A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings
Abstract
:1. Introduction
2. Zeolites: Microstructure and Properties
3. Zeolite-Based Anti-Corrosion Coatings
4. Composite Zeolite Coatings
4.1. Zeolite Painting
4.2. Sol-Gel Coatings
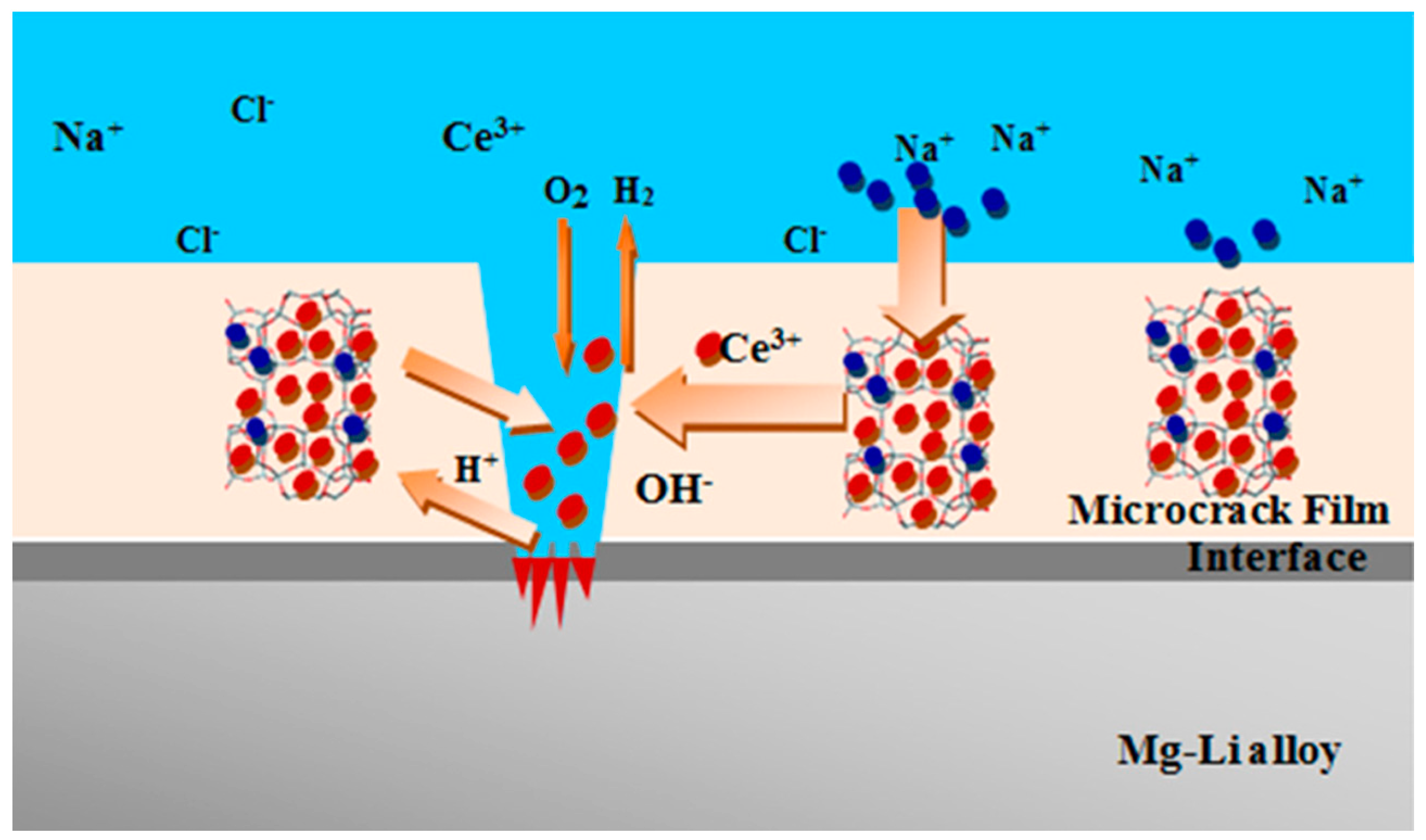
- Inhibition action: The inhibition action can be related to a reaction of zeolite surface with the OH− ions generated by the cathodic reaction that leads to the formation of SiO3− polar groups which are able to interact with the Al3+ ions, generated in the anodic area to form a passivating silica-alumina layer [86].
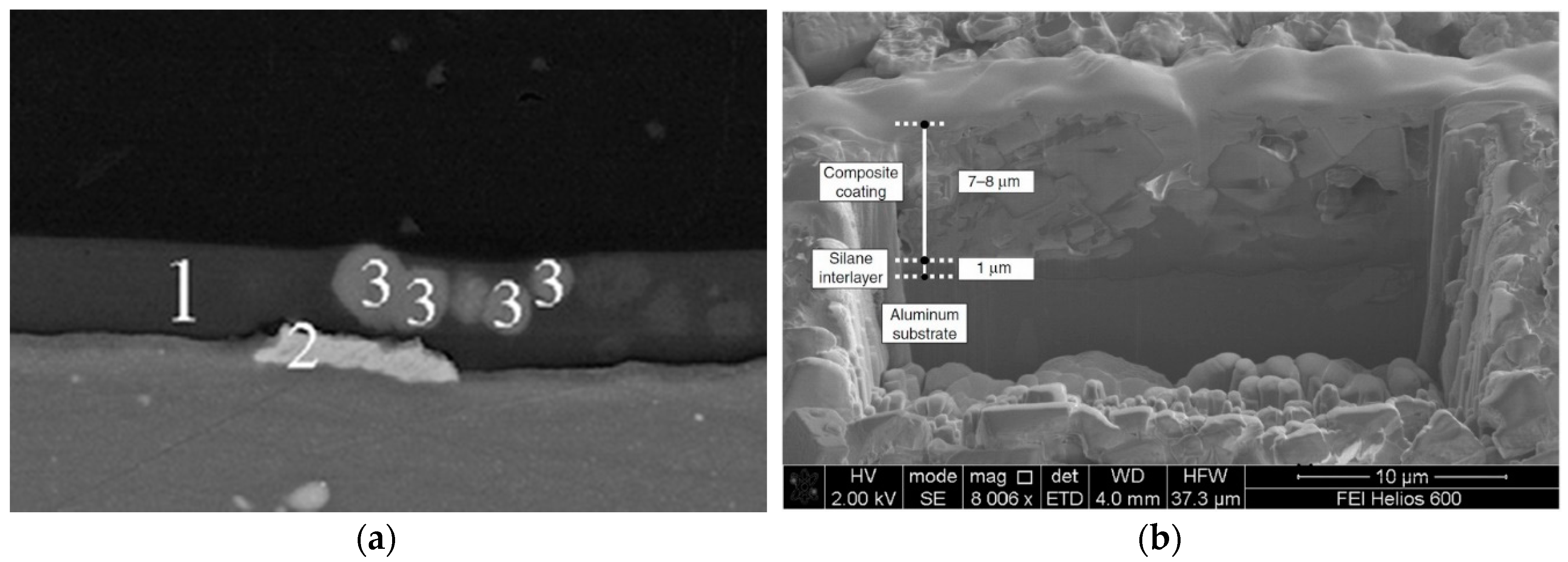
- Barrier action: The barrier protection offered by the silane–zeolite composite coatings is related to limited diffusion of aggressive species, through the coating, toward the metal substrate thanks to low liquid water permeability of silane matrix [87]. At the same time, the presence of zeolite filler in the silane layer increases the film thickness and reduces the layer porosity, exalting the anti-corrosion and barrier properties of the coating [88].
