Assessment of Super-Hydrophobic Textured Coatings on AA6082 Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Treatment
- Boiling H2O: aluminum substrates were treated in ultrapure boiling water for 5 min and dried at 70° for 60 min.
- HNO3/HCl: the cleaned samples were immersed in a mixture of HNO3 and HCl (volume ratio 1:3) in ultra-pure water solution for 1 h.
- HF/HCl: a mixture acid solution was prepared by adding 73% by volume of HCl and 5% HF in ultra-pure water (22%). The aluminum substrates were immersed in this solution for 15 s.
2.3. Wettability Measurements
2.4. Morphology Analysis
3. Results and Discussion
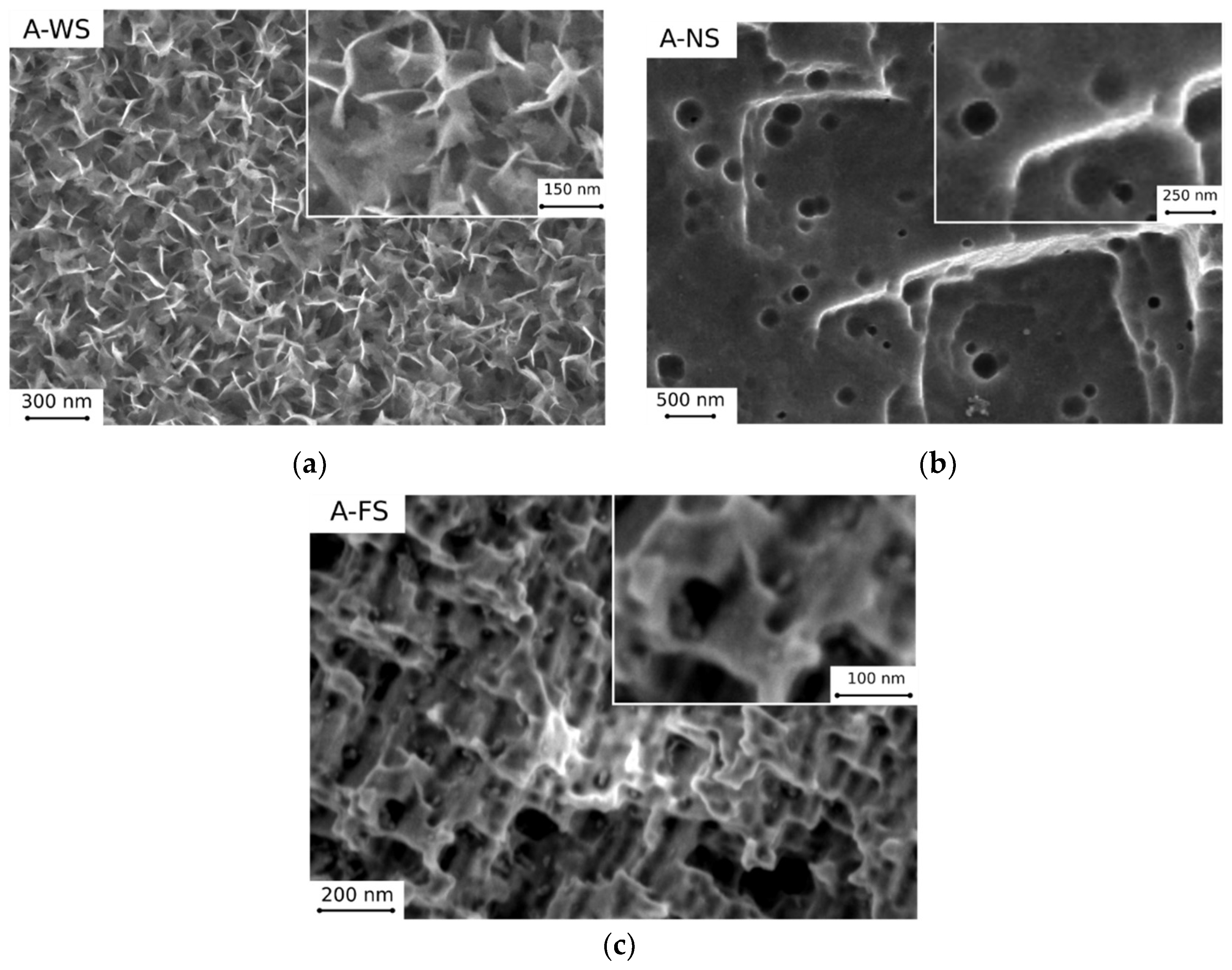
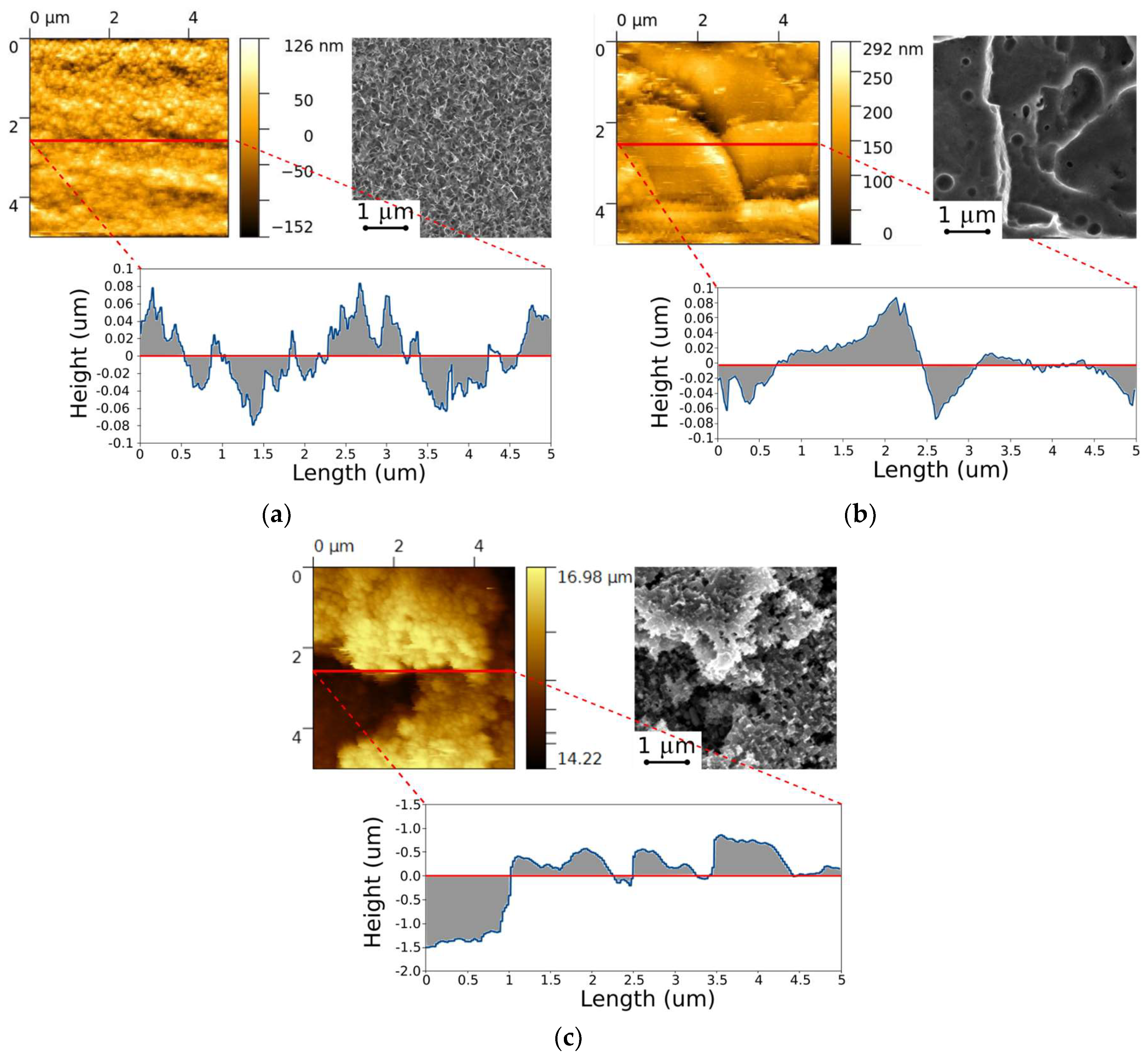
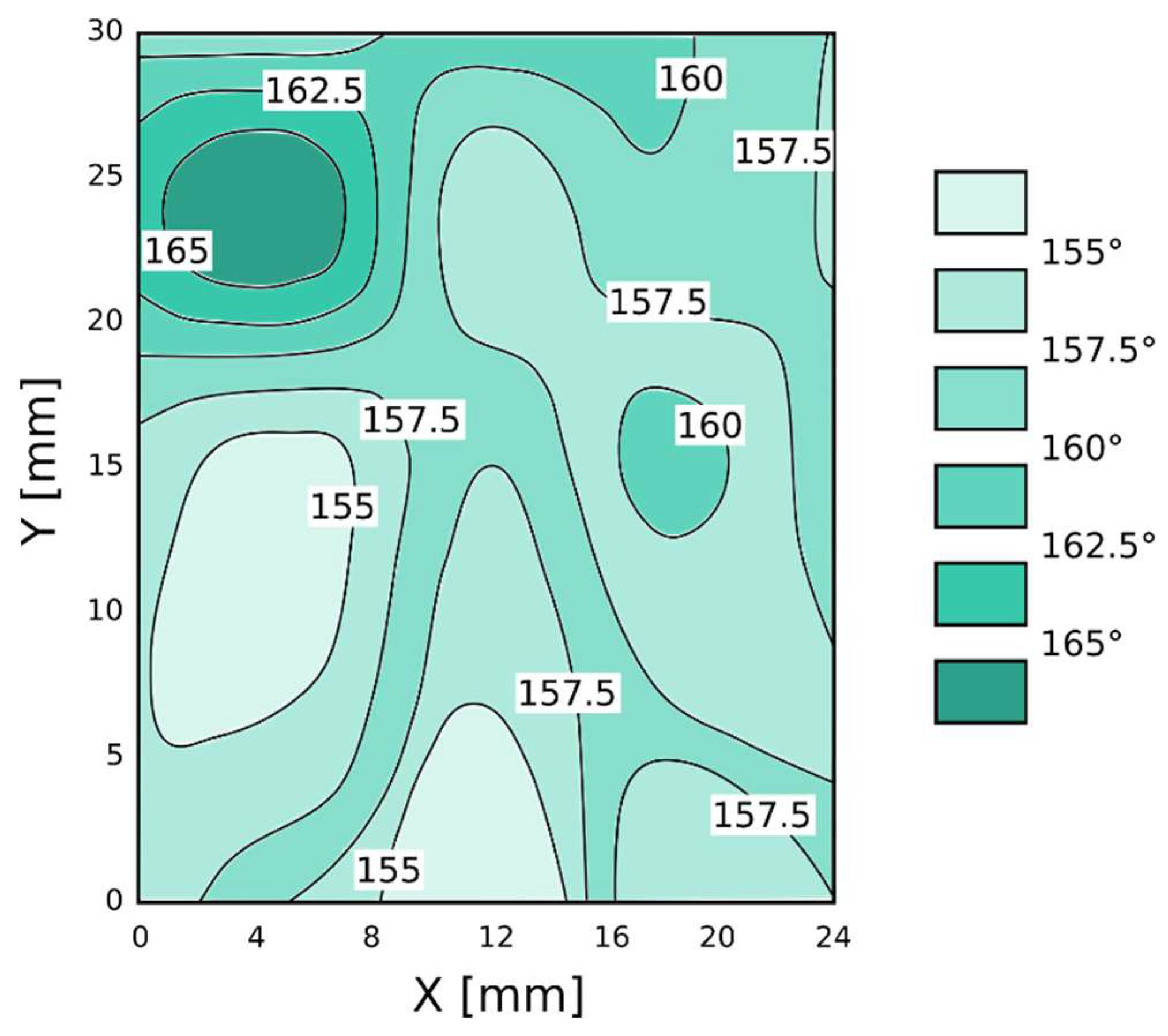
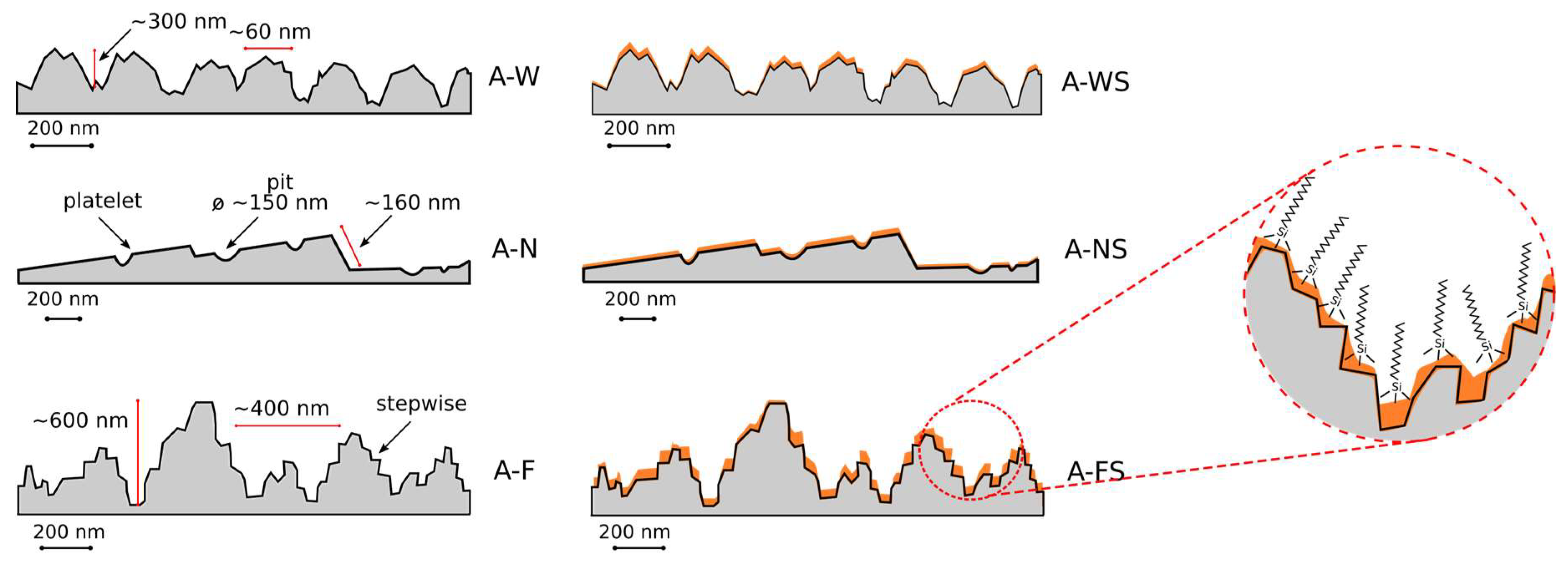
3.1. Morphological Analysis
- Boiling H2O: the surfaces appeared as a 3D flower-like structure. In fact, many 3D randomly oriented flakes clusters grew on the substrate. These flower-like clusters consisted of numerous petal-like flakes with a thickness of 30 nm. These neighboring platelets overlapped and connected with each other, thus generating a complex micro- and nano-scale upper-structure during growth. This result is a consequence of the formation of a protective surface film composed mainly by an amorphous or pseudo-amorphous boehmite with approximate AlO(OH)·H2O [24,25,26]. The boehmite formation method is called boehmitage, and it is used in the industrial field as a surface engineering process in order to improve hydrophobicity and corrosion resistance of aluminum alloys [27].
