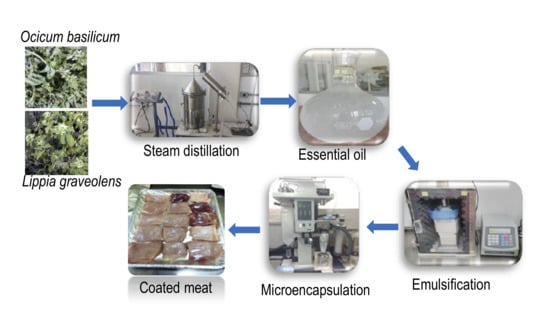
Microbiological and Physicochemical Properties of Meat Coated with Microencapsulated Mexican Oregano (Lippia graveolens Kunth) and Basil (Ocimum basilicum L.) Essential Oils Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plant Material and Extraction of EOs
2.2.2. GC/MS Analysis of Plant EOs
2.2.3. Bacteria
2.2.4. Minimum Inhibitory Concentration (MIC) of EOs on E. coli O157:H7
2.2.5. Combined Effect of EOs Mixture on E. coli O157:H7
2.2.6. Microencapsulation of EOs Mixture
2.2.7. Active Coating Preparation
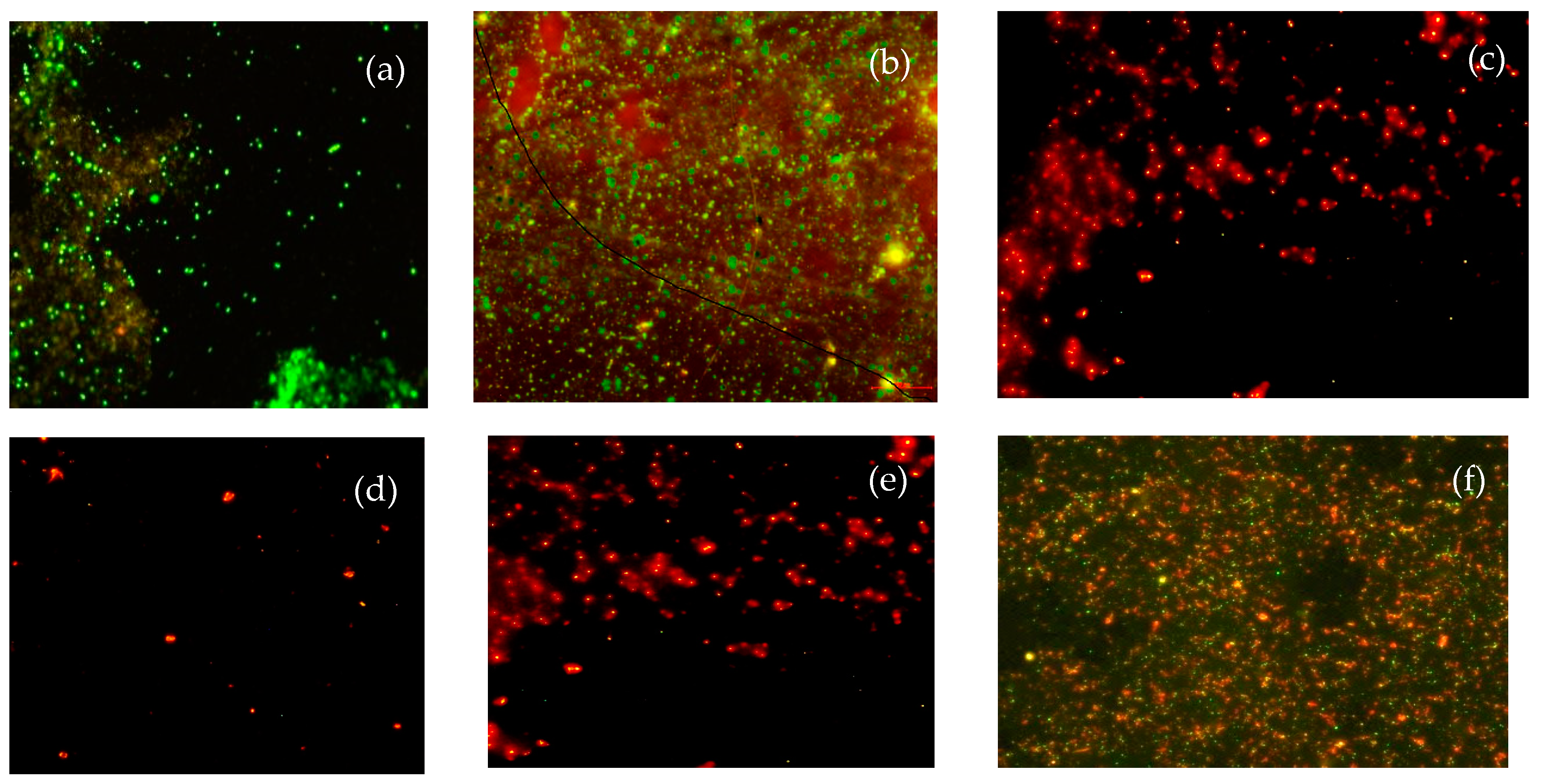
2.2.8. Cells Population Viability
2.2.9. Active Coating Application on Meat
2.2.10. Effect of Coatings on Fresh Meat Properties
Antimicrobial Properties
