemi, E.; Serlo, W.; Niemela, E.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J. Biomed. Mater. Res. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Chan, C.; Trigwell, C.; Duerig, T. Oxidation of a NiTi alloy. Surf. Interface Anal. 1990, 15, 349–354. [Google Scholar] [CrossRef]
- Espinos, J.P.; Fernandes, A.; Gonzales-Elipe, A.R. Oxidation and diffusion proccesses in nickel-titanium oxide systems. Surf. Sci. 1993, 295, 402–410. [Google Scholar] [CrossRef]
- Heravi, F.; Moayed, M.H.; Mokhber, N. Effects of fluoride on nickel-titanium and stainless-steel orthodontic archwires: An in vitro study. J. Dent. 2015, 12, 49–59. [Google Scholar]
- Qu, J.; Blau, P.J.; Watkins, T.R.; Cavin, O.B.; Kulkarni, N.S. Friction and wear of titanium alloys sliding against metal, polymer and ceramic counterfaces. Wear 2005, 258, 1348–1356. [Google Scholar] [CrossRef]
- Zhenping, S.; Jiqiang, W.; Zhengbin, W.; Yanxin, Q.; Tianying, X.; Yugui, Z. Cavitation erosion and jet impingement erosion of the NiTi coating produced by air plasma spraying. Coatings 2018, 8, 346. [Google Scholar]
- Freiberg, K.E.; Bremer-Streck, S.; Kiehntopf, M.; Rettenmayr, M.; Undisz, A. Effect of thermomechanical pre-treatment on short- and long-term Ni release from biomedical NiTi. Acta Biomater. 2014, 10, 2290–2295. [Google Scholar] [CrossRef]
- Jia, W.; Beatty, M.W.; Reinhardt, R.A.; Petro, T.M.; Cohen, D.M.; Maze, C.R.; Strom, E.A.; Hoffman, M. Nickel release from orthodontic arch wires and cellular immune response to various nickel concentrations. J. Biomed. Mater. Res. 1999, 48, 488–495. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Pagano, S.; Cianetti, S.; Lombardo, G.; Kenny, J.M.; Torre, L. Effect of fibre post, bone losses and fibre content on the biomechanical behaviour of endodontically treated teeth: 3D-finite alement analysis. Mater. Sci. Eng. C 2017, 74, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Fercec, J.; Anzel, I.; Rudolf, R. Stress dependent electrical resistivity of orthodontic wire from the shape memory alloy NiTi. Mater. Des. 2014, 55, 699–706. [Google Scholar] [CrossRef]
- Vázquez-Sánchez; Ángeles, M. Available online: https://readycell.com/cacoready/ (accessed on 19 July 2018).
- Calabro, A.R.; Gazarian, D.I.; Barile, F.A. Effect of metals on β-actin and total protein synthesis in cultured human intestinal epithelial cells. J. Pharmacol. Toxicol. Methods 2011, 63, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.W.; Shull, G.M.; Fountain, J.H.; Guo, Z.; Musselman, L.P.; Fiumera, A.C.; Mahler, G.J. Titanium dioxide nanoparticle exposure alters metabolic homeostasis in a cell culture model of the intestinal epithelium and drosophila melanogaster. Nanotoxicology 2018, 12, 390–406. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, A.; Vila, L.; Cortés, C.; Hernández, A.; Marcos, R. Effects of differently shaped TiO2NPs (nanospheres, nanorods and nanowires) on the in vitro model (Caco-2/HT29) of the intestinal barrier. Part. Fibre Toxicol. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Staffolani, N.; Damiani, F.; Lilli, C.; Guerra, M.; Staffolani, N.J.; Belcastro, S.; Locci, P. Ion release from orthodontic appliances. J. Dent. 1999, 27, 449–454. [Google Scholar] [CrossRef]
- Cisse, O.; Savagodo, O.; Wu, M.; Yahia, L. Effect of surface treatment of NiTi alloy on its corrosion behavior in Hanks solution. J. Biomed. Mater. Res. 2002, 61, 339–345. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohgoe, Y.; Ozeki, K.; Sato, K.; Sumiya, T.; Hirakuri, K. Diamond-like carbon coatings on orthodontic archwires. Diam. Relat. Mater. 2005, 14, 1094–1097. [Google Scholar] [CrossRef]