- HNO3/HCl: the surfaces obtained after nitric and chloridric acid diluted solution had a bimodal structure characterized by step like structure, at micro-scale level, and by regular small pits at nano-scale level. This two-level surface morphology significantly increased the effective surface area and the roughness surface profile. The smooth platelets had a length in the range 2–4 µm. The pits (diameter about 150 nm) were randomly distributed on the apparently smooth and planar areas of the platelets. This microstructure is a consequence of aluminum grain orientation, preferred dissolution planes and secondary phases distribution. The AA6082 alloy is indeed characterized by Fe–Mn inclusions with dimensions in the range 5–8 μm, randomly distribute in the whole surface [28]. Furthermore, several dislocations and defects are presents in the aluminum alloy matrix. These defects were more sensitive to the acidic etching solution than other locations of the metal substrate [29].
- HF/HCl: a bimodal structure was observed on the surface of treated aluminum. A main micro-structure was like a coral network structure with the presence of large deeper corrosion attack zones, while the nano-structure was similar to a pixel like structure with clear edges and corners. Only on this surface was a thin silane film observed on SEM images. The presence of the octadecylsilane film was mainly evidenced on the profile asperities. In fact, “pixel” corners, which were covered by the polymer monolayer, looked less acute still preserving the original shape.
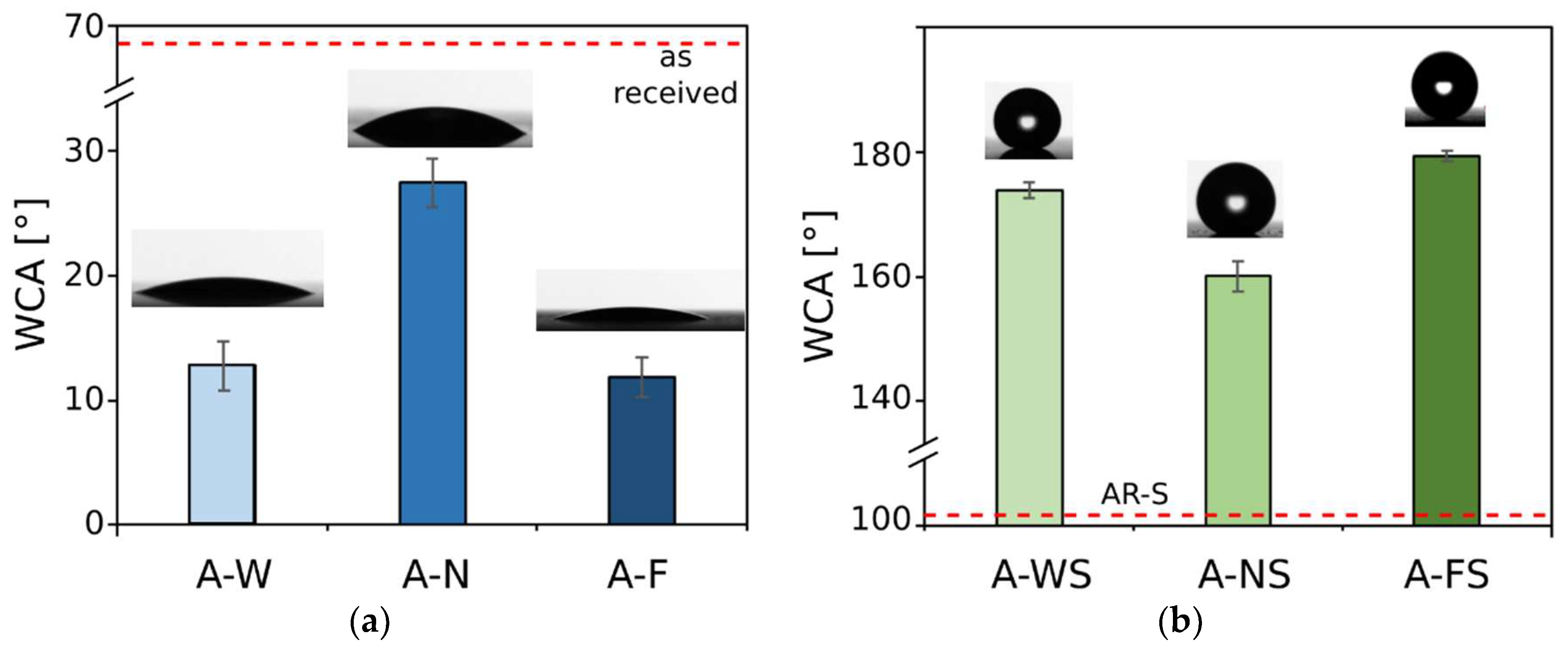
3.2. Wettability Analysis
3.3. Structural Surface Scheme of Super-Hydrophobic Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Joseph, G.B.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Cui, M.; Shen, Y.; Tian, H.; Yang, Y.; Feng, H.; Li, J. Influence of water adhesion of superhydrophobic surfaces on their anti-corrosive behavior. Surf. Coat. Technol. 2018, 347, 38–45. [Google Scholar] [CrossRef]
- Lai, D.-L.; Kong, G.; Li, X.-C.; Che, C.-S. Corrosion resistance of ZnO nanorod superhydrophobic coatings with rose petal effect or lotus leaf effect. J. Nanosci. Nanotechnol. 2019, 19, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Wang, G.; Liu, A.; Lin, Y.; Chen, H.; Wang, G.; Liu, A. Recent progress in preparation and anti-icing applications of superhydrophobic coatings. Coatings 2018, 8, 208. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Li, S.; Han, Z.; Yu, S.; Ren, L. Fabrication of biomimetic super-hydrophobic surface on aluminum alloy. J. Mater. Sci. 2014, 49, 1624–1629. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, T.; Wang, F. Fabrication of Superhydrophobic AA5052 aluminum alloy surface with improved corrosion resistance and self cleaning property. Coatings 2018, 8, 390. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Wu, Y.; Zhu, B.; Song, H. One-step method for fabrication of bioinspired hierarchical superhydrophobic surface with robust stability. Appl. Surf. Sci. 2019, 473, 493–499. [Google Scholar] [CrossRef]
- Urata, C.; Masheder, B.; Cheng, D.F.; Miranda, D.F.; Dunderdale, G.J.; Miyamae, T.; Hozumi, A. Why can organic liquids move easily on smooth alkyl-terminated surfaces? Langmuir 2014, 30, 4049–4055. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Z.; Liub, W. Outmatching superhydrophobicity: Bio-inspired re-entrant curvature for mighty superamphiphobicity in air. Mater. Chem. A 2017, 5, 14480–14507. [Google Scholar] [CrossRef]
- Kadlečková, M.; Minařík, A.; Smolka, P.; Mráček, A.; Wrzecionko, E.; Novák, L.; Musilová, L.; Gajdošík, R. Preparation of textured surfaces on aluminum-alloy substrates. Materials 2018, 12, 109. [Google Scholar] [CrossRef]
- Minařík, M.; Wrzecionko, E.; Minařík, A.; Grulich, O.; Smolka, P.; Musilová, L.; Junkar, I.; Primc, G.; Ptošková, B.; Mozetič, M. Preparation of hierarchically structured polystyrene surfaces with superhydrophobic properties by plasma-assisted fluorination. Coatings 2019, 9, 201. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Ran, M.; Zheng, W.; Wang, H. Fabrication of superhydrophobic surfaces for corrosion protection: A review. Mater. Sci. Technol. 2019, 35, 313–326. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Petr, M.; Hanuš, J.; Kylián, O.; Kratochvíl, J.; Solař, P.; Slavínská, D.; Biederman, H. Superhydrophobic fluorine-free hierarchical coatings produced by vacuum based method. Mater. Lett. 2016, 167, 30–33. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Y.; Fang, S. A facial approach to fabricate superhydrophobic aluminum surface. Surf. Interface Anal. 2010, 42, 1–6. [Google Scholar] [CrossRef]