Physicochemical Properties
2.2.11. Sensory Evaluation
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. GC/MS Analysis of Plant EOs
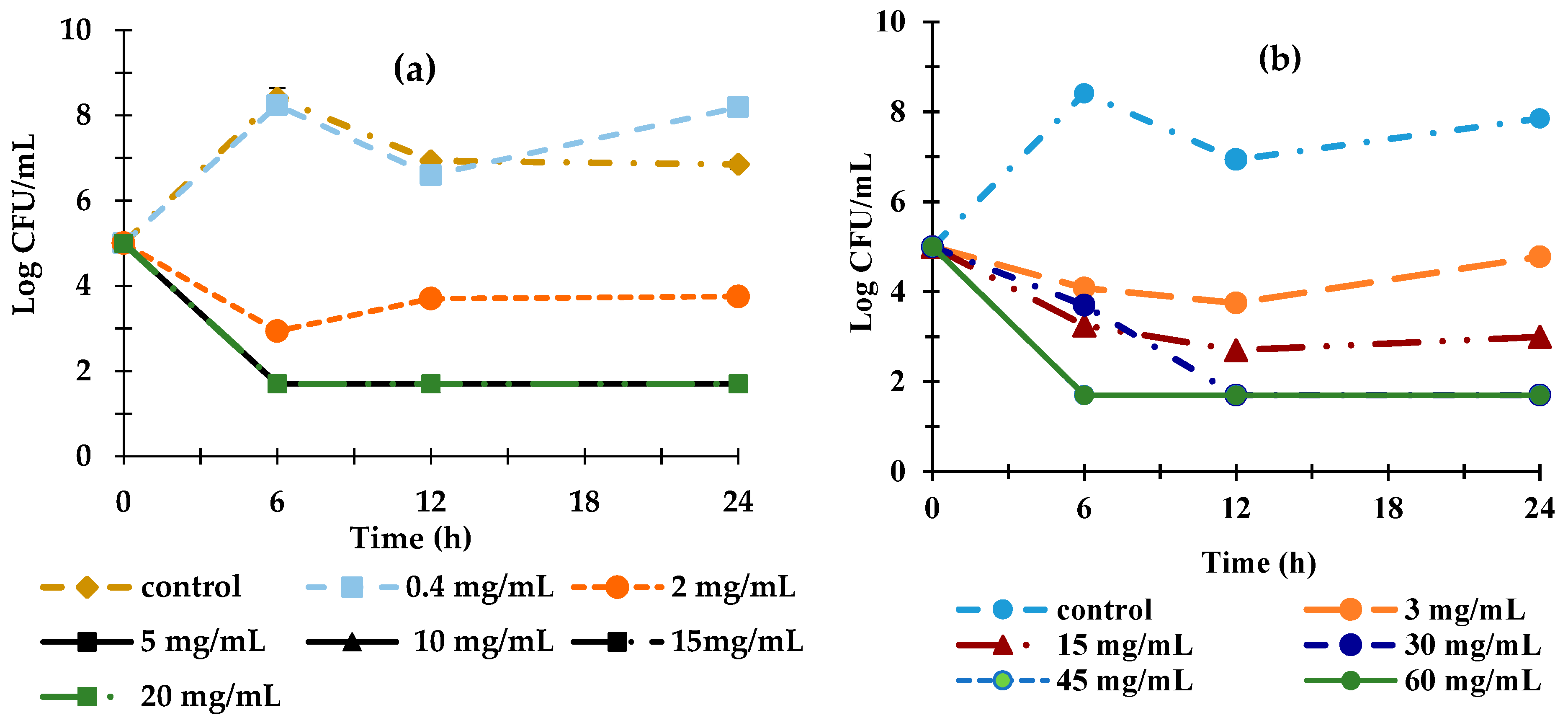
3.2. Minimum Inhibitory Concentration (MIC) of EOs Against Escherichia coli O157:H7
3.3. Combined Effect of EOs Mixture Against E. coli O157:H7
3.4. Determination of Cells Population Viability
3.5. Effect of Coatings on Fresh Meat Properties
3.5.1. Antimicrobial Properties
3.5.2. Physicochemical Properties
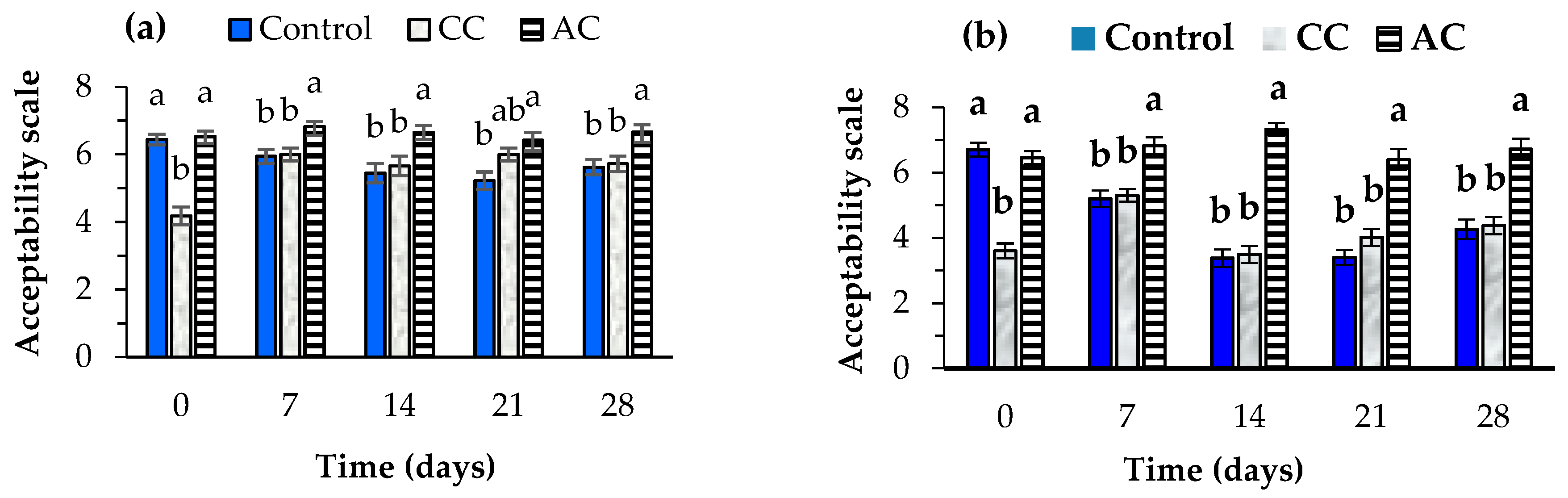
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gómez, B.; Barba, F.J.; Domínguez, R.; Putnik, P.; Bursać-Kovaĉević, D.B.; Pateiro, M.; Toldrá, F.; Lorenzo, J.M. Microencapsulation of antioxidant compounds through innovative technologies and its specific application in meat processing. Trends Food Sci. Technol. 2018, 182, 135–147. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Lira-Moreno, C.Y.; Guerrero-Legarreta, I.; Wild-Padua, G.; Di Pierro, P.; García-Almendárez, B.E.; Regalado-González, C. Effect of nanoemulsified and microencapsulated Mexican oregano (Lippia graveolens Kunth) essential oil coatings on quality of fresh pork meat. J. Food Sci. 2017, 82, 1423–1432. [Google Scholar] [CrossRef]
- Food Safety and Inspection Service (FSIS). United States Department of Agriculture. La Rosita Fresh Market Inc. Recalls Ground Beef Products Due to Possible E. coli O157:H7 Contamination. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2019/recall-033-2019-release (accessed on 20 March 2019).
