Surface Functionalization of Bioactive Glasses with Polyphenols from Padina pavonica Algae and In Situ Reduction of Silver Ions: Physico-Chemical Characterization and Biological Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Padina pavonica Macroalgae Selection, Collection, and Storage
2.2. Determination of Polyphenols, Carotenoids, and Chlorophylls from P. pavonica Extract
2.3. Specimen Preparation and Physico-Chemical Characterization
2.3.1. Specimen Preparation
2.3.2. Specimens Surface Characterization
2.4. Antibacterial Evaluation
2.4.1. Strain
2.4.2. Biofilm Formation
2.4.3. Metabolic Evaluation
2.5. Cytotoxicity Evaluation
2.5.1. Cells
2.5.2. Direct Metabolic Evaluation
2.6. Statistical Analysis of Data
3. Results and Discussion
3.1. Polyphenols, Carotenoids, and Chlorophylls Quantification from P. pavonica Extract
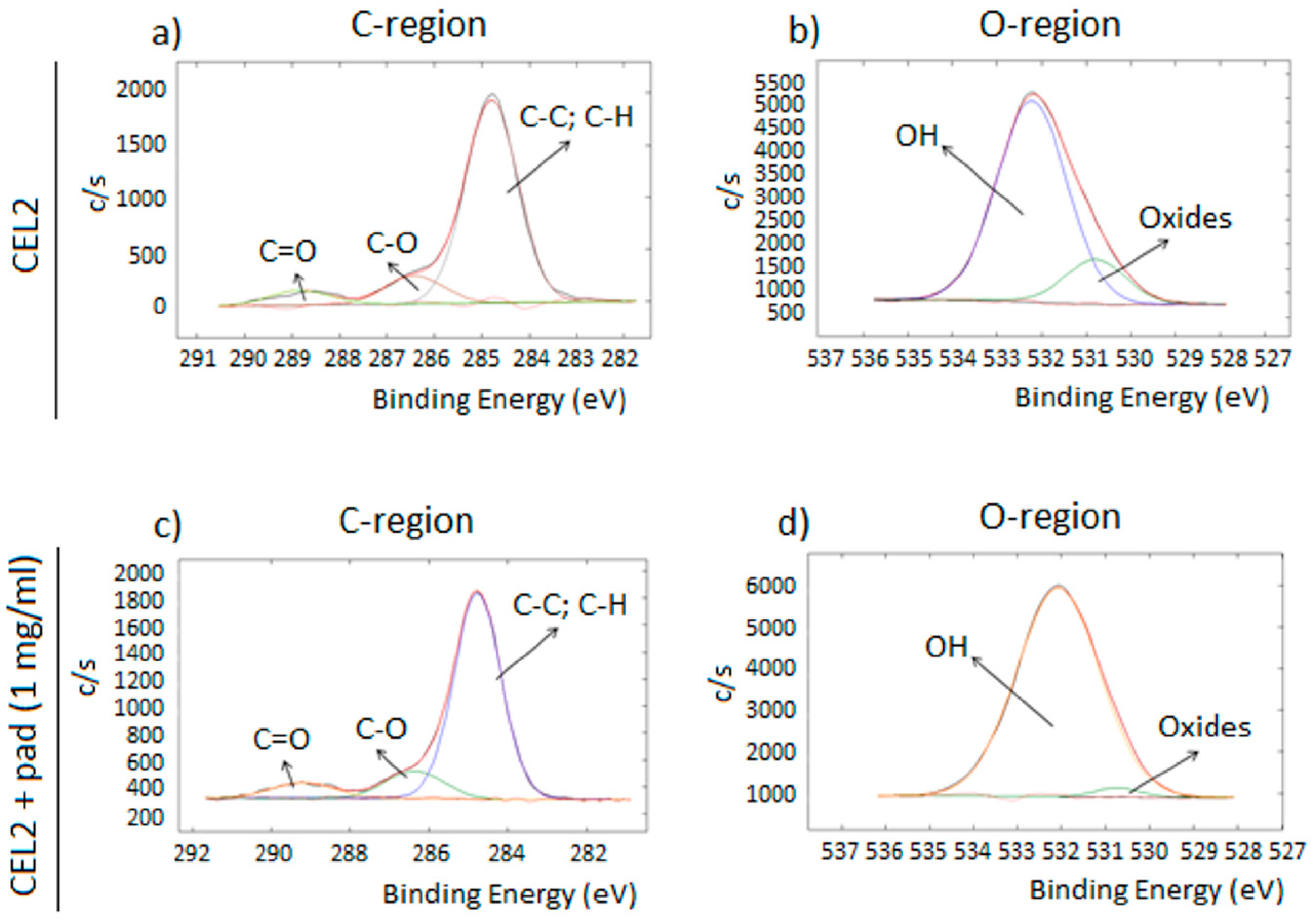
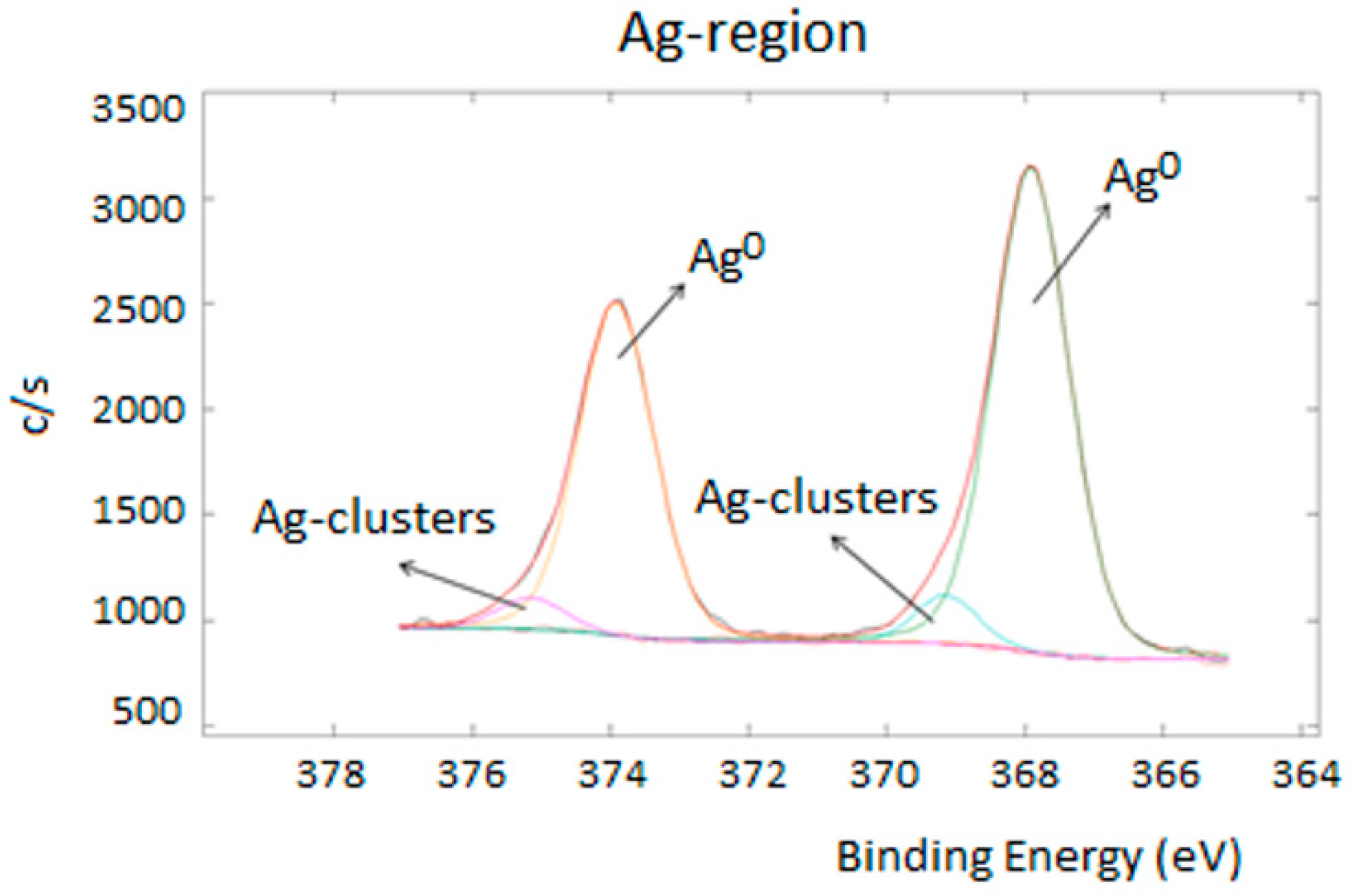
3.2. Specimens’ Physico-Chemical Characterization
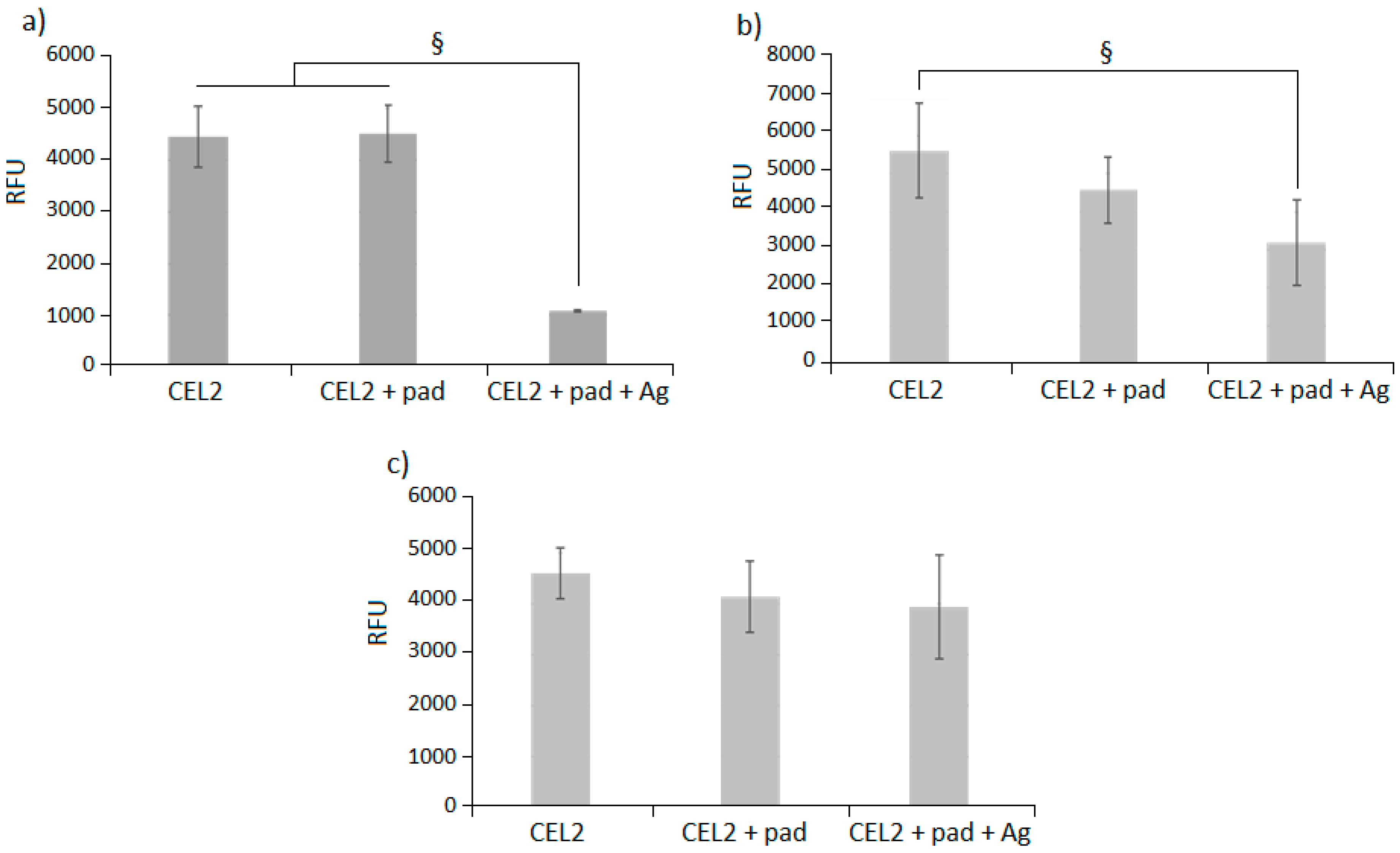
3.3. Antibacterial Evaluation
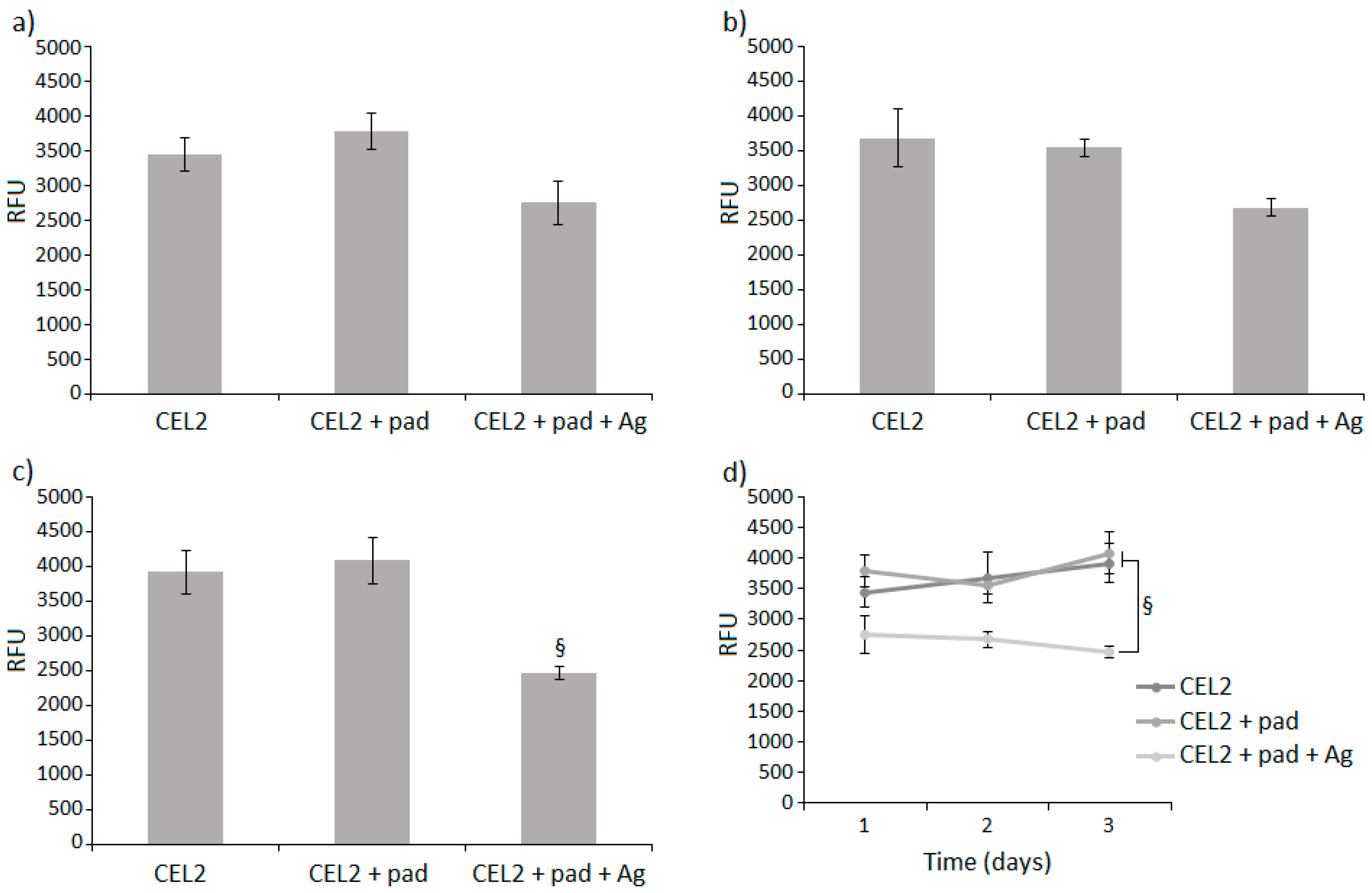
3.4. Cytocompatibility Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Collignon, A.M.; Lesieur, J.; Vacher, C.; Chaussain, C.; Rochefort, G.Y. Strategies developed to induce, direct, and potentiate bone healing. Front. Physiol. 2017, 8, 927. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with antibacterial and osteoinductive properties to repair infected bone defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Low, S.B. Bioactive ceramics for periodontal treatment: Comparative studies in the Patus monkey. J. Appl. Biomater. Funct. Mater. 