Effect of Linseed (Linum usitatissimum) Mucilage and Chitosan Edible Coatings on Quality and Shelf-Life of Fresh-Cut Cantaloupe (Cucumis melo)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mucilage Extraction Process
2.3. Development and Application of Coating-Forming Solutions
2.4. Analysis of Quality and Shelf Life of the Product
2.4.1. Microbiological Quality Analysis
2.4.2. Physicochemical Quality Analysis
- Juice Leakage, Firmness, and Color
- pH, Titratable Acidity, Total Soluble Solids, and Ascorbic Acid Determinations
2.4.3. Sensory Quality Analysis
2.5. Statistical Analysis
3. Results and Discussion
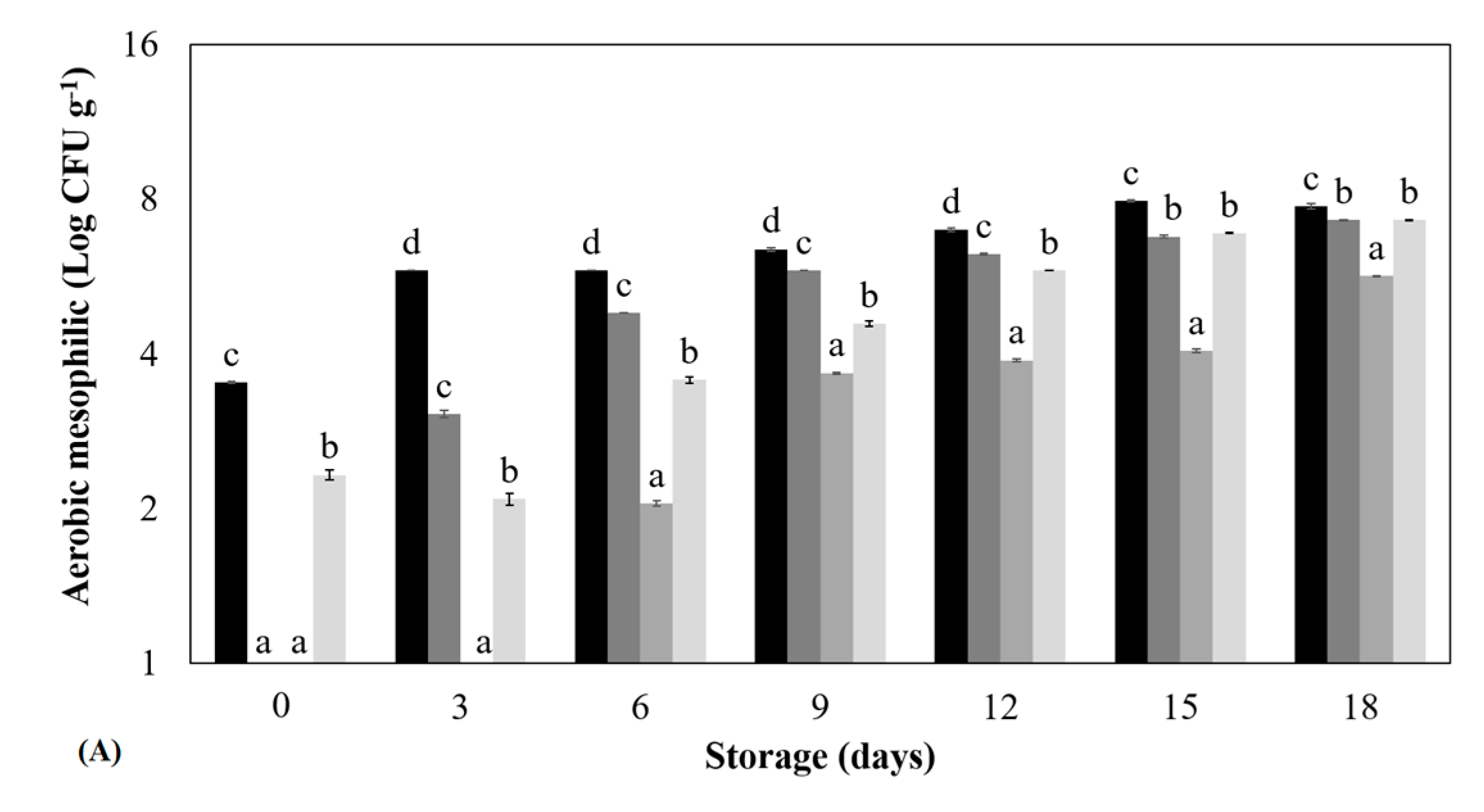
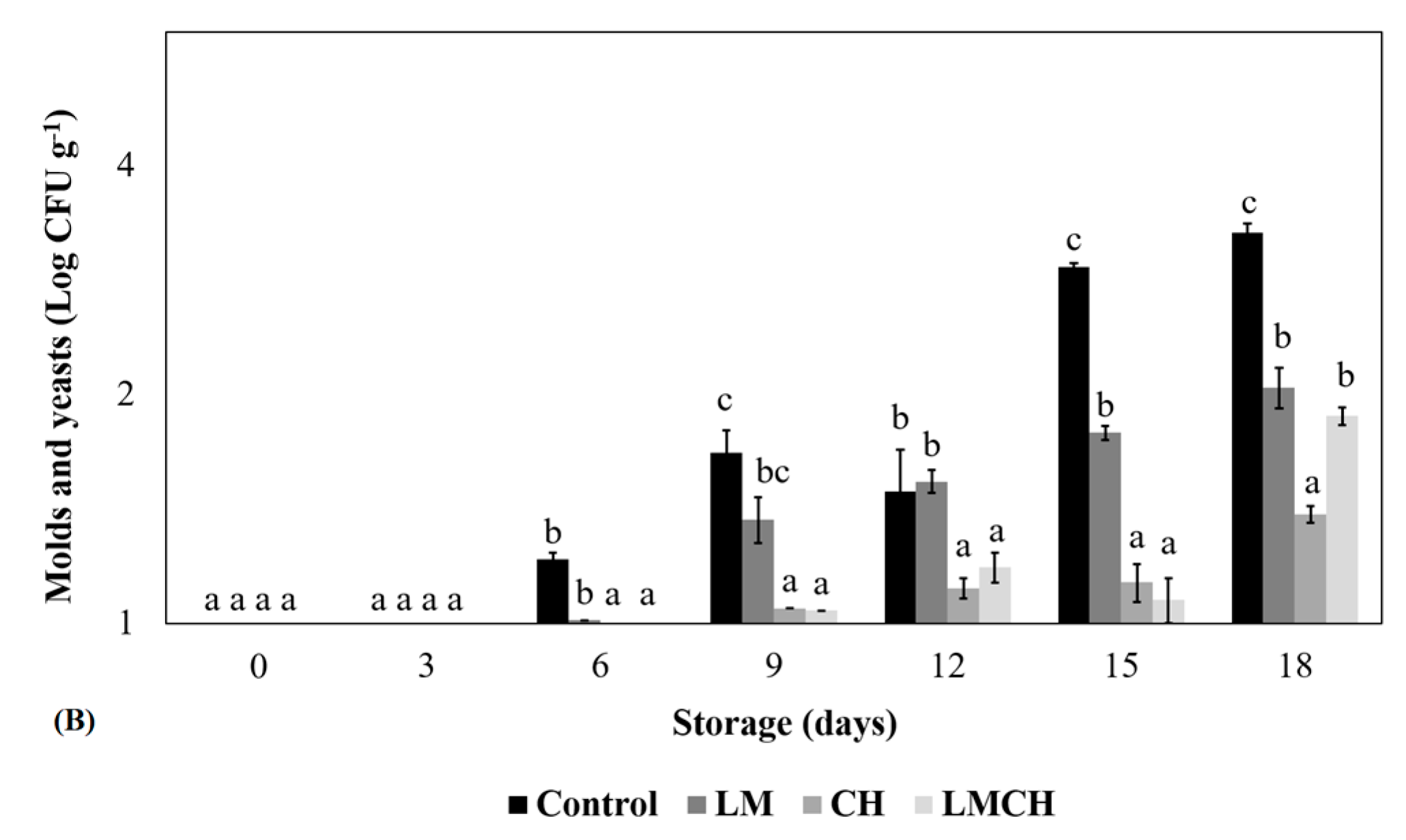
3.1. Microbiological Quality Testing
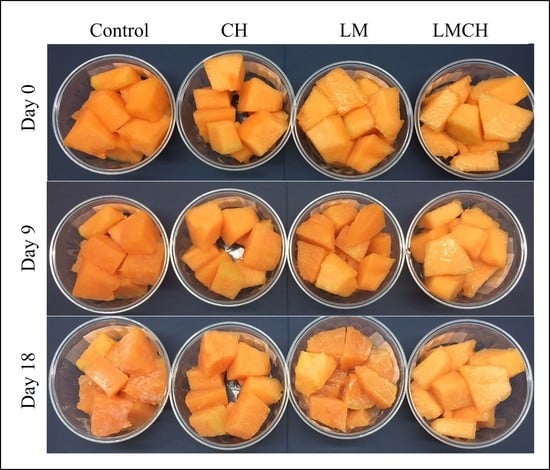
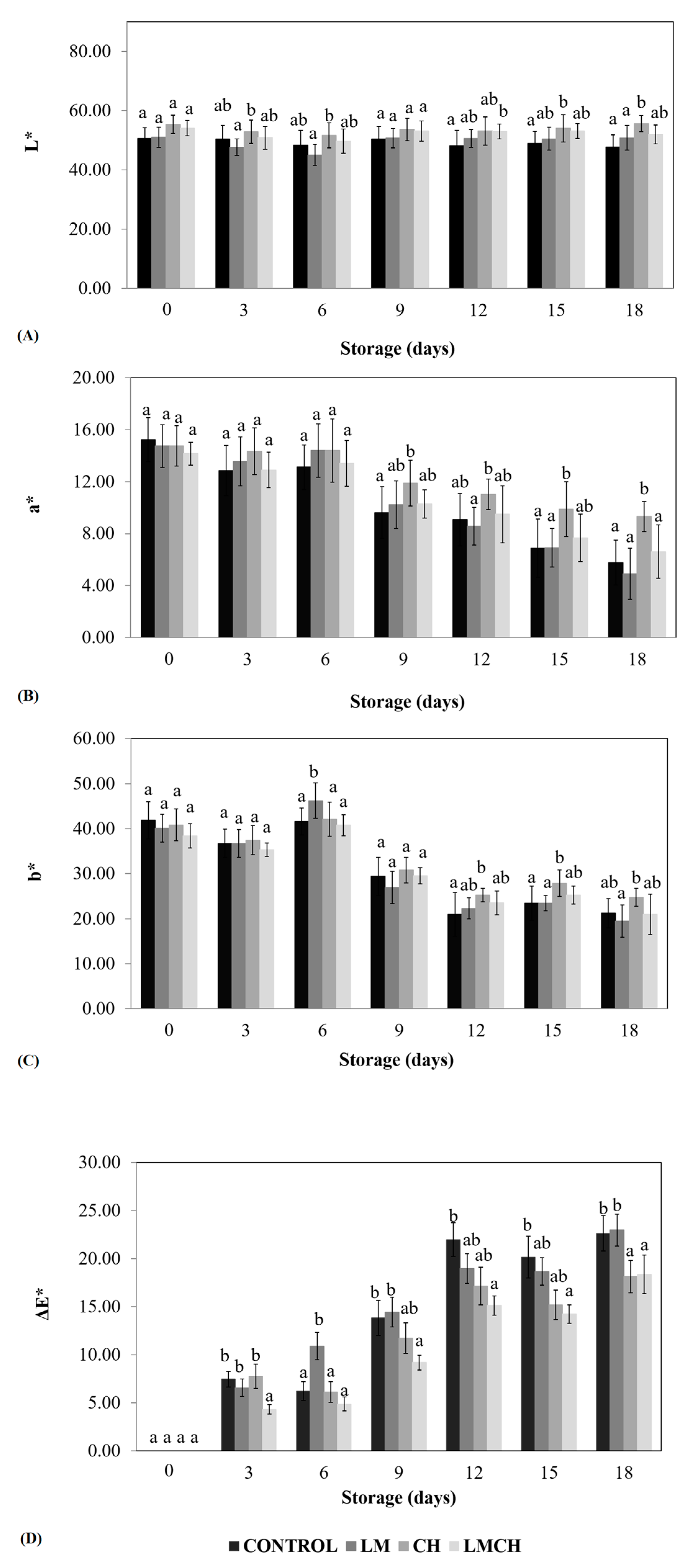
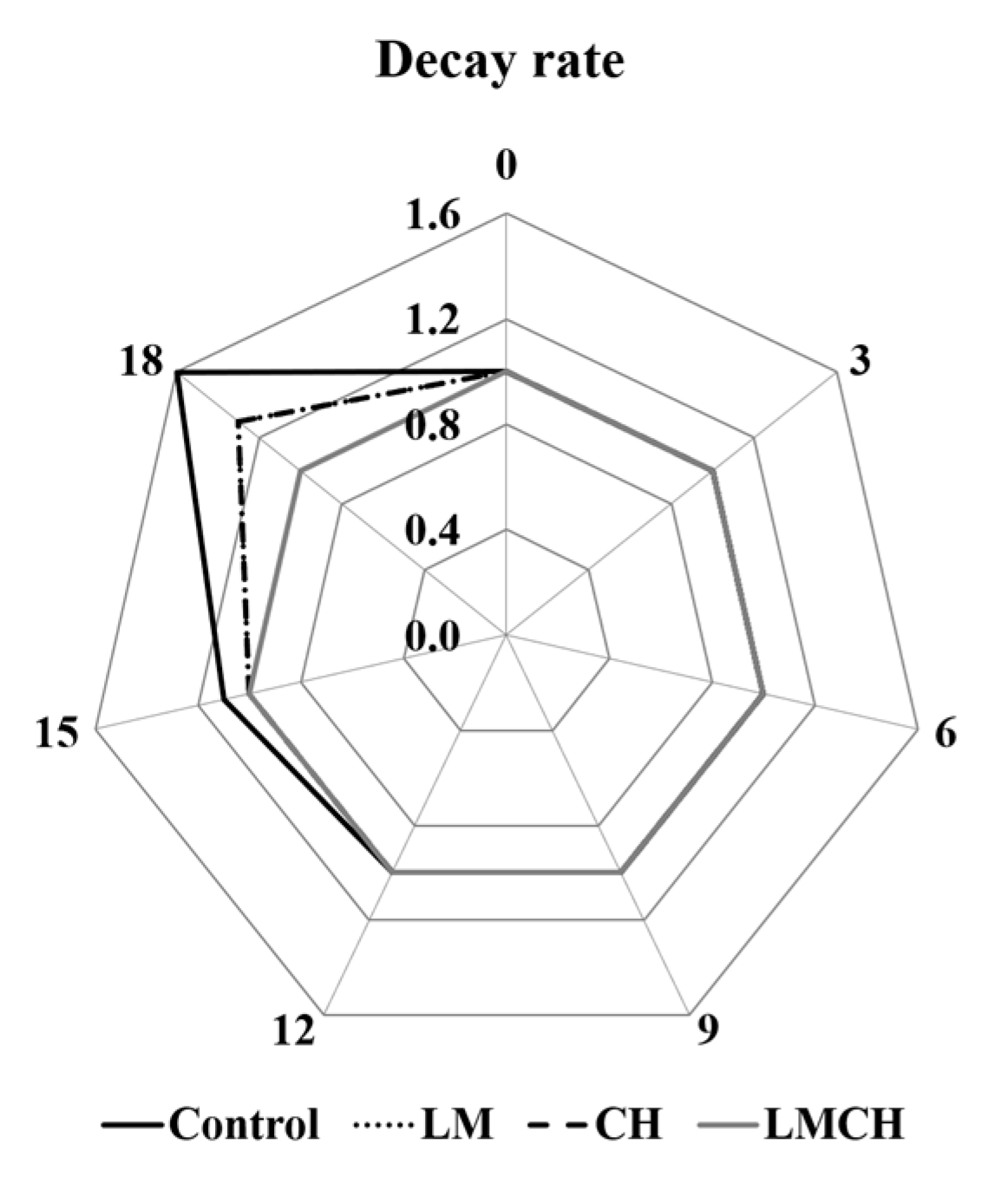
3.2. Juice Leakage, Firmness, and Color Tests
pH, TA, TSS, and AA Content