- Hernández-Hernández, E.; Regalado-González, C.; Vázquez-Landaverde, P.; Guerrero-Legarreta, I.; García-Almendárez, B.E. Microencapsulation, chemical characterization, and antimicrobial activity of Mexican (Lippia graveolens H.B. K.) and European (Origanum vulgare L.) oregano essential oils. Sci. World J. 2014, 2014, 641814. [Google Scholar] [CrossRef] [PubMed]
- Karagöz-Emiroğlu, Z.; Polat-Yemiş, G.; Kodal-Coşkun, B.; Candoğan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Ortega-Nieblas, M.M.; Robles-Burgueño, M.R.; Acedo-Félix, E.; González-León, A.; Morales-Trejo, A.; Vázquez-Moreno, L. Chemical composition and antimicrobial activity of oregano (Lippia palmeri S. Wats) essential oil. Rev. Fitotec. Mex. 2011, 34, 11–17. [Google Scholar]
- Helal, I.M.; El-Bessoumy, A.; Al-Bataineh, E.; Joseph, M.R.P.; Rajagopalan, P.; Chandramoorthy, H.C.; Ahmed, S.B.H. Antimicrobial efficiency of essential oils from traditional medicinal plants of Asir Region, Saudi Arabia, over drug resistant isolates. BioMed Res. Int. 2019, 2019, 8928306. [Google Scholar] [CrossRef] [PubMed]
- Kayode, R.M.O.; Afolayan, A.J. Cytotoxicity and effect of extraction methods on the chemical composition of essential oils of Moringa oleifera seeds. J. Zhejiang Univ. Sci. B (Biomed. Biotechnol.) 2015, 16, 680–689. [Google Scholar] [CrossRef]
- Martínez-Natarén, D.A.; Parra-Tabla, V.; Ferrer-Ortega, M.M.; Calvo-Irabién, L.M. Genetic diversity and genetic structure in wild populations of Mexican oregano (Lippia graveolens H. B. K.) and its relationship with the chemical composition of the essential oil. Plant Syst. Evol. 2014, 300, 535–547. [Google Scholar] [CrossRef]
- Cristani, M.T.; D’arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.V.N.; Capello, T.M.; Siqueira, L.J.A.; Lago, J.H.G.; Caseli, L. Mechanism of action of thymol on cell membranes investigated through lipid Langmuir monolayers at the air−water interface and molecular simulation. Langmuir 2016, 32, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Navikaite-Snipaitiene, V.; Ivanauskas, L.; Jakstas, V.; Rüegg, N.; Rutkaite, R.; Wolfram, E.; Yildirim, S. Development of antioxidant food packaging materials containing eugenol for extending display life of fresh beef. Meat Sci. 2018, 145, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Khaneghah, A.M.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Mukumbo, F.M.; Idamokoro, E.M.; Afolayan, A.J.; Muchenje, V. Phytochemical constituents and antioxidant activity of sweet basil (Ocimum basilicum L.) essential oil on ground beef from boran and nguni cattle. Int. J. Food Sci. 2019, 2019, 2628747. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Torrico-Vieira, É.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Adrar, N.; Oukil, N.; Bedjou, F. Antioxidant and antibacterial activities of Thymus numidicus and Salvia officinalis essential oils alone or in combination. Ind. Crops Prod. 2016, 88, 112–119. [Google Scholar] [CrossRef]
- Sedlaříková, J.; Janalíková, M.; Rudolf, O.; Pavlačková, J.; Egner, P.; Peer, P.; Varad’ová, V.; Krejčí, J. Chitosan/thyme oil systems as affected by stabilizing agent: Physical and antimicrobial properties. Coatings 2019, 9, 165. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Hernández-Zamoran, E.; López-Mendoza, I.; Palou, E.; Jiménez-Munguía, M.T.; Nevárez-Moorillón, G.V.; López-Malo, A. Fungal inactivation by Mexican oregano (Lippia berlandieri Schauer) essential oil added to amaranth, chitosan, or starch edible films. J. Food Sci. 2010, 75, M127–M133. [Google Scholar] [CrossRef] [PubMed]