1992, 3, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Wilson, J. Surface-active biomaterials. Science 1984, 226, 630. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Newby, P.J.; El-Gendy, R.; Kirkham, J.; Yang, X.B.; Thompson, I.D.; Boccaccini, A.R. Ag-doped 45S5 Bioglass®-based bone scaffolds by molten salt ion exchange: Processing and characterisation. J. Mater. Sci. Mater. Med. 2011, 22, 557–569. [Google Scholar] [CrossRef]
- Caridade, S.G.; Merino, E.G.; Alves, N.M.; Mano, J.O.F. Bioactivity and viscoelastic characterization of chitosan/bioglass® composite membranes. Macromol. Biosci. 2012, 12, 1106–1113. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Vernè, E.; Ferraris, S.; Vitale-Brovarone, C.; Cochis, A.; Rimondini, L. Bioactive glass functionalized with alkaline phosphatase stimulates bone extracellular matrix deposition and calcification in vitro. Appl. Surf. Sci. 2014, 313, 372–381. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.M.; Radhakrishnan, A.; Kurup, G.M. Antioxidant and antimitotic activities of sulfated polysaccharide from marine brown algae Padina tetrastromatica. J. Phytol. 2015, 7, 39. [Google Scholar] [CrossRef]
- El-Aty, A.M.A.; Mohamed, A.A.; Samhan, F.A. In vitro antioxidant and antibacterial activities of two fresh water Cyanobacterial species, Oscillatoria agardhii and Anabaena sphaerica. JAPS 2014, 4, 69–75. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Sheen, J.M.; Hu, W.L.; Hung, Y.C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. JAPS 2007, 103, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Dua, A.; Garg, G.; Mahajan, R. Polyphenols, flavonoids and antimicrobial properties of methanolic extract of fennel (Foeniculum vulgare Miller). Eur. J. Exp. Biol. 2013, 3, 203–208. [Google Scholar]