- Sui, J.; Cai, W. Effect of diamond-like carbon (DLC) on the properties of NiTi alloys. Diam. Relat. Mater. 2006, 15, 1720–1726. [Google Scholar] [CrossRef]
- Clarke, B.; Carroll, W.; Rochev, Y.; Hynes, M.; Bradley, D.; Plumley, D. Influence of nitinol wire surface treatment on oxide thickness and composition and its subsequent effect on corrosion resistance and nickel ion release. J. Biomed. Mater. Res. A 2006, 79, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Shabalovskaya, S.; Anderegg, J.; Laab, F.; Thiel, P.A.; Rondelli, G. Surface conditions of Nitinol wires, tubing, and as-cast alloys. The effect of chemical etching, aging in boiling water, and heat treatment. J. Biomed. Mater. Res. 2003, 65B, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Chieruzzi, M.; Balloni, S.; Lombardo, G.; Torre, L.; Bodo, M.; Cianetti, S.; Mairnucci, L. Biological, thermal and mechanical characterization of modified dlass ionomer cements: The role of nanohydroxyapatite, ciprofloxacin and zinc L-carnosine. Mater. Sci. Eng. C 2019, 94, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Shabalovskaya, S.; Anderegg, J. Surface spectroscopic characterization of TiNi nearly equiatomic shape memory alloys for implants. J. Vac. Sci. Technol. A 1995, 13, 2624–2632. [Google Scholar] [CrossRef]
- Uchil, J.; Mahesh, K.K.; Ganesh-Kumara, K. Electrical resistivity and strain recovery studies on the effect of thermal cycling under constant stress on R-phase in NiTi shape memory alloy. Phys. B Condens. Mater. 2002, 324, 419–428. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A. On the nature of the biocompatibility an medical applications of NiTi shape memory and superelastic alloys. Biomed. Mater. Eng. 1996, 6, 267–289. [Google Scholar] [PubMed]
- Colic, M.; Tomic, S.; Rudolf, R.; Markovic, E.; Scepan, I. Differeces in citocompatibility, dynamics of the oxide layers’ formation, and nickel release between superelastic and thermo-elastic activated nickel-titanium archwires. J. Mater. Sci. Mater. Med. 2016, 27, 128. [Google Scholar]
- Ramazanzadeh, B.A.; Ahrari, F.; Sabzevari, B.; Habibi, S. Nickel ion release from three types of nickel-titanium-based orthodontic archwires in the as-received state and after oral simulation. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 71–76. [Google Scholar]
- Azizi, A.; Jamilian, A.; Nucci, F.; Kamali, Z.; Hosseinikhoo, N.; Perillo, L. Release of metal ions from round and rectangular NiTi wires. Prog. Orthod. 2016, 17, 10. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Balassa, J.J.; Tipton, I.H. Abnormal trace metals in man-nickel. J. Chron. Dis. 1962, 15, 51–65. [Google Scholar] [CrossRef]
- Kaaber, K.; Veien, N.K.; Tjell, J.C. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br. J. Dermatol. 1978, 98, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L.; Wataha, C.T.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Wataha, J.C.; Lockwood, P.E.; Marek, M.; Ghazi, M. Ability of Ni-containing biomedical alloys to activate monocytes and endothelial cells in vitro. J. Biomed. Mater. Res. 1999, 45, 251–257. [Google Scholar] [CrossRef]
- Cederbrant, K.; Anderson, C.; Andersson, T.; Marcusson-Stahl, M.; Hultman, P. Cytokine production, lymphocyte proliferation and T-cell receptor Vβ expression in primary peripheral blood mononuclear cell cultures from nickel-allergic individuals. Int. Arch. Allergy Immunol. 2003, 132, 373–379. [Google Scholar] [CrossRef] [PubMed]
- ISO 15841:2014: Dentistry—Wires for Use in Orthodontics; International Organization for Standardization: Geneva, Switzerland, 2014.