- Avila-Sosa, R.; Palou, E.; Jiménez-Munguía, M.T.; Nevárez-Moorillón, G.V.; Navarro-Cruz, A.R.; López-Malo, A. Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. Int. J. Food Microbiol. 2012, 153, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nevárez-Moorillón, G.V.; Ortiz-López, J.L.; Dávila-Márquez, R.M.; Meneses-Sánchez, M.C.; Navarro-Cruz, A.R.; Avila-Sosa, R. Anthracnose control by Mexican oregano (Lippia berlandieri Schauer) essential oil added to edible films. Int. Food Res. J. 2014, 21, 1863–1867. [Google Scholar]
- Rodríguez-García, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodriguez, M.R.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Vargas, M.; Atarés, L.; Chiralt, A. Physical properties of chitosan-basil essential oil edible films as affected by oil content and homogenization conditions. Procedia Food Sci. 2011, 1, 50–56. [Google Scholar] [CrossRef]
- Riveros, C.G.; Nepote, V.; Grosso, N.R. Thyme and basil essential oils included in edible coatings as a natural preserving method of oilseed kernels. J. Sci. Food. Agric. 2016, 96, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.P.; Moita de Carvalho, W.; Costa-Alexandrino, A.; Bezerra de Paula, H.C.; Passos-Rodrigues, M.C.; Wilane de Figueiredo, R.; Arraes-Maia, G.; Teixeira de Figueiredo, E.M.A.; Montenegro-Brasil, I. Freshness retention of minimally processed melon using different packages and multilayered edible coating containing microencapsulated essential oil. Int. J. Food Sci. Technol. 2014, 49, 2192–2203. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential oils: Antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Jemaa, M.B.; Falleh, H.; Serairi, R.; Neves, M.A.; Snoussi, M.; Isoda, H.; Nakajima, M.; Ksouri, R. Nanoencapsulated Thymus capitatus essential oil as natural preservative. Innov. Food Sci. Emerg. Technol. 2018, 45, 92–97. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Passerini de Rossi, B.; García, C.; Alcaraz, E.; Franco, M. Stenotrophomonas maltophilia interferes via the DSF-mediated quorum sensing system with Candida albicans filamentation and its planktonic and biofilm modes of growth. Rev. Argent. Microbiol. 2014, 46, 288–297. [Google Scholar]
- Shange, N.; Makasi, T.; Gouws, P.; Hoffman, L.C. Preservation of previously frozen black wildebeest meat (Connochaetes gnou) using oregano (Oreganum vulgare) essential oil. Meat Sci. 2019, 148, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; King, A.; Barbut, S.; Claus, J.; Cornforth, D.; Hanson, D.; Lindahl, G.; Mancini, R.; Milkowski, A.; Mohan, A.; et al. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Cheliku, N.; Cvetkovikj-Karanfilova, I.; Stefkov, G.; Karapandzova, M.; Bardhi, N.; Qjazimi, B.; Kulevanova, S. Essential oil composition of five basil cultivars (Ocimum basilicum) from Albania. Maced. Pharm. Bull. 2015, 61, 11–18. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Sweet basil essential oil composition: Relationship between cultivar, foliar feeding with nitrogen and oil content. J. Essent. Oil Res. 2012, 24, 217–227. [Google Scholar] [CrossRef]
- Beatović, D.; Krstić-Milošević, D.; Trifunović, S.; Šiljegović, J.; Glamočlija, J.; Ristić, M.; Jelačić, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75. [Google Scholar]
- Instituto Nacional de Estadística (INEGI). Geografía e Informática. 1. Aspectos geográficos. In Anuario Estadístico y Geográfico de Querétaro 2017; INEGI: Aguascalientes, Mexico, 2017; Cuadro 1.2. [Google Scholar]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- Poonkodi, K. Chemical composition of essential oil of Ocimum basilicum L. (Basil) and its biological activities-an overview. J. Crit. Rev. 2016, 3, 56–62. [Google Scholar]