- Alhussaini, M.S.; Saadabi, A.M.; Alghonaim, M.I.; Ibrahim, K.E. An evaluation of the Antimicrobial activity of Commiphora myrrha Nees (Engl.) oleo-gum resins from Saudi Arabia. J. Med. Sci. 2015, 15, 198–203. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Shukla, S.; Nandy, S.; Sahoo, H.B. Synthesis of novel coumarin derivatives and its biological evaluations. Eur. J. Exp. Biol. 2012, 2, 899–908. [Google Scholar]
- Ferraris, S.; Miola, M.; Cochis, A.; Azzimonti, B.; Rimondini, L.; Prenesti, E.; Vernè, E. In situ reduction of antibacterial silver ions to metallic silver nanoparticles on bioactive glasses functionalized with polyphenols. Appl. Surf. Sci. 2017, 396, 461–470. [Google Scholar] [CrossRef]
- Miola, M.; Cochis, A.; Kumar, A.; Arciola, C.; Rimondini, L.; Verné, E. Copper-doped bioactive glass as filler for PMMA-based bone cements: Morphological, mechanical, reactivity, and preliminary antibacterial characterization. Materials 2018, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, T.; Myc, A.; Donovan, B.; Shih, A.Y.; Reuter, J.D.; Baker, J.R. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol. Res. 2001, 156, 1–7. [Google Scholar] [CrossRef]
- Russel, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 2231, 606. [Google Scholar] [CrossRef]
- Cazzola, M.; Corazzari, I.; Prenesti, E.; Bertone, E.; Vernè, E.; Ferraris, S. Bioactive glass coupling with natural polyphenols: Surface modification, bioactivity and antioxidant ability. Appl. Surf. Sci. 2016, 367, 237–248. [Google Scholar] [CrossRef]