- Ruan, M.; Li, W.; Wang, B.; Luo, Q.; Ma, F.; Yu, Z. Optimal conditions for the preparation of superhydrophobic surfaces on al substrates using a simple etching approach. Appl. Surf. Sci. 2012, 258, 7031–7035. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Wang, T. Facile fabrication of superhydrophobic surface with micro/nanoscale binary structures on aluminum substrate. Appl. Surf. Sci. 2011, 253, 5831–5836. [Google Scholar] [CrossRef]
- Shi, Y.; Xiao, X.; Zhang, W. Facile fabrication of superhydrophobic surface with needle-like microflower structure on aluminum substrate. J. Coat. Technol. Res. 2015, 12, 1143–1151. [Google Scholar] [CrossRef]
- Chen, F.F.; Yang, Z.Y.; Zhu, Y.J.; Xiong, Z.C.; Dong, L.Y.; Lu, B.Q.; Wu, J.; Yang, R.L. Low-cost and scaled-up production of fluorine-free, substrate-independent, large-area superhydrophobic coatings based on hydroxyapatite nanowire bundles. Chem. A Eur. J. 2018, 24, 416–424. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef]
- Geiculescu, A.C.; Strange, T.F. A microstructural investigation of low-temperature crystalline alumina films grown on aluminum. Thin Solid Films 2003, 426, 160–171. [Google Scholar] [CrossRef]
- Underhill, P.R.; Rider, A.N. Hydrated oxide film growth on aluminium alloys immersed in warm water. Surf. Coat. Technol. 2005, 192, 199–207. [Google Scholar] [CrossRef]
- Feng, L.; Yan, Z.; Qiang, X.; Liu, Y.; Wang, Y. Facile formation of superhydrophobic aluminum alloy surface and corrosion-resistant behavior. Appl. Phys. A Mater. Sci. Process. 2016, 122, 165. [Google Scholar] [CrossRef]
- Jafari, R.; Farzaneh, M. Fabrication of superhydrophobic nanostructured surface on aluminum alloy. Appl. Phys. A Mater. Sci. Process. 2011, 102, 195–199. [Google Scholar] [CrossRef]
- Calabrese, L.; Bruzzaniti, P.; Proverbio, E. Pitting corrosion of aluminum alloys in anhydrous ethanol. Mater. Corros. 2018, 69, 1815–1826. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Yu, X.; Wu, H. Low-cost one-step fabrication of superhydrophobic surface on Al alloy. Appl. Surf. Sci. 2011, 257, 7928–7931. [Google Scholar] [CrossRef]
- Rahimi, M.; Fojan, P.; Gurevich, L.; Afshari, A. Effects of aluminium surface morphology and chemical modification on wettability. Appl. Surf. Sci. 2014, 296, 124–132. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhang, X.M.; Ma, J.P.; Chen, S.C.; Chen, W.X.; Feng, L.F. Experimental study on film thickness and the problem of free surface film flow in dip coating. Asia-Pac. J. Chem. Eng. 2016, 11, 695–704. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog. Org. Coat. 2014, 77, 1341–1350. [Google Scholar] [CrossRef]