- National Institute of Standards and Technology (NIST). U. S. Department of Commerce. NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 15 April 2019).
- Heipieper, H.J.; Weber, F.J.; Sikkema, J.; Keweloh, H.; de Bont, J.A.M. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994, 12, 409–415. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef]
- Gao, C.; Tian, C.; Lu, Y.; Xu, J.; Luo, J.; Guo, X. Essential oil composition and antimicrobial activity of Sphallerocarpus gracilis seeds against selected food-related bacteria. Food Control. 2011, 22, 517–522. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food ingredients. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Microorganisms in foods. Fresh meat and poultry. In Modern Food Microbiology, 7th ed.; Heldman, D.R., Ed.; Springer: New York, NY, USA, 2005; pp. 61–100. [Google Scholar]
- Lulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 2015, 14, 316–326. [Google Scholar]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Nabrdalik, M.; Grata, K. Antibacterial activity of Ocimum basilicum L. essential oil against Gram-negative bacteria. Post Fitoter. 2016, 17, 80–86. [Google Scholar]
- Predoi, D.; Iconaru, S.I.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial activity of new materials based on lavender and basil essential oils and hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Langroodi, A.M.; Tajik, H.; Mehdizadeh, T.; Moradi, M.; Kia, E.M.; Mahmoudian, A. Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss oil on the shelf-life of meat in modified atmosphere packaging. LWT Food Sci. Technol. 2018, 98, 372–380. [Google Scholar] [CrossRef]
- Gómez, M.; Lorenzo, J.M. Effect of packaging conditions on shelf-life of fresh foal meat. Meat Sci. 2012, 91, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic-Radic, Z.; Pejcic, M.; Jokovic, N.; Jokanovic, M.; Ivic, M.; Sojic, B.; Skaljac, S.; Stojanovic, P.; Mihajilov-Krstev, T. Inhibition of Salmonella Enteritidis growth and storage stability in chicken meat treated with basil and rosemary essential oils alone or in combination. Food Control. 2018, 90, 332–343. [Google Scholar] [CrossRef]
- Lorenzo, M.; Battle, R.; Gomez, M. Extension of the shelf life of foal meat with two antioxidant active packing systems. Food Sci. Technol. 2014, 59, 181–188. [Google Scholar]
- Wang, G.Y.; Wang, H.H.; Han, Y.W.; Xing, T.; Ye, K.P.; Xu, X.L.; Zhou, G.H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | MOEO | BEO | KI1 | KI2 | Ref. |
|---|---|---|---|---|---|---|
| 1 | Bicyclo[3.1.0]hexane, 4-methyl-1-(1-methylethyl)-, didehydro deriv. | 0.06 | 0.03 | 929 | 929 | [4] |
| 2 | α-Pinene | 0.6 | 0.03 | 935 | 937 | [41] |
| 3 | Camphene | 0.13 | 0.04 | 951 | 951 | [41] |
| 4 | 3,7,7-Trimethyl-1,3,5-cycloheptatriene | 0.01 | – | 973 | 972.6 | [41] |
| 5 | β-Thujene | – | 0.31 | 975 | 978 | [41] |
| 6 | β-Pinene | – | 0.52 | 980 | 980 | [41] |
| 7 | 1-Octen-3-ol | 0.06 | – | 989 | 986 | [41] |
| 8 | β-Myrcene | 1.06 | – | 993 | 991 | [41] |
| 9 | α-Phellandrene | 0.66 | 0.03 | 1007 | 1005 | [41] |
| 10 | 3-Carene | 0.62 | – | 1013 | 1012 | [4] |
| 11 | α-Terpinene | – | 0.04 | 1019 | 1019 | [41] |
| 12 | 2-Carene | 0.38 | – | 1020 | 1018 | [41] |
| 13 | p-Cymene | 6.61 | 0.34 | 1030 | 1030 | [41] |
| 14 | D-Limonene | – | 0.41 | 1032 | 1033 | [41] |
| 15 | o-Cymene | 2.05 | – | 1034 | 1031 | [4] |
| 16 | Eucalyptol | 16.07 | 8.12 | 1036 | 1035 | [41] |
| 17 | trans-β-Ocimene | – | 0.04 | 1040 | 1039 | [41] |
| 18 | β-Ocimene | – | 0.55 | 1050 | 1050 | [41] |