- Verne, E.; Vitale-Brovarone, C.; Bui, E.; Bianchi, C.L.; Boccaccini, A.R. Surface functionalization of bioactive glasses. J. Biomed. Mater. Res. Part A 2009, 90, 981–992. [Google Scholar] [CrossRef]
- Verné, E.; Ferraris, S.; Vitale-Brovarone, C.; Spriano, S.; Bianchi, C.L.; Naldoni, A.; Morra, M.; Cassinelli, C. Alkaline phosphatase grafting on bioactive glasses and glass ceramics. Acta Biomater. 2010, 6, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Zhang, X.; Prenesti, E.; Corazzari, I.; Turci, F.; Tomatis, M.; Vernè, E. Gallic acid grafting to a ferrimagnetic bioactive glass-ceramic. J. Non-Cryst Solids 2016, 432, 167–175. [Google Scholar] [CrossRef]
- Ferraris, S.; Giachet, F.T.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Antibacterial effectiveness meets improved mechanical properties: Manuka honey/gellan gum composite hydrogels for cartilage repair. Carbohydr. Polym. 2018, 198, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Rimondini, L.; Pourroy, G.; Stanic, V.; Palkowski, H.; Carradò, A. Biomimetic calcium–phosphates produced by an auto-catalytic route on stainless steel 316L and bio-inert polyolefin. RSC Adv. 2013, 3, 11255–11262. [Google Scholar] [CrossRef]
- Galindo, R.E.; Benito, N.; Palacio, C.; Cavaleiro, A.; Carvalho, S. Ag+ release inhibition from ZrCN–Ag coatings by surface agglomeration mechanism: Structural characterization. J. Phys. D Appl. Phys. 2013, 46, 325303. [Google Scholar] [CrossRef]
- Ferraris, M.; Ferraris, S.; Miola, M.; Perero, S.; Balagna, C.; Verne, E.; Gautier, G.; Manfredotti, C.; Battiato, A.; Vittone, E.; et al. Effect of thermal treatments on sputtered silver nanocluster/silica composite coatings on soda-lime glasses: Ionic exchange and antibacterial activity. J. Nanopart. Res. 2012, 14, 1287. [Google Scholar] [CrossRef]