- Hydrophobic action: The surface hydrophobicity of the coating, that can be characterized by water contact angle above 140° [89], is due to the high chemical interaction between the composite constituents of the coating. In particular, the surface of the zeolite crystals is characterized by a large amount silanol functional groups that are able to create a chemical reaction with the silane matrix [90]. These Si–OH groups externally located in the zeolite particles act as preferential sites for the physical and chemical adsorption of the hydrolyzed silane molecules [91,92], reducing the surface polarity and the interaction with water molecules [93]. In fact, due to the functionalization of the zeolite with silane coupling agent, the hydrophobicity of the external surface can be easily triggered [94]. Furthermore, the high chemical affinity between zeolite filler and silane matrix could also increase the crosslinking density [95], thus limiting the hydrophilic sites in the matrix and exalting the coating hydrophobicity [96]. Finally, caused by the reaction of the zeolite surface with the hydrolyzed silane groups, a strong covalent bond is formed between the two constituents and silane long alkyl chains tend to gather on the peripheral areas of the zeolite crystal. The resulting structure can therefore be characterized by functional micelles with a preferential orientation of hydrophobic alkyl chains, which reduce surface energy and enhance the hydrophobic properties of the coating [53].
5. Anti-Corrosion Mechanisms of Zeolite Coatings
5.1. Barrier Mechanisms
5.2. Active Mechanisms
6. Conclusions and Future Trends
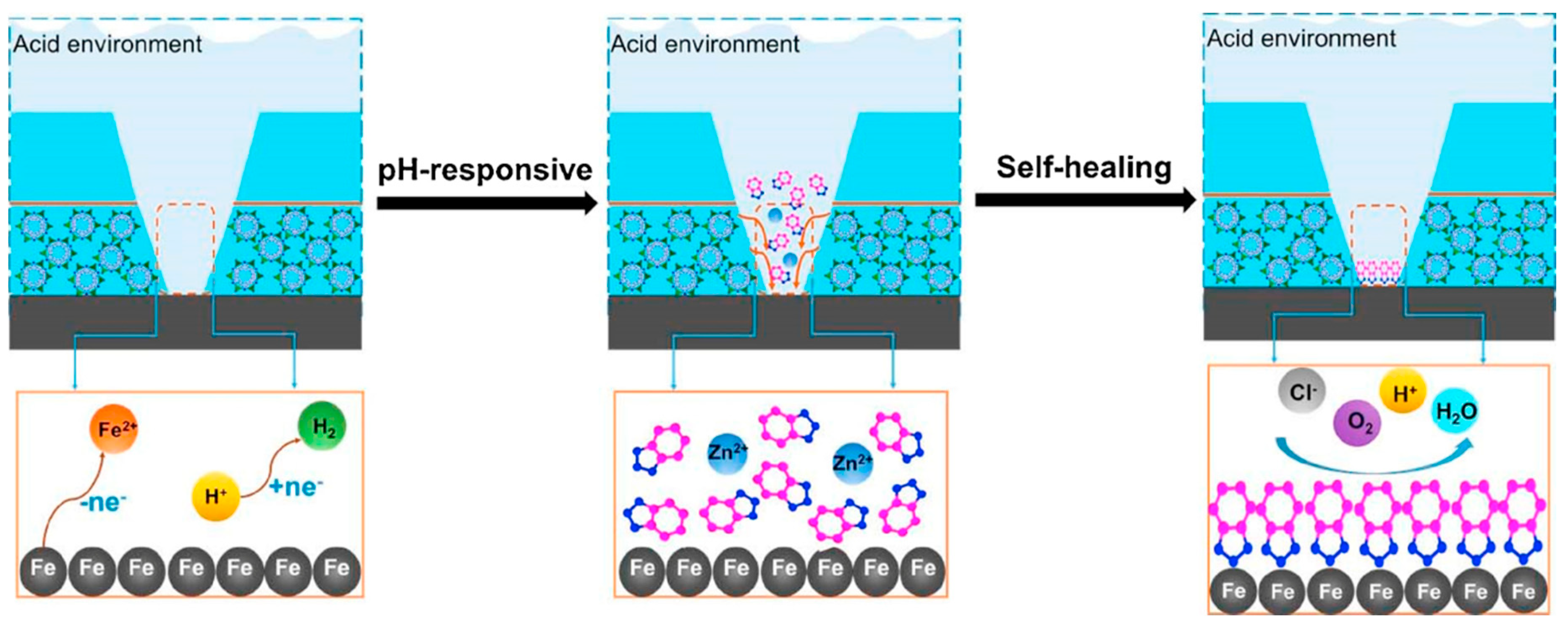
- Inhibition action: The inhibition action is strictly related to the release capability of the inhibitor due to interaction with the electrolyte. It is worth noting that, on this protection mechanism, the environmental conditions, such as the pH of the electrolyte, play a relevant role in the inhibitory efficiency of the coating.
- Barrier action: The barrier protection is related to the limited diffusion of aggressive species, through the coating, toward the metal substrate thanks to low liquid water permeability of the matrix and, at the same time, to the reduction of the layer porosity due to the presence of zeolite filler that increases the film thickness, exalting the anti-corrosion properties of the coating.
- Hydrophobic action: The surface hydrophobicity of the coating, that can be characterized by water contact angle above 140°, is due to the high chemical interaction between the composite constituents of the coating. In particular, the surface of the zeolite crystals is characterized by a large amount silanol functional groups that are able to create a chemical reaction with the organic chains of the matrix, reducing the surface polarity and the interaction with water molecules.