- Possart, W.; Kamusewitz, H. Wetting and scanning force microscopy on rough polymer surfaces: Wenzel’s roughness factor and the thermodynamic contact angle. Appl. Phys. A Mater. Sci. Process. 2003, 76, 899–902. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Electrochemical behavior of hydrophobic silane–zeolite coatings for corrosion protection of aluminum substrate. J. Coat. Technol. Res. 2014, 11, 883–898. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Belaud, V.; Valette, S.; Stremsdoerfer, G.; Bigerelle, M.; Benayoun, S. Wettability versus roughness: Multi-scales approach. Tribol. Int. 2015, 82, 343–349. [Google Scholar] [CrossRef]
- Xiang, T.; Zhang, M.; Li, C.; Zheng, S.; Ding, S.; Wang, J.; Dong, C.; Yang, L. A facile method for fabrication of superhydrophobic surface with controllable water adhesion and its applications. J. Alloy. Compd. 2017, 704, 170–179. [Google Scholar] [CrossRef]
- Cheng, Z.; Du, M.; Lai, H.; Zhang, N.; Sun, K. From petal effect to lotus effect: A facile solution immersion process for the fabrication of super-hydrophobic surfaces with controlled adhesion. Nanoscale 2013, 5, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. The original presentation of Boyle’s law. J. Appl. Physiol. 1999, 87, 1543–1545. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Q.; Li, Y.; Hou, B. Facile fluorine-free one step fabrication of superhydrophobic aluminum surface towards self-cleaning and marine anticorrosion. Chem. Eng. J. 2018, 352, 625–633. [Google Scholar] [CrossRef]
- Varshney, P.; Mohapatra, S.; Kumar, A. Fabrication of Mechanically Stable Superhydrophobic Aluminium Surface with Excellent Self-Cleaning and Anti-Fogging Properties. Biomimetics 2017, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Yohe, S.T.; Herrera, V.L.M.; Colson, Y.L.; Grinstaff, M.W. 3D superhydrophobic electrospun meshes as reinforcement materials for sustained local delivery against colorecal cancer cells. J. Control. Release 2012, 162, 92–101. [Google Scholar] [CrossRef] [PubMed]
- van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes-An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Effect of silane matrix composition on performances of zeolite composite coatings. Prog. Org. Coat. 2016, 101, 100–110. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. Alkylsilane self-assembled monolayers: Modeling their wetting characteristics. Appl. Surf. Sci. 2004, 230, 232–240. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Capri, A.; Proverbio, E. Effect of silane matrix on corrosion protection of zeolite based composite coatings. Metall. Ital. 2014, 106, 35–39. [Google Scholar]
- Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion protection of aluminum 6061 in NaCl solution by silane-zeolite composite coatings. J. Coat. Technol. Res. 2012, 9, 597–607. [Google Scholar] [CrossRef]
| Ultimate Tensile Strength | Yield Tensile Strength | Elongation to Break |
|---|---|---|
| 290 MPa | 250 MPa | 10% |
| Code | Surface Treatment | Silane |
|---|---|---|
| As received | – | – |
| AR-S | – | S18 |
| A-F | HF/HCl solution | – |
| A-FS | HF/HCl solution | S18 |
| A-N | HNO3/HCl solution | – |
| A-NS | HNO3/HCl solution | S18 |
| A-W | Boiling water | – |
| A-WS | Boiling water | S18 |
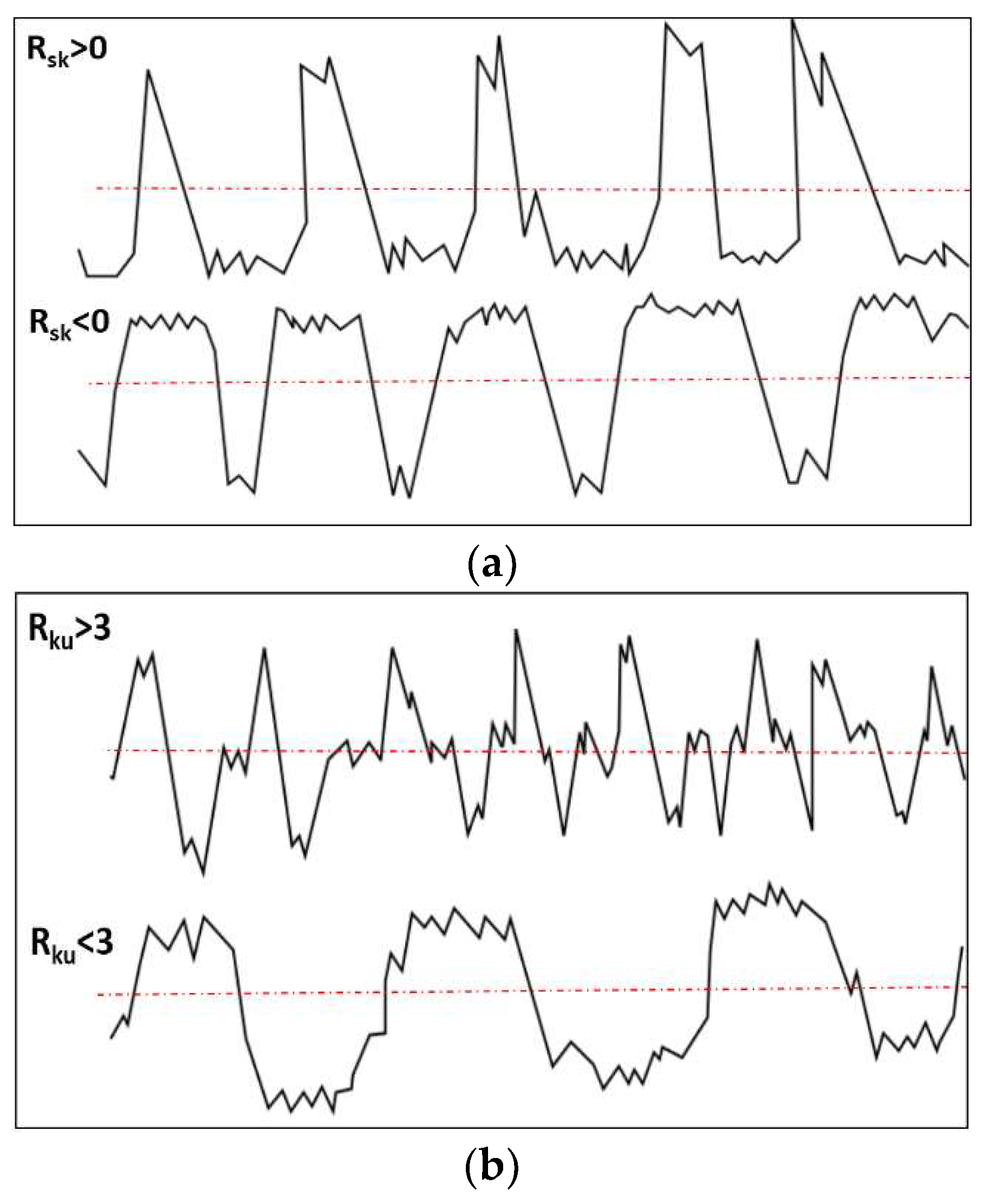
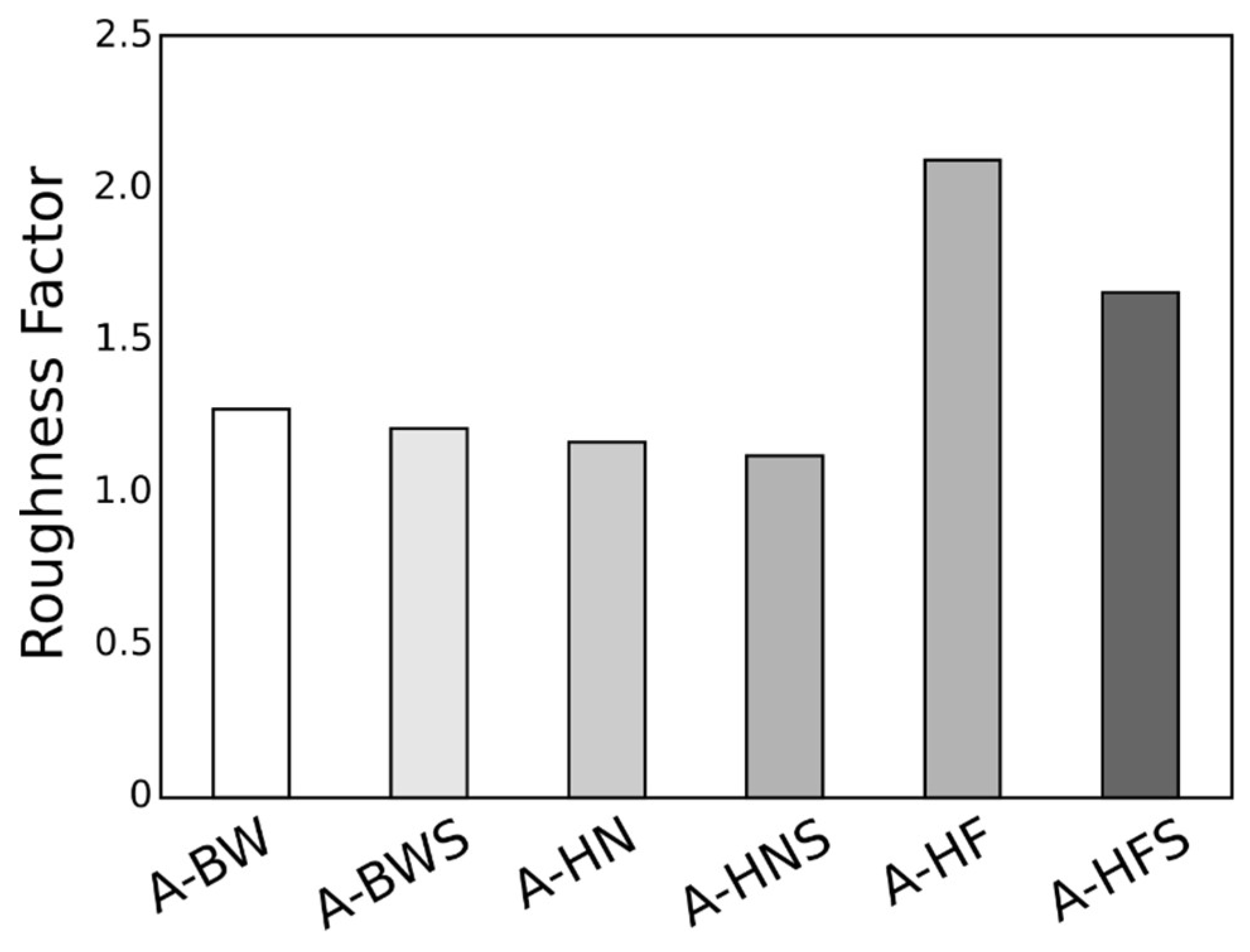
| Code | Ra [nm] | Rq [nm] | Rsk | Rku | RSA [μm2] |
|---|---|---|---|---|---|
| As-received | 11.26 | 15.55 | 0.11 | 3.49 | 26.87 |
| A-F | 172.0 | 225.8 | 1.22 | 1.98 | 52.33 |
| A-FS | 136.5 | 171.5 | -0.57 | 0.195 | 41.37 |
| A-N | 30.37 | 40.39 | 0.076 | 0.767 | 28.92 |
| A-NS | 15.12 | 20.40 | 0.046 | 2.11 | 27.85 |
| A-W | 29.48 | 39.28 | 0.177 | 1.397 | 31.94 |
| A-WS | 28.22 | 35.39 | -0.121 | 0.057 | 30.46 |
| Code | RA [°] |
|---|---|
| As-received | >90 |
| AR-S | >90 |
| A-F | >90 |
| A-FS | <3 |
| A-N | >90 |
| A-NS | 25 ± 5 |
| A-W | >90 |
| A-WS | >90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, L.; Khaskhoussi, A.; Patane, S.; Proverbio, E. Assessment of Super-Hydrophobic Textured Coatings on AA6082 Aluminum Alloy. Coatings 2019, 9, 352. https://doi.org/10.3390/coatings9060352
Calabrese L, Khaskhoussi A, Patane S, Proverbio E. Assessment of Super-Hydrophobic Textured Coatings on AA6082 Aluminum Alloy. Coatings. 2019; 9(6):352. https://doi.org/10.3390/coatings9060352
Chicago/Turabian StyleCalabrese, Luigi, Amani Khaskhoussi, Salvatore Patane, and Edoardo Proverbio. 2019. "Assessment of Super-Hydrophobic Textured Coatings on AA6082 Aluminum Alloy" Coatings 9, no. 6: 352. https://doi.org/10.3390/coatings9060352
APA StyleCalabrese, L., Khaskhoussi, A., Patane, S., & Proverbio, E. (2019). Assessment of Super-Hydrophobic Textured Coatings on AA6082 Aluminum Alloy. Coatings, 9(6), 352. https://doi.org/10.3390/coatings9060352