- Maliszewska, I.; Sadowski, Z. Synthesis and antibacterial activity of silver nanoparticles. J. Phys. Conf. Ser. 2009, 146, 012024. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Sun, Y.; Hung, W.C.; Chen, F.Y.; Lee, C.C.; Huang, H.W. Interaction of tea catechin (−)-epigallocatechin gallate with lipid bilayers. Biophys. J. 2009, 96, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol uses in biomaterials engineering. Biomaterials 2018, 167, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Surget, G.; Roberto, V.P.; Le Lann, K.; Mira, S.; Guérard, F.; Laizé, V.; Poupart, N.; Cancela, M.L.; Stiger-Pouvreau, V. Marine green macroalgae: A source of natural compounds with mineralogenic and antioxidant activities. J. Appl. Phycol. 2017, 29, 575–584. [Google Scholar] [CrossRef]
- Kurt, O.; Özdal-Kurt, F.; Akçora, C.M.; Özkut, M.; Tuğlu, M.I. Neurotoxic, cytotoxic, apoptotic and antiproliferative effects of some marine algae extracts on the NA2B cell line. Biotech. Histochem. 2018, 93, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017, 8013850. [Google Scholar] [CrossRef]
- Skalska, J.; Strużyńska, L. Toxic effects of silver nanoparticles in mammals—Does a risk of neurotoxicity exist? Folia Neuropathol. 2015, 53, 281. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.B.; Milić, M.; Pongrac, I.M.; Marjanović, A.M.; Mlinarić, H.; Pavičić, I.; Gajović, S.; Vrček, I.V. Impact of surface functionalization on the uptake mechanism and toxicity effects of silver nanoparticles in HepG2 cells. Food Chem. Toxicol. 2017, 107, 349–361. [Google Scholar] [CrossRef]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle-based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Saallah, S.; Lenggoro, I.W. Nanoparticles carrying biological molecules: Recent advances and applications. Kona Powder Part J. 2018, 35. [Google Scholar] [CrossRef]