Author Contributions
Funding
Conflicts of Interest
References
- Davis, J.R. Surface Engineering for Corrosion and Wear Resistance; ASM International: Cleveland, OH, USA, 2001; ISBN 1615030727. [Google Scholar]
- Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Zheng, H.; Shao, Y.; Wang, Y.; Meng, G.; Liu, B. Reinforcing the corrosion protection property of epoxy coating by using graphene oxide-poly (urea-formaldehyde) composites. Corros. Sci. 2017, 123, 267–277. [Google Scholar] [CrossRef]
- Tavandashti, N.P.; Ghorbani, M.; Shojaei, A.; Mol, J.M.C.; Terryn, H.; Baert, K.; Gonzalez-Garcia, Y. Inhibitor-loaded conducting polymer capsules for active corrosion protection of coating defects. Corros. Sci. 2016, 112, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice; Wiley: Singapore, 2011; ISBN 9780470452103. [Google Scholar]
- Forsyth, M.; Hinton, B. Rare Earth-Based Corrosion Inhibitors; Woodhead Publishing: Cambridge, OK, USA, 2014; ISBN 9780857093585. [Google Scholar]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G. Mechanism of corrosion inhibition of AA2024 by rare-earth compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Heras, M.; Jimenez-Morales, A.; Casal, B.; Galvan, J.C.; Radzki, S.; Villegas, M.A. Preparation and electrochemical study of cerium-silica sol-gel thin films. J. Alloys Compd. 2004, 380, 219–224. [Google Scholar] [CrossRef]
- Khramov, A.N.; Voevodin, N.N.; Balbyshev, V.N.; Mantz, R.A. Sol-gel-derived corrosion-protective coatings with controllable release of incorporated organic corrosion inhibitors. Thin Solid Films 2005, 483, 191–196. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Grigoriev, D.; Shchukina, E.; Shchukin, D.G. Nanocontainers for self-healing coatings. Adv. Mater. Interfaces 2017, 4, 1600318. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 2011, 5, 1939–1946. [Google Scholar] [CrossRef]
- Nesterova, T.; Dam-Johansen, K.; Pedersen, L.T.; Kiil, S. Microcapsule-based self-healing anticorrosive coatings: Capsule size, coating formulation, and exposure testing. Prog. Org. Coat. 2012, 75, 309–318. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; van der Zwaag, S. A critical appraisal of the potential of self healing polymeric coatings. Prog. Org. Coat. 2011, 72, 211–221. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Loveridge, M.J. Inhibition of corrosion-driven organic coating disbondment on galvanised steel by smart release group II and Zn (II)—Exchanged bentonite pigments. Electrochim. Acta 2010, 55, 1740–1748. [Google Scholar] [CrossRef]
- Ghazi, A.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B.; Rostami, M. The application of benzimidazole and zinc cations intercalated sodium montmorillonite as smart ion exchange inhibiting pigments in the epoxy ester coating. Corros. Sci. 2015, 94, 207–217. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, M.; Xiang, T.; Yang, L.; Chan, W.; Li, C. Novel self-healing anticorrosion coating based on L-valine and MBT-loaded halloysite nanotubes. J. Mater. Sci. 2018, 53, 7793–7808. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Fu, J. An intelligent anticorrosion coating based on pH-responsive smart nanocontainers fabricated via a facile method for protection of carbon steel. J. Mater. Chem. A 2015, 3, 6423–6431. [Google Scholar] [CrossRef]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G. Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef]
- Hollamby, M.J.; Fix, D.; Dönch, I.; Borisova, D.; Möhwald, H.; Shchukin, D. Hybrid polyester coating incorporating functionalized mesoporous carriers for the holistic protection of steel surfaces. Adv. Mater. 2011, 23, 1361–1365. [Google Scholar] [CrossRef]
- Borisova, D.; Akçakayıran, D.; Schenderlein, M.; Möhwald, H.; Shchukin, D.G. Nanocontainer-based anticorrosive coatings: Effect of the container size on the self-healing performance. Adv. Funct. Mater. 2013, 23, 3799–3812. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar] [CrossRef]
- Calabrese, L. Anticorrosion behavior of zeolite coatings obtained by in situ crystallization: A critical review. Materials 2018, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Javierre, E. Modeling self-healing mechanisms in coatings: Approaches and perspectives. Coatings 2019, 9, 122. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Zagorodni, A.A. Ion Exchange Materials: Properties and Applications; Elsevier: Oxford, UK, 2006; ISBN 9780080467535. [Google Scholar]
- Mahesh, M.; Thomas, J.; Arun Kumar, K.; Bhople, B.S.; Saresh, N.V.; Vaid, S.K.; Sahu, S.K. Zeolite farming: A sustainable agricultural prospective. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2912–2924. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y.; Diaz, I. Synthesis of zeolite A using kaolin from Ethiopia and its application in detergents. New J. Chem. 2016, 40, 3440–3446. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Sourinejad, I.; Kazemian, H.; Rohani, S. Application of zeolites in aquaculture industry: A review. Rev. Aquac. 2018, 10, 75–95. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Memb. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Mandal, S.; Planells, A.D.; Hunt, H.K. Impact of deposition and laser densification of Silicalite-1 films on their optical characteristics. Microporous Mesoporous Mater. 2016, 223, 68–78. [Google Scholar] [CrossRef]