| 19 | Ocimene | 0.02 | – | 1051 | 1050 | [41] |
| 20 | γ-Terpinene | – | 0.17 | 1062 | 1062 | [41] |
| 21 | Linalool oxide | 0.03 | – | 1077 | 1078 | [41] |
| 22 | α-Terpinolene | 0.3 | 0.31 | 1092 | 1090 | [41] |
| 23 | p-Cymenene | 0.12 | – | 1093 | 1090 | [41] |
| 24 | Linalool | 3.64 | 23.74 | 1108 | 1106 | [41] |
| 25 | 1-Octen-3-yl-acetate | – | 0.08 | 1114 | 1110 | [41] |
| 26 | Fenchol | 0.05 | – | 1122 | 1121 | [41] |
| 27 | trans-2-Menthen-1-ol | 0.26 | – | 1129 | 1124 | [41] |
| 28 | 4-Acetil-1-methylcyclohexene | 0.01 | – | 1136 | 1135 | [41] |
| 29 | Pinocarveol | 0.48 | – | 1148 | 1142 | [41] |
| 30 | Camphor | – | 0.23 | 1152 | 1150 | [41] |
| 31 | Borneol | 1.1 | – | 1177 | 1177 | [41] |
| 32 | Terpinen-4-ol | 3.9 | 1.46 | 1188 | 1193 | [41] |
| 33 | Octyl ester acetic acid | – | 0.13 | 1194 | 1196 | [41] |
| 34 | p-Cymene-8-ol | 0.24 | – | 1196 | 1193 | [41] |
| 35 | Estragole | – | 3.77 | 1198 | 1196 | [41] |
| 36 | α-Terpineol | 2.62 | – | 1201 | 1200 | [41] |
| 37 | cis-Piperitol | 0.16 | – | 1212 | 1211 | [41] |
| 38 | cis-Carveol | 0.14 | – | 1217 | 1215 | [41] |
| 39 | Thymol ether | 0.96 | – | 1218 | 1217 | [41] |
| 40 | D-Carvone | 0.09 | – | 1230 | 1231 | [41] |
| 41 | p-Menth-1(7)-en-2-one | 0.08 | – | 1234 | 1231 | [41] |
| 42 | Piperitone | 0.13 | – | 1241 | 1250 | [41] |
| 43 | Bornyl acetate | – | 1.48 | 1285 | 1285 | [41] |
| 44 | Thymol | 28.88 | – | 1308 | 1306 | [41] |
| 45 | Eugenol | – | 1.13 | 1361 | 1358 | [41] |
| 46 | β-Elemene | – | 1.61 | 1400 | 1401 | [4] |
| 47 | Methyleugenol | 0.08 | – | 1403 | 1403 | [41] |
| 48 | Isocaryophyllene | – | 0.11 | 1411 | 1411 | [41] |
| 49 | Caryophyllene | 2.01 | – | 1412 | 1415 | [41] |
| 50 | β-Cubebene | 0.06 | 0.1 | 1414 | 1419 | [41] |
| 51 | α-Bergamotene | 0.29 | 3.39 | 1416 | 1414 | [41] |
| 52 | α-Guaiene | – | 0.49 | 1417 | 1413 | [41] |
| 53 | β-cis-Farnesene | 0.04 | 0.1 | 1423 | 1428 | [41] |
| 54 | Humulene | 1.3 | 0.37 | 1425 | 1432 | [41] |
| 55 | Germacrene-D | – | 2.09 | 1437 | 1442 | [41] |
| 56 | β-Selinene | 0.35 | – | 1440 | 1436 | [41] |
| 57 | Aromandendrene | – | 0.06 | 1441 | 1439 | [41] |
| 58 | Amorphene | – | 1.66 | 1454 | 1452 | [41] |
| 59 | Guaiol | 0.2 | – | 1602 | 1602 | [41] |
| 60 | Humulene epoxide II | 0.56 | – | 1610 | 1609 | [41] |
| 61 | Cubenol | – | 0.34 | 1613 | 1623 | [41] |
| 62 | γ-Eudesmol | 0.1 | – | 1624 | 1629 | [41] |
| 63 | Methyl jasmonate | – | 0.1 | 1632 | 1629 | [41] |
| 64 | β-Eudesmol | 0.17 | 0.07 | 1635 | 1638 | [41] |
| 65 | α-Cadinol | 0.28 | 0.09 | 1637 | 1635 | [41] |
| 66 | Hexahydrofarnesylacetone | 0.02 | – | 1826 | 1828 | [41] |
| MOEO 1 (mg/mL) | BEO 2 (mg/mL) | MOEO FIC 3 | BEO FIC | FICIndex |
|---|---|---|---|---|
| 3 | 23 | 0.6 | 0.51 | 1.11 |
| 3 | 34 | 0.6 | 0.76 | 1.36 |
| 3 | 45 | 0.6 | 1 | 1.6 |
| 4 | 11 | 0.8 | 0.24 | 1.04 |
| 4 | 23 | 0.8 | 0.51 | 1.31 |
| 4 | 34 | 0.8 | 0.76 | 1.56 |
| 4 | 45 | 0.8 | 1 | 1.8 |
| 5 | 11 | 1 | 0.24 | 1.24 |
| 5 | 23 | 1 | 0.51 | 1.51 |
| 5 | 34 | 1 | 0.76 | 1.76 |
| 5 | 45 | 1 | 1 | 2 |
| Microorganism | Time (days) | Treatment | ||
|---|---|---|---|---|
| Control | CC | AC | ||
| Mesophilic | 0 | 5.28cA ± 0.02 | 5.28eA ± 0.02 | 5.28aA ± 0.02 |
| 7 | 5.50cA ± 0.09 | 5.81dA ± 0.03 | 4.78aB ± 0.15 | |
| 14 | 7.29bB ± 0.01 | 7.63cA ± 0.03 | 5.25aC ± 0.08 | |
| 21 | 8.58aA ± 0.04 | 8.00bB ± 0.03 | 2.92bC ±0.05 | |
| 28 | 8.42aA ± 0.03 | 8.65aA ± 0.02 | 2.29cB ± 0.06 | |
| B. thermosphacta | 0 | 3.75eA ± 0.01 | 3.75eA ± 0.01 | 3.75aA ± 0.01 |
| 7 | 4.24dB ± 0.01 | 4.73dA ± 0.04 | 3.35bC ± 0.08 | |
| 14 | 5.64cA ± 0.02 | 5.21cB ± 0.02 | 3.32bC ± 0.13 | |