- Xu, L.Q.; Neoh, K.G.; Kang, E.T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. OJSTA 2016, 5. [Google Scholar] [CrossRef]
- Moulton, M.C.; Braydich-Stolle, L.K.; Nadagouda, M.N.; Kunzelman, S.; Hussain, S.M.; Varma, R.S. Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale 2010, 2, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, S.; Mousavi, S.E.; Ashtiani, H.R.A.; Doust, S.R.H.; Rezayat, S.M. Antibacterial activity of silver nanoparticles and their combination with zataria multiflora essential oil and methanol extract. Jundishapur J. Microbiol. 2016, 9, e36070. [Google Scholar] [CrossRef] [PubMed]
- Matei, P.; Martín-Gil, J.; Michaela Iacomi, B.; Pérez-Lebeña, E.; Barrio-Arredondo, M.; Martín-Ramos, P. Silver nanoparticles and polyphenol inclusion compounds composites for Phytophthora cinnamomi mycelial growth inhibition. Antibiotics 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]


| Sample | pH |
|---|---|
| pad (1 mg/mL) | 6.76 |
| pad (5 mg/mL) | 7.44 |
| CEL2 + pad (1 mg/mL) | 7.53 (± 0.06) |
| CEL2 + pad (5 mg/mL) | 8.28 (± 0.04) |
| Sample | Polyphenols (GAE mg/mL) |
|---|---|
| CEL2 + pad (1 mg/mL) | 0.001 |
| CEL2 + pad (5 mg/mL) | 0.001 |
| Element | CEL2 | CEL2 + pad (1 mg/mL) | CEL2 + pad (1 mg/mL) + Ag |
|---|---|---|---|
| O | 43.3 | 46.9 | 35.1 |
| C | 36.9 | 28 | 41.9 |
| Si | 13.5 | 12 | 8.0 |
| Na | 2.1 | 2 | 2.0 |
| Ca | 1.8 | 3.7 | 3.9 |
| Al | 1.4 | – | – |
| Mg | 0.9 | 7.5 | 4.8 |