- Lew, C.M.; Cai, R.; Yan, Y. Zeolite thin films: From computer chips to space stations. Acc. Chem. Res. 2010, 43, 210–219. [Google Scholar] [CrossRef]
- Katariya, M.N.; Jana, A.K.; Parikh, P.A. Corrosion inhibition effectiveness of zeolite ZSM-5 coating on mild steel against various organic acids and its antimicrobial activity. J. Ind. Eng. Chem. 2013, 19, 286–291. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Popovici, D.; Danaila, A.I.; Butnaru, M.; Rimbu, C.; Carp-Carare, C.; Kalvachev, Y. Biocompatible poly (ether-ether-ketone)/Ag-zeolite L composite films with antimicrobial properties. Mater. Lett. 2018, 212, 339–342. [Google Scholar] [CrossRef]
- Fernández, A.; Soriano, E.; Hernández-Muñoz, P.; Gavara, R. Migration of antimicrobial silver from composites of polylactide with silver zeolites. J. Food Sci. 2010, 75, E186–E193. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 zeolite filled macrocellular foams for adsorption heat pump applications: A preliminary study. Appl. Therm. Eng. 2017, 124, 1312–1318. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Freni, A.; Proverbio, E.; Restuccia, G. Synthesis of SAPO-34 on graphite foams for adsorber heat exchangers. Appl. Therm. Eng. 2013, 61, 848–852. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P.; Schäfer, R. Zeolite membranes-state of their development and perspective. Microporous Mesoporous Mater. 2000, 38, 3–24. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Hao, H.; Dutta, P.K. Fabrication of high-performance antifogging and antireflective coatings using faujasitic nanozeolites. Microporous Mesoporous Mater. 2018, 263, 62–70. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J. Layer-by-layer approach to superhydrophobic zeolite antireflective coatings. CJC 2018, 36, 51–54. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Freni, A.; Proverbio, E. Hydrothermal and microwave synthesis of SAPO (CHA) zeolites on aluminium foams for heat pumping applications. Microporous Mesoporous Mater. 2013, 167, 30–37. [Google Scholar] [CrossRef]
- Tatlier, M. Performances of MOF vs. zeolite coatings in adsorption cooling applications. Appl. Therm. Eng. 2017, 113, 290–297. [Google Scholar] [CrossRef]
- Snelders, D.J.M.; Valega Mackenzie, F.O.; Boersma, A.; Peeters, R.H.M. Zeolites as coating materials for Fiber Bragg Grating chemical sensors for extreme conditions. Sens. Actuators B Chem. 2016, 235, 698–706. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, J.; Sun, Y.; Li, X.; Wang, X.; Zhao, Z. Gas-sensing properties of composites of Y-zeolite and SnO2. J. Mater. Sci. 2018, 53, 6729–6740. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Z.; Yan, Y. Corrosion-resistant zeolite coatings by in situ crystallization. Electrochem. Solid-State Lett. 2001, 4, B23–B26. [Google Scholar] [CrossRef]
- Mitra, A.; Wang, Z.; Cao, T.; Wang, H.; Huang, L.; Yan, Y. Synthesis and corrosion resistance of high-silica zeolite MTW, BEA, and MFI coatings on steel and aluminum. J. Electrochem. Soc. 2002, 149, B472–B478. [Google Scholar] [CrossRef]
- Cai, R.; Yan, Y. Corrosion-resistant zeolite coatings. Corrosion 2008, 64, 271–278. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Proverbio, E. Low temperature single-step synthesis of zeolite Y coatings on aluminium substrates. Microporous Mesoporous Mater. 2011, 144, 40–45. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion protection of aluminum 6061 in NaCl solution by silane-zeolite composite coatings. J. Coat. Technol. Res. 2012, 9, 597–607. [Google Scholar] [CrossRef]
- Bein, T. Synthesis and applications of molecular sieve layers and membranes. Chem. Mater. 1996, 8, 1636–1653. [Google Scholar] [CrossRef]
- Zaarour, M.; Dong, B.; Naydenova, I.; Retoux, R.; Mintova, S. Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater. 2014, 189, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, L.; Bonaccorsi, L.; Pietro, D.D.; Proverbio, E. Effect of process parameters on behaviour of zeolite coatings obtained by hydrothermal direct synthesis on aluminium support. Ceram. Int. 2014, 40, 12837–12845. [Google Scholar] [CrossRef]
- Dong, Y.; Peng, Y.; Wang, G.; Wang, Z.; Yan, Y. Corrosion-resistant zeolite silicalite-1 coatings synthesized by seeded growth. Ind. Eng. Chem. Res. 2012, 51, 3646–3652. [Google Scholar] [CrossRef]
- Romagnoli, R.; del Amo, B.; Revuelta, M.; Roselli, S.; Deyá, C.; Bellotti, N. Lanthanum-exchanged zeolite and clay as anticorrosive pigments for galvanized steel. J. Rare Earths 2014, 32, 352–359. [Google Scholar]
- Ahmed, N.M.; Emira, H.S.; Selim, M.M. Anticorrosive performance of ion-exchange zeolites in alkyd-based paints. Pigment Resin Technol. 2011, 40, 91–99. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Nogueira, C.A.; Diamantino, T.C.; Ferreira, M.G.S. Sol-gel coatings modified with zeolite fillers for active corrosion protection of AA2024. Corros. Sci. 2012, 62, 153–162. [Google Scholar] [CrossRef]