- Rincic Mlinaric, M.; Karlovic, S.; Ciganj, Z.; Acev, D.P.; Pavlic, A.; Spalj, S. Oral antiseptics and nickel-titanium alloys: Mechanical and chemical effects of interaction. Odontology 2019, 107, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Trolić, I.M.; Turco, G.; Contardo, L.; Serdarević, N.L.; Ćurković, H.O.; Špalj, S. Corrosion of nickel-titanium orthodontic archwires in saliva and oral probiotic supplements. Acta Stomatol. Croat. 2017, 51, 316–325. [Google Scholar]
- D’Attilio, M.; Rodolfino, D.; Filippakos, A.; Saccucci, M.; Festa, F.; Tripodi, D. Second class resolver: A retrospective analysis. Eur. J. Paediatr. Dent. 2014, 15, 78–82. [Google Scholar]
- Baldini, A.; Nota, A.; Santariello, C.; Assi, V.; Ballanti, F.; Gatto, R.; Cozza, P. Sagittal dentoskeletal modifications associated with different activation protocols of rapid maxillary expansion. Eur. J. Paediatr. Dent. 2018, 19, 151–155. [Google Scholar]
- Kamat, A.M.; Copley, S.M.; Segal, A.E.; Todd, J.A. Laser-sustained plasma (LSP) nitriding of titanium: A review. Coatings 2019, 9, 283. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S.; Fehrman, J.; Barnes, A.C.; Zapf, A.M.; Zinelis, S.; Berzins, D.W. Titanium nitride and nitrogen ion implanted coated dental materials. Coatings 2012, 2, 160–178. [Google Scholar] [CrossRef]
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and surface treatments on orthodontic metallic materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Iijima, M.; Endo, K.; Mizoguchi, I. Electrophoretic deposition as a new bioactive glass coating process for orthodontic stainless steel. Coatings 2017, 7, 199. [Google Scholar] [CrossRef]



| Sample | Conc Ni (µg/L) | Conc Ti (µg/L) |
|---|---|---|
| Control | 1.240 | <0.05 |
| SMAS Testing | 1.310 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepojević, N.; Šćepan, I.; Glišić, B.; Jenko, M.; Godec, M.; Hočevar, S.; Rudolf, R. Characterisation of NiTi Orthodontic Archwires Surface after the Simulation of Mechanical Loading in CACO2-2 Cell Culture. Coatings 2019, 9, 440. https://doi.org/10.3390/coatings9070440
Lepojević N, Šćepan I, Glišić B, Jenko M, Godec M, Hočevar S, Rudolf R. Characterisation of NiTi Orthodontic Archwires Surface after the Simulation of Mechanical Loading in CACO2-2 Cell Culture. Coatings. 2019; 9(7):440. https://doi.org/10.3390/coatings9070440
Chicago/Turabian StyleLepojević, Nikola, Ivana Šćepan, Branislav Glišić, Monika Jenko, Matjaž Godec, Samo Hočevar, and Rebeka Rudolf. 2019. "Characterisation of NiTi Orthodontic Archwires Surface after the Simulation of Mechanical Loading in CACO2-2 Cell Culture" Coatings 9, no. 7: 440. https://doi.org/10.3390/coatings9070440
APA StyleLepojević, N., Šćepan, I., Glišić, B., Jenko, M., Godec, M., Hočevar, S., & Rudolf, R. (2019). Characterisation of NiTi Orthodontic Archwires Surface after the Simulation of Mechanical Loading in CACO2-2 Cell Culture. Coatings, 9(7), 440. https://doi.org/10.3390/coatings9070440