| 21 | 6.12bA ± 0.03 | 6.14bA ± 0.01 | 1.73cB ± 0.04 | |
| 28 | 6.51aA ± 0.03 | 6.32aB ± 0.01 | 1.69cC ± 0.03 | |
| LAB | 0 | 2.33eA ± 0.07 | 2.33cA ± 0.07 | 2.33bA ± 0.07 |
| 7 | 2.62dA ± 0.02 | 2.60cA ± 0.02 | 2.62aA ± 0.02 | |
| 14 | 3.12cB ± 0.09 | 3.45bA ± 0.10 | 1.69dC ± 0.02 | |
| 21 | 4.23bA ± 0.07 | 3.55bB ± 0.07 | 1.92cC ± 0.08 | |
| 28 | 5.11aA ± 0.01 | 4.45aB ± 0.04 | 1.87cC ± 0.08 | |
| Pseudomonas | 0 | 3.26dA ± 0.16 | 3.26cA ± 0.16 | 3.26eA ± 0.16 |
| 7 | 4.84cA ± 0.10 | 4.37bA ± 0.28 | 3.71dB ± 0.05 | |
| 14 | 6.12bA ± 0.09 | 5.47aB ± 0.08 | 5.37aB ± 0.08 | |
| 21 | 5.00cB ± 0.08 | 5.58aA ± 0.06 | 4.15cC ± 0.09 | |
| 28 | 6.68aA ± 0.04 | 6.12aB ± 0.09 | 4.64bC ± 0.03 | |
| E. coli 0157:H7 | 0 | 4.03dA ± 0.05 | 4.03dA ± 0.05 | 4.03aA ± 0.05 |
| 7 | 4.95cA ± 0.03 | 4.59cB ± 0.08 | 3.84abC ± 0.13 | |
| 14 | 4.85cA ± 0.06 | 5.00cA ± 0.05 | 3.29cB ± 0.03 | |
| 21 | 6.37bA ± 0.01 | 5.63bB ± 0.06 | 3.63bC ± 0.06 | |
| 28 | 7.67aA ± 0.02 | 6.58aB ± 0.02 | 1.97dC ± 0.02 | |
| Physicochemical Properties | Time (days) | Treatment | ||
|---|---|---|---|---|
| Control | CC | AC | ||
| TBARS | 0 | 0.04bA ± 0.02 | 0.04aA ± 0.02 | 0.04aA ± 0.02 |
| 7 | 0.08bA ± 0.004 | 0.04aB ± 0.00 | 0.04aB ± 0.00 | |
| 14 | 0.08bA ± 0.00 | 0.03aB ± 0.00 | 0.01aC ± 0.00 | |
| 21 | 0.09bA ± 0.00 | 0.04aB ± 0.00 | 0.02aC ± 0.00 | |
| 28 | 0.28aA ± 0.01 | 0.08aB ± 0.00 | 0.01aC ± 0.00 | |
| pH | 0 | 5.23bA ± 0.09 | 5.27bcA ± 0.08 | 5.25abA ± 0.06 |
| 7 | 5.45bA ± 0.12 | 5.40abA ± 0.15 | 5.06bcA ± 0.04 | |
| 14 | 4.80cB ± 0.02 | 4.91cA ± 0.01 | 4.74dB ± 0.02 | |
| 21 | 6.00aA ± 0.15 | 5.74aAB ± 0.10 | 5.35aB ± 0.06 | |
| 28 | 5.14bcA ± 0.06 | 5.05bcA ± 0.06 | 4.93cdA ± 0.06 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Hernández, E.; Castillo-Hernández, G.; González-Gutiérrez, C.J.; Silva-Dávila, A.J.; Gracida-Rodríguez, J.N.; García-Almendárez, B.E.; Di Pierro, P.; Vázquez-Landaverde, P.; Regalado-González, C. Microbiological and Physicochemical Properties of Meat Coated with Microencapsulated Mexican Oregano (Lippia graveolens Kunth) and Basil (Ocimum basilicum L.) Essential Oils Mixture. Coatings 2019, 9, 414. https://doi.org/10.3390/coatings9070414
Hernández-Hernández E, Castillo-Hernández G, González-Gutiérrez CJ, Silva-Dávila AJ, Gracida-Rodríguez JN, García-Almendárez BE, Di Pierro P, Vázquez-Landaverde P, Regalado-González C. Microbiological and Physicochemical Properties of Meat Coated with Microencapsulated Mexican Oregano (Lippia graveolens Kunth) and Basil (Ocimum basilicum L.) Essential Oils Mixture. Coatings. 2019; 9(7):414. https://doi.org/10.3390/coatings9070414
Chicago/Turabian StyleHernández-Hernández, Elvia, Gustavo Castillo-Hernández, Claudia J. González-Gutiérrez, Areli J. Silva-Dávila, Jorge N. Gracida-Rodríguez, Blanca E. García-Almendárez, Prospero Di Pierro, Pedro Vázquez-Landaverde, and Carlos Regalado-González. 2019. "Microbiological and Physicochemical Properties of Meat Coated with Microencapsulated Mexican Oregano (Lippia graveolens Kunth) and Basil (Ocimum basilicum L.) Essential Oils Mixture" Coatings 9, no. 7: 414. https://doi.org/10.3390/coatings9070414
APA StyleHernández-Hernández, E., Castillo-Hernández, G., González-Gutiérrez, C. J., Silva-Dávila, A. J., Gracida-Rodríguez, J. N., García-Almendárez, B. E., Di Pierro, P., Vázquez-Landaverde, P., & Regalado-González, C. (2019). Microbiological and Physicochemical Properties of Meat Coated with Microencapsulated Mexican Oregano (Lippia graveolens Kunth) and Basil (Ocimum basilicum L.) Essential Oils Mixture. Coatings, 9(7), 414. https://doi.org/10.3390/coatings9070414