- Shabani-nooshabadi, M.; Allahyary, E.; Jafari, Y. Enhanced anti-corrosive properties of electrosynthesized polyaniline/zeolite nanocomposite coatings on steel. J. Nanostructure 2018, 8, 131–143. [Google Scholar]
- Roselli, S.; Deyá, C.; Revuelta, M.; Di Sarli, A.R.; Romagnoli, R. Zeolites as reservoirs for Ce (III) as passivating ions in anticorrosion paints. Corros. Rev. 2018, 36, 305–322. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavian, M.; Arabi, A.M. Synthesis and characterization of zeolites for anti-corrosion application: The effect of precursor and hydrothermal treatment. J. Mater. Eng. Perform. 2018, 27, 4625–4634. [Google Scholar] [CrossRef]
- Al-Subaie, M.S.M.; Al-Turkustani, A.M.A.; Selvin, R.; Al-Mhayawi, S.R. Anticorrosion nanocrystalline beta zeolite thin film for advanced applications. J. Chem. 2015, 2015, 693730. [Google Scholar] [CrossRef]
- Padhy, R.R.; Shaw, R.; Tiwari, S.; Tiwari, S.K. Ultrafine nanocrystalline mesoporous NaY zeolites from fly ash and their suitability for eco-friendly corrosion protection. J. Porous Mater. 2015, 22, 1483–1494. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Zheng, X.; Zhu, Y.; Yan, Y.; Zhang, M.; Shchukin, D.G.; Li, C.; Wang, Y.; Song, D. Smart epoxy coating containing Ce-MCM-22 zeolites for corrosion protection of Mg-Li alloy. Appl. Surf. Sci. 2016, 369, 384–389. [Google Scholar] [CrossRef]
- Devaki, H.; Priya, P.G. Corrosion studies using zeolite synthesized from fly ash. Indian J. Sci. Technol. 2016, 9, 93147. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavain, M. The role of micro/nano zeolites doped with zinc cations in the active protection of epoxy ester coating. Appl. Surf. Sci. 2017, 423, 571–583. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavian, M. Study of the active corrosion protection properties of epoxy ester coating with zeolite nanoparticles doped with organic and inorganic inhibitors. J. Taiwan Inst. Chem. Eng. 2018, 85, 207–220. [Google Scholar] [CrossRef]
- Abdolah Zadeh, M.; Tedim, J.; Zheludkevich, M.; van der Zwaag, S.; Garcia, S.J. Synergetic active corrosion protection of AA2024-T3 by 2D-anionic and 3D-cationic nanocontainers loaded with Ce and mercaptobenzothiazole. Corros. Sci. 2018, 135, 35–45. [Google Scholar] [CrossRef]
- Olad, A.; Naseri, B. Preparation, characterization and anticorrosive properties of a novel polyaniline/clinoptilolite nanocomposite. Prog. Org. Coat. 2010, 67, 233–238. [Google Scholar] [CrossRef]
- Abdel Aziz, A.H.; Jamil, T.S.; Shalaby, M.S.; Shaban, A.M.; Souaya, E.R.; Abdel Ghany, N.A. Application of (polyaniline/zeolite X) composite as anticorrosion coating for energy recovery devices in RO desalination water plants. Int. J. Ind. Chem. 2019, 10, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Shabani-Nooshabadi, M.; Mollahoseiny, M.; Jafari, Y. Electropolymerized coatings of polyaniline on copper by using the galvanostatic method and their corrosion protection performance in HCl medium. Surf. Interface Anal. 2014, 46, 472–479. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Zhang, D.; Qi, T.; Li, G.L. pH-responsive self-healing anticorrosion coatings based on benzotriazole-containing zeolitic imidazole framework. Colloids Surf. A Physicochem. Eng. Asp. 2018, 561, 1–8. [Google Scholar] [CrossRef]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of sol-gel dip coating. Thin Solid Films 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Thibault, M.; Bavay, J.; Hernandez, J.; Leroy, J.-M. Adhesion improvement mechanism of sol-gel silicone coatings. Surf. Eng. 1998, 14, 256–258. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Marques, A.; Lamaka, S.V.; Simões, A.; Diamantino, T.C.; Ferreira, M.G.S. The role of Ce (III)-enriched zeolites on the corrosion protection of AA2024-T3. Electrochim. Acta 2013, 112, 549–556. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Diamantino, T.C.; Ferreira, M.G.S. Synergistic protection against corrosion of AA2024-T3 by sol-gel coating modified with La and Mo-enriched zeolites. J. Electrochem. Soc. 2014, 161, C215–C222. [Google Scholar] [CrossRef]
- Caprì, A.; Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion resistance of cerium based silane-zeolite coatings on AA6061. Solid State Phenom. 2015, 227, 163–166. [Google Scholar] [CrossRef]
- Ferrer, E.L.; Rollon, A.P.; Mendoza, H.D.; Lafont, U.; Garcia, S.J. Double-doped zeolites for corrosion protection of aluminium alloys. Microporous Mesoporous Mater. 2014, 188, 8–15. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Electrochemical behavior of hydrophobic silane-zeolite coatings for corrosion protection of aluminum substrate. J. Coat. Technol. Res. 2014, 11, 883–898. [Google Scholar] [CrossRef]