| Ag | – | – | 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed Abdelgeliel, A.; Ferraris, S.; Cochis, A.; Vitalini, S.; Iriti, M.; Mohammed, H.; Kumar, A.; Cazzola, M.; Salem, W.M.; Verné, E.; et al. Surface Functionalization of Bioactive Glasses with Polyphenols from Padina pavonica Algae and In Situ Reduction of Silver Ions: Physico-Chemical Characterization and Biological Response. Coatings 2019, 9, 394. https://doi.org/10.3390/coatings9060394
Sayed Abdelgeliel A, Ferraris S, Cochis A, Vitalini S, Iriti M, Mohammed H, Kumar A, Cazzola M, Salem WM, Verné E, et al. Surface Functionalization of Bioactive Glasses with Polyphenols from Padina pavonica Algae and In Situ Reduction of Silver Ions: Physico-Chemical Characterization and Biological Response. Coatings. 2019; 9(6):394. https://doi.org/10.3390/coatings9060394
Chicago/Turabian StyleSayed Abdelgeliel, Asmaa, Sara Ferraris, Andrea Cochis, Sara Vitalini, Marcello Iriti, Hiba Mohammed, Ajay Kumar, Martina Cazzola, Wesam M. Salem, Enrica Verné, and et al. 2019. "Surface Functionalization of Bioactive Glasses with Polyphenols from Padina pavonica Algae and In Situ Reduction of Silver Ions: Physico-Chemical Characterization and Biological Response" Coatings 9, no. 6: 394. https://doi.org/10.3390/coatings9060394
APA StyleSayed Abdelgeliel, A., Ferraris, S., Cochis, A., Vitalini, S., Iriti, M., Mohammed, H., Kumar, A., Cazzola, M., Salem, W. M., Verné, E., Spriano, S., & Rimondini, L. (2019). Surface Functionalization of Bioactive Glasses with Polyphenols from Padina pavonica Algae and In Situ Reduction of Silver Ions: Physico-Chemical Characterization and Biological Response. Coatings, 9(6), 394. https://doi.org/10.3390/coatings9060394