- Calabrese, L.; Caprì, A.; Proverbio, E. Anti-corrosion performances of hybrid silane coatings on AZ31 alloy. Anti-Corrosion Methods Mater. 2018, 65, 317–324. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Assessment of hydrophobic and anticorrosion properties of composite silane-zeolite coatings on aluminum substrate. J. Coat. Technol. Res. 2016, 13, 287–297. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Enhancement of the hydrophobic and anti-corrosion properties of a composite zeolite coating on Al6061 substrate by modification of silane matrix. Corros. Eng. Sci. Technol. 2017, 52, 61–72. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavain, M. Study of the impact of sequence of corrosion inhibitor doping in zeolite on the self-healing properties of silane sol-gel film. J. Ind. Eng. Chem. 2018, 66, 221–230. [Google Scholar] [CrossRef]
- Palanivel, V.; Zhu, D.; van Ooij, W.J. Nanoparticle-filled silane films as chromate replacements for aluminum alloys. Prog. Org. Coat. 2003, 47, 384–392. [Google Scholar] [CrossRef]
- Woo, R.S.C.; Zhu, H.; Chow, M.M.K.; Leung, C.K.Y.; Kim, J.K. Barrier performance of silane-clay nanocomposite coatings on concrete structure. Compos. Sci. Technol. 2008, 68, 2828–2836. [Google Scholar] [CrossRef]
- Montemor, M.F.; Cabral, A.M.; Zheludkevich, M.L.; Ferreira, M.G.S. The corrosion resistance of hot dip galvanized steel pretreated with Bis-functional silanes modified with microsilica. Surf. Coat. Technol. 2006, 200, 2875–2885. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog. Org. Coat. 2014, 77, 1341–1350. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Proverbio, E. Organosilanes functionalization of alumino-silica zeolites for water adsorption applications. Microporous Mesoporous Mater. 2016, 234, 113–119. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, Q. Preparation of nanosized NaA zeolite and its surface modification by KH-550. Mater. Sci. 2019, 36, 638–643. [Google Scholar] [CrossRef]
- Matsumoto, A.; Tsutsumi, K.; Schumacher, K.; Unger, K.K. Surface functionalization and stabilization of mesoporous silica spheres by silanization and their adsorption characteristics. Langmuir 2002, 18, 4014–4019. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Alioto, S.; Bruzzaniti, P.; Proverbio, E. Surface silanation of alumina-silica zeolites for adsorption heat pumping. Renew. Energy 2017, 110, 79–86. [Google Scholar] [CrossRef]
- Wang, X.G.; Fang, X.; Che, S.; Du, T.; Liu, L.Y. Preparation of hydrophobic zeolite 13X@SiO2 and their adsorption properties of CO2 and H2O. Adv. Mater. Res. 2014, 1053, 311–316. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Kim, S. Surface tension of silane treated natural zeolite. Mater. Chem. Phys. 2000, 63, 251–255. [Google Scholar] [CrossRef]
- Deymier, P.; Roop, R.; Monk, D.J.; Almanza-Workman, A.M.; Raghavan, S. Water dispersible silanes for wettability modification of polysilicon. J. Electrochem. Soc. 2002, 149, H6–H11. [Google Scholar]
- Takatani, Y.; Yamakawa, K.; Yoshizawa, S. Corrosion behaviour of aluminum alloys in saturated calcium hydroxide solution. Zair. Soc. Mater. Sci. Jpn. 1983, 32, 1218–1222. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sugiyama, S.; Matsuoka, O.; Kohmura, K.; Honda, T.; Banno, Y.; Nozoye, H. Dissolution of zeolite in acidic and alkaline aqueous solutions as revealed by AFM imaging. J. Phys. Chem. 1996, 100, 18474–18482. [Google Scholar] [CrossRef]
- Kumaraguru, S.P.; Veeraraghavan, B.; Popov, B.N. Development of an electroless method to deposit corrosion-resistant silicate layers on metallic substrates. J. Electrochem. Soc. 2006, 153, B253–B259. [Google Scholar] [CrossRef]
- Deyá, C.; Romagnoli, R.; Del Amo, B. A new pigment for smart anticorrosive coatings. J. Coat. Technol. Res. 2007, 4, 167–175. [Google Scholar] [CrossRef]
- Chico, B.; Simancas, J.; Vega, J.M.; Granizo, N.; Díaz, I.; de la Fuente, D.; Morcillo, M. Anticorrosive behaviour of alkyd paints formulated with ion-exchange pigments. Prog. Org. Coat. 2008, 61, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Schenderlein, M.; Huang, X.; Brownbill, N.J.; Blanc, F.; Shchukin, D. Influence of functionalization of nanocontainers on self-healing anticorrosive coatings. ACS Appl. Mater. Interfaces 2015, 7, 22756–22766. [Google Scholar] [CrossRef]
- Ghosh, S.K. Functional Coatings: By Polymer Microencapsulation; Wiley: Weinheim, Germany, 2006; ISBN 352731296X. [Google Scholar]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Garza, M.; Olguín, M.T.; García-Sosa, I.; Alcántara, D.; Rodríguez-Fuentes, G. Silver supported on natural Mexican zeolite as an antibacterial material. Microporous Mesoporous Mater. 2000, 39, 431–444. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Williams, C.; Kenward, M.A.; Radecka, I. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 2008, 104, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Fonseca, A.M.; Botelho, G.; Aguiar, C.A.; Neves, I.C. Antimicrobial activity of faujasite zeolites doped with silver. Microporous Mesoporous Mater. 2012, 160, 126–132. [Google Scholar] [CrossRef]
- McDonnell, A.M.P.; Beving, D.; Wang, A.; Chen, W.; Yan, Y. Hydrophilic and antimicrobial zeolite coatings for gravity-independent water separation. Adv. Funct. Mater. 2005, 15, 336–340. [Google Scholar] [CrossRef]
- Chanda, R.; Selvam, T.; Herrmann, R.; Schwieger, W. Reactive coating process for binder-free zeolite FAU films on metallic aluminum supports. Mater. Lett. 2018, 211, 103–106. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Bonaccorsi, L.; Frazzica, A.; Caprì, A.; Freni, A.; Proverbio, E. Development and characterization of silane-zeolite adsorbent coatings for adsorption heat pump applications. Appl. Therm. Eng. 2017, 116, 364–371. [Google Scholar] [CrossRef]
- Aramaki, K. Preparation of protective films containing molybdate for self-healing of a scratched iron surface. Corrosion 2000, 56, 901–909. [Google Scholar] [CrossRef]
- Lin, B.L.; Lu, J.T.; Kong, G. Effect of molybdate post-sealing on the corrosion resistance of zinc phosphate coatings on hot-dip galvanized steel. Corros. Prot. 2007, 50, 962–967. [Google Scholar] [CrossRef]
- Trabelsi, W.; Cecilio, P.; Ferreira, M.G.S.; Montemor, M.F. Electrochemical assessment of the self-healing properties of Ce-doped silane solutions for the pre-treatment of galvanised steel substrates. Prog. Org. Coat. 2005, 54, 276–284. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Geerlings, P.; Pidko, E.A.; Van Santen, R.A. The framework basicity of zeolites. J. Mater. Chem. 2012, 22, 18705–18717. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Han, E.H. Corrosion characterization of Mg-8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]
- Majdi, M.R.; Danaee, I.; Afghahi, S.S.S. Preparation and anti-corrosive properties of cerium oxide conversion coatings on steel X52. Mat. Res. 2017, 20, 445–451. [Google Scholar] [CrossRef]
- Volarič, B.; Milošev, I. Rare earth chloride and nitrate salts as individual and mixed inhibitors for aluminium alloy 7075-T6 in chloride solution. Corros. Eng. Sci. Technol. 2017, 52, 201–211. [Google Scholar] [CrossRef]
- Yan, W.; Ong, W.K.; Wu, L.Y.; Wijesinghe, S.L.; Yan, W.; Ong, W.K.; Wu, L.Y.; Wijesinghe, S.L. Investigation of using sol-gel technology for corrosion protection coating systems incorporating colours and inhibitors. Coatings 2019, 9, 52. [Google Scholar] [CrossRef]
- Wang, T.; Du, J.; Ye, S.; Tan, L.; Fu, J. Triple-stimuli-responsive smart nanocontainers enhanced self-healing anticorrosion coatings for protection of aluminum alloy. ACS Appl. Mater. Interfaces 2019, 11, 4425–4438. [Google Scholar] [CrossRef] [PubMed]
- Kanu, N.J.; Gupta, E.; Vates, U.K.; Singh, G.K. Self-healing composites: A state-of-the-art review. Compos. A Appl. Sci. Manuf. 2019, 121, 474–486. [Google Scholar] [CrossRef]
- Den Brabander, M.; Fischer, H.R.; Garcia, S.J. Self-healing polymeric systems: Concepts and applications. In Smart Polymers and their Applications, 2nd ed.; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 379–409. ISBN 978-0-08-102416-4. [Google Scholar]
- Samiee, R.; Ramezanzadeh, B.; Mahdavian, M.; Alibakhshi, E. Assessment of the smart self-healing corrosion protection properties of a water-base hybrid organo-silane film combined with non-toxic organic/inorganic environmentally friendly corrosion inhibitors on mild steel. J. Clean. Prod. 2019, 220, 340–356. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Li, B.; Hu, T.; Yang, Y.; Li, L.; Zhang, J. Environmentally benign and durable superhydrophobic coatings based on SiO2 nanoparticles and silanes. J. Colloid Interface Sci. 2019, 542, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, H.; Jia, Y.; Liu, J.; Zhang, H.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of biomimetic polydimethylsiloxane (PDMS) films with superhydrophobic, self-healing and drag reduction properties via replication of shark skin and SI-ATRP. Chem. Eng. J. 2019, 356, 318–328. [Google Scholar] [CrossRef]
- Lopez de Armentia, S.; Pantoja, M.; Abenojar, J.; Martinez, M.; Lopez de Armentia, S.; Pantoja, M.; Abenojar, J.; Martinez, M.A. Development of silane-based coatings with zirconia nanoparticles combining wetting, tribological, and aesthetical properties. Coatings 2018, 8, 368. [Google Scholar] [CrossRef]
- Ma, L.; Svec, F.; Tan, T.; Lv, Y. In-situ growth of highly permeable zeolite imidazolate framework membranes on porous polymer substrate using metal chelated polyaniline as interface layer. J. Membrane Sci. 2019, 576, 1–8. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, F.; Zhang, J. Design and synthesis of multifunctional metal-organic zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, X.; Wang, J.; Cui, X.; Zhou, Q.; Qi, T.; Li, G.L. Smart-sensing polymer coatings with autonomously reporting corrosion dynamics of self-healing systems. Adv. Mater. Interfaces 2019, 6, 1900055. [Google Scholar] [CrossRef]
- Karami, Z.; Maleki, S.; Moghaddam, A.; Jahandideh, A. Self-healing bio-composites: Concepts, developments, and perspective. In Sustainable Polymer Composites and Nanocomposites; Springer: Cham, Switzerland, 2019; pp. 1323–1343. [Google Scholar]
- Catauro, M.; Vecchio Ciprioti, S. Sol-gel synthesis and characterization of hybrid materials for biomedical applications. In Thermodynamics and Biophysics of Biomedical Nanosystems; Demetzos, C., Pippa, N., Eds.; Springer: Singapore, 2019; pp. 445–475. [Google Scholar]


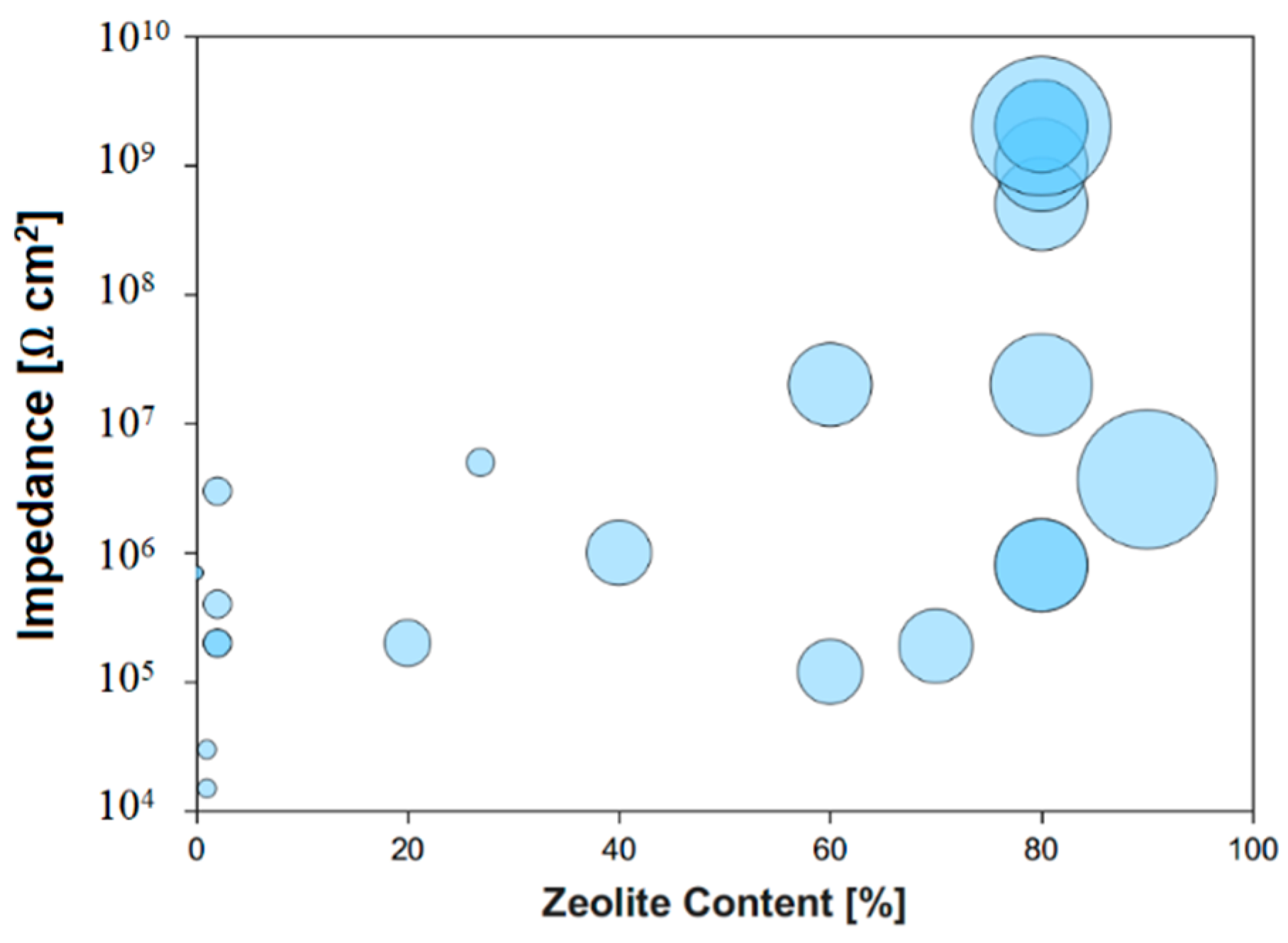


| Authors | Ref. | Year | Zeolite | Matrix | Substrate | Coating Thickness | Testing Electrolyte | Icor [A/cm2] | |Z| 0.01 Hz [Ω/cm2] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Content | Inhibitor | Coating | Substrate | Coating | Substrate | |||||||
| Olad et al. | [71] | 2010 | clinoptilolite | 0% 1% 3% 5% | none | polyaniline | iron | 20 μm | 3.5% NaCl | 7.5 × 10−7 3.1 × 10−5 1.0 × 10−6 1.3 × 10−6 | – | – | – |
| Olad et al. | [71] | 2010 | clinoptilolite | 0% 1% 3% 5% | none | polyaniline | iron | 20 μm | 1 M H2SO4 | 5.6 × 10−6 1.2 × 10−5 1.4 × 10−6 3.2 × 10−6 | – | – | – |
| Olad et al. | [71] | 2010 | clinoptilolite | 0% 1% 3% 5% | none | polyaniline | iron | 20 μm | 1 M HCl | 7.5 × 10−5 4.2 × 10−5 3.1 × 10−6 7.9 × 10−6 | – | – | – |
| Padhy et al. | [65] | 2015 | Y | 2% | none Zn | epoxy | mild steel | 45 μm | 3.5% NaCl | – | – | 8 × 106 2.5 × 107 | – |
| Zhang et al. | [66] | 2016 | MCM-22 | – | none Ce | epoxy | Mg-Li alloy | 50 μm | 0.35% NaCl | – | – | 2 × 107 2 × 109 | – |
| Devaki et al. | [67] | 2016 | – | 76% | none | – | mild steel | 35 μm | 3.5% NaCl | 3.0 × 10−5 | 8.3 × 10−4 | – | – |
| Rassouli et al. | [68] | 2017 | X | 0% 1% | Zn | epoxy ester | mild steel | 27 μm | 3.5% NaCl | 1.1 × 10−2 1.3 × 10−3 | – | 1 × 103 1 × 103.5 | – |
| Roselli et al. | [62] | 2018 | – | 30% | Zn phosphate Ce Ce + Zn phosp. | alkyd | SAE 1010 steel | 70 μm | 0.05 M NaCl | – | – | 1 × 104 – 1 × 106 | – |
| Guo et al. | [74] | 2018 | ZIF-7 | 1% | none benzotriazole | epoxy | Q235 steel | 70 μm | 0.1 M HCl | – | – | 3.5 × 104 4.0 × 104 | – |
| Abdolah et al. | [70] | 2018 | Y | – | none Ce MBZ Ce + MBZ | epoxy | AA2024-T3 | 30 μm | 0.05 M NaCl | – | – | 6 × 105 1.0 × 106 1.0 × 106 7.0 × 106 | – |
| Rassouli et al. | [69] | 2018 | X | 1% | none MBI Zn MBI + Zn | epoxy ester | mild steel | 28 μm | 3.5% NaCl | – | – | 1 × 108.4 1 × 108.1 1 × 109 1 × 1010.4 | – |
| Shabani et al. | [61] | 2018 | Y | 1% | none | polyaniline | SS 304 | – | 0.5 M HCl | 2.0 × 10−5 | 6.8 × 10−5 | 5.8 × 104 | 2.6 × 102 |
| Aziz et al. | [72] | 2019 | X | 1% | none | polyaniline | SS 304 SS316 Al Carbon steel | – | 3.5% NaCl | 1.0 × 10−6 1.0 × 10−6 3.2 × 10−5 2.4 × 10−5 | 5.3 × 10−6 5.4 × 10−6 8.4 × 10−5 7.7 × 10−5 | – | – |
| Authors | Ref. | Year | Zeolite | Matrix | Substrate | Coating Thickness | Electrolyte | Icor (A/cm2) | |Z| 0.01 Hz (Ω/cm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Content | Inhibitor | Coating | Substrate | Coating | Substrate | |||||||
| Calabrese et al. | [53] | 2012 | Y | 2% 4% 8% 16% | none | TMS | AA6061 | 10 μm 10 μm 10 μm 10 μm | 3.5% NaCl | 2 × 10−5 1 × 10−5 2 × 10−6 2 × 10−5 | 2 × 10−5 | – | – |
| Dias et al. | [60] | 2012 | X | – | none Ce | GLYMO + ZTP | AA2024-T3 | 1.5 μm | 3.0% NaCl | – | – | 7 × 105 7 × 105 | 5 × 105 |
| Dias et al. | [77] | 2013 | X | 26.9% 26.9% | none Ce | GLYMO + ZTP | AA2024-T3 | 3.0 μm | 0.05 M NaCl | – | – | 5 × 106 5 × 106 | 3 × 106 |
| Ferrer et al. | [80] | 2014 | Y | 10% | none Ce DEDTC Ce + DETC | GLYMO + TEOS + epoxy resin | AA2024-T3 | 100 μm | 0.05 M NaCl | 4 × 10−6 4 × 10−7 2.5 × 10−6 1 × 10−7 | 5 × 10−6 | – | – |
| Calabrese et al. | [81] | 2014 | SAPO-34 | 60% 70% 80% 90% | none | TMS | AA6061 | 7 μm 8 μm 10 μm 15 μm | 3.5% NaCl | 2 × 10−5 3 × 10−7 7 × 10−7 5 × 10−6 | 1.5 × 10−5 | 1.2 × 105 1.9 × 105 8 × 105 3.7 × 106 | 1.2 × 104 |
| Dias et al. | [78] | 2014 | X | 2% | none La Mo La + Mo | silane | AA2024-T3 | 3.0 μm | 0.05 M NaCl | – | – | 2 × 105 4 × 105 2 × 105 3 × 106 | – |
| Caprì et al. | [79] | 2015 | SAPO-34 | 80% | none Ce | TMS | AA6061 | 10 μm | 3.5% NaCl | – | – | 8 × 105 5 × 108 | 2 × 104 |
| Calabrese et al. | [83] | 2016 | SAPO-34 | 80% | none | TMS DMS + TMS OTS | AA6061 | 10 μm | 3.5% NaCl | 2 × 10−10 5 × 10−11 2 × 10−11 | 2 × 10−5 | 1 × 109 2 × 109 2 × 109 | 2 × 104 |
| Calabrese et al. | [84] | 2017 | SAPO-34 | 80% | none | DMS + TMS | AA6061 | 15 μm | 3.5% NaCl | 5 × 10−11 | 2 × 10−5 | 2 × 109 | 1.2 × 104 |
| Calabrese et al. | [82] | 2018 | SAPO-34 | 20% 40% 60% 80% | none | TMS | – | 5 μm 7 μm 9 μm 11 μm | 3.5% NaCl | – | – | 2 × 105 1 × 106 2 × 107 2 × 107 | 8 × 103 |
| Rassolui et al. | [85] | 2018 | X | 1 | none Zn + MBI | MTES + TEOS + GLYMO | mild steel | – | 3.5% NaCl | 1 × 10−6 5 × 0−7 | 1.4 × 10−5 | 1.5 × 104 3 × 104 | 0.7 × 103 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, L.; Proverbio, E. A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings. Coatings 2019, 9, 409. https://doi.org/10.3390/coatings9060409
Calabrese L, Proverbio E. A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings. Coatings. 2019; 9(6):409. https://doi.org/10.3390/coatings9060409
Chicago/Turabian StyleCalabrese, Luigi, and Edoardo Proverbio. 2019. "A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings" Coatings 9, no. 6: 409. https://doi.org/10.3390/coatings9060409