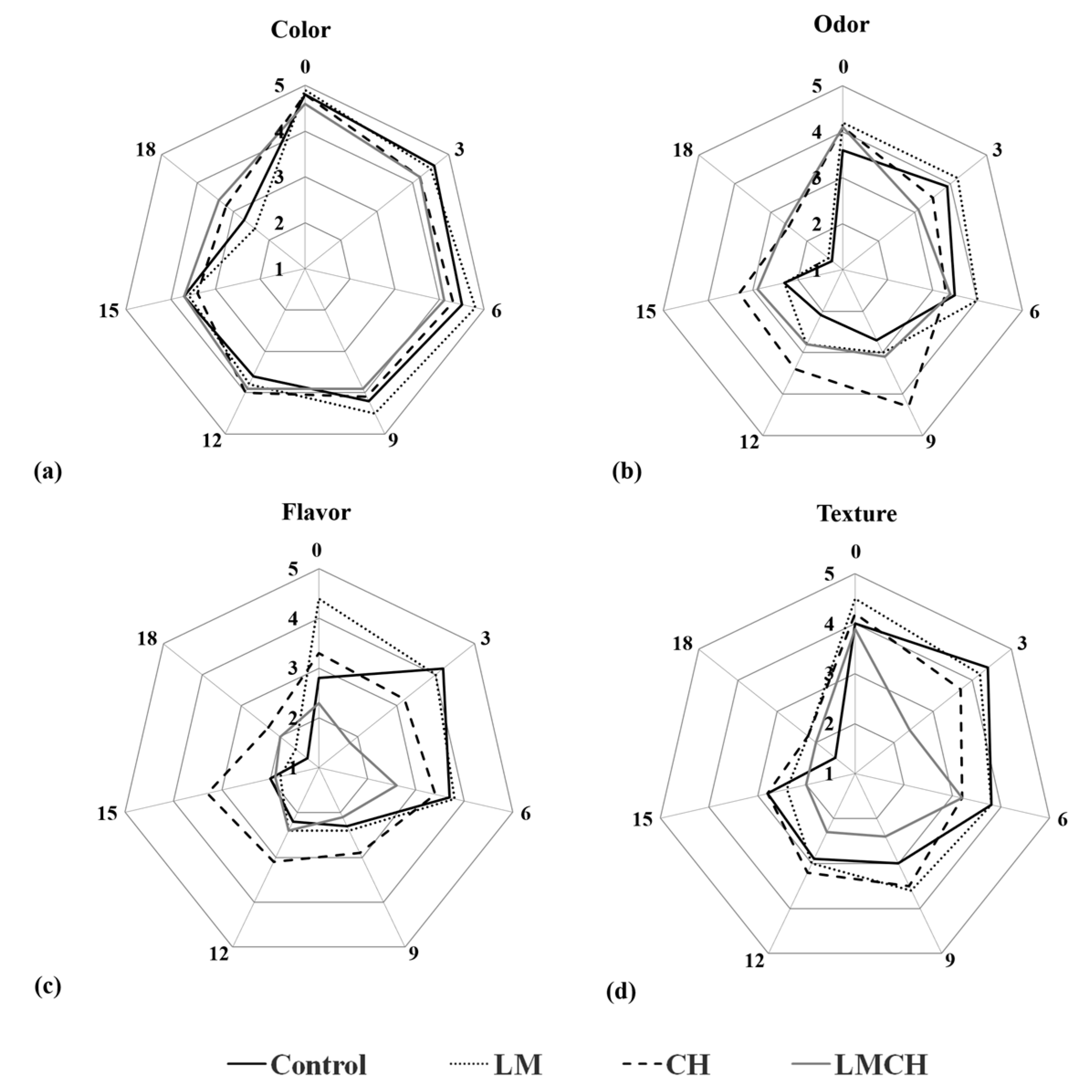
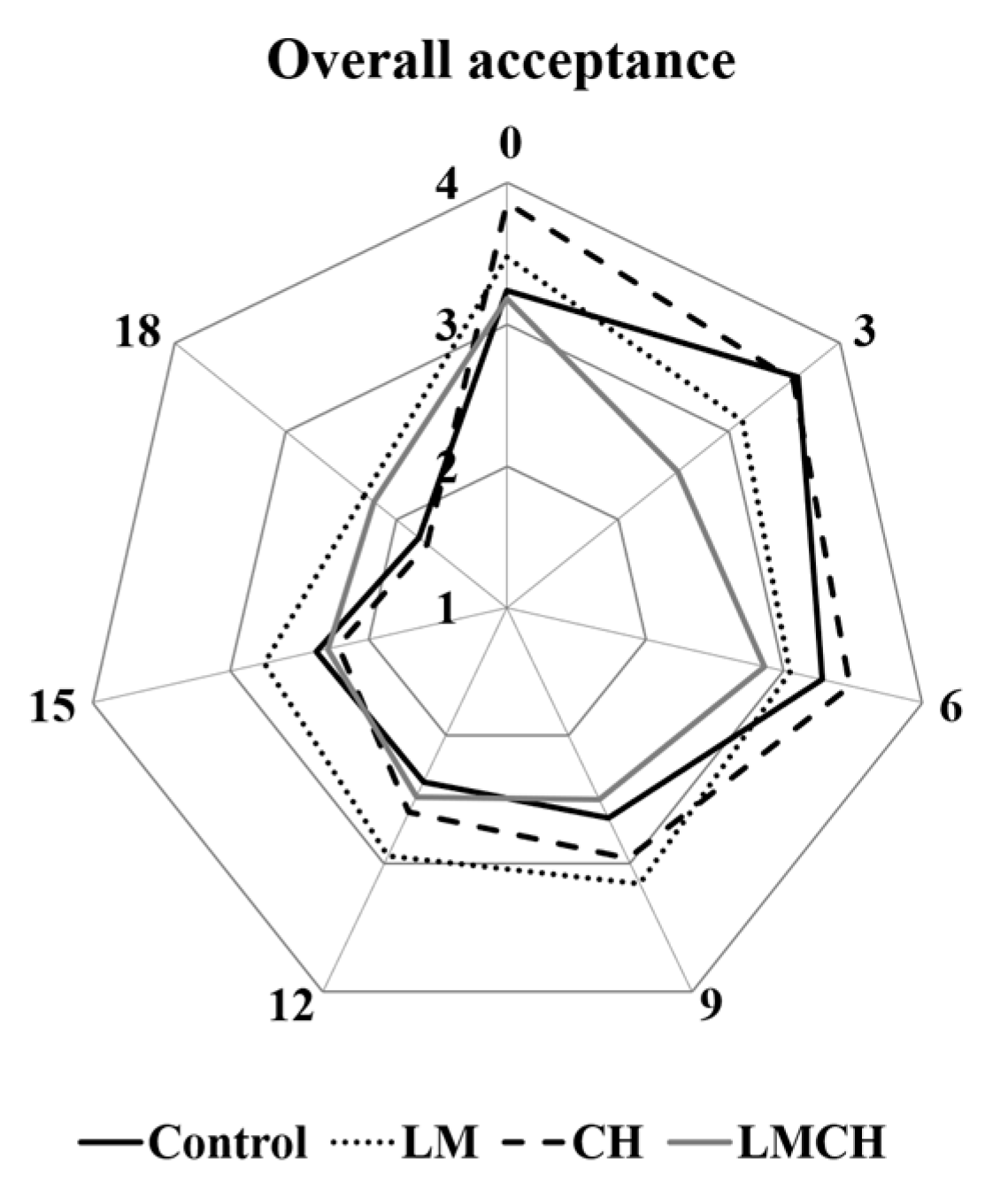
3.3. Sensory Quality Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vodnar, D.C.; Pop, O.L.; Dulf, F.V.; Socaciu, C. Antimicrobial efficiency of edible films in food industry. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 302–312. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Rojas-Grau, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.; Munuera, I.P.; Fiszman, S.; Martin-Belloso, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Yousuf, B.; Kumar, S.A. Flaxseed gum in combination with lemongrass essential oil as aneffective edible coating for ready-to-eat pomegranate arils. Int. J. Biol. Macromol. 2017, 104, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]
- Riaie, S.; Saadatian, M.; Aghaie, M.; Alizadeh, M.; Hajitaghiloo, R. Application salicylic acid and aloevera gel as edible coating layer to preserving fresh-cut melon slices in cold storage. Int. Food Res. J. 2017, 24, 2456–2459. [Google Scholar]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A.; Lasik-Kurdyś, M. Combination of alginate coating and repetitive pulsed light for shelf life extension of fresh-cut cantaloupe (Cucumis melo L. reticulatus cv. Glamour). J. Food Process. Preserv. 2018, 42, e13786. [Google Scholar] [CrossRef]
- Kasim, R.; Kasim, M.U. Biochemical and color changes of fresh-cut melon (Cucumis melo L. cv. Galia) treated with UV-C. Food Sci. Technol. 2014, 34, 547–551. [Google Scholar] [CrossRef]
- Martiñon, M.E.; Moreira, R.G.; Castell-Perez, M.E.; Gomes, C. Development of a multilayered antimicrobial edible coating for shelf life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 °C. LWT-Food Sci. Technol. 2014, 56, 341–350. [Google Scholar] [CrossRef]
- Chong, J.X.; Lai, S.; Yang, H. Chitosan combined with calcium chloride impacts fresh-cut honeydew melon by stabilising nanostructures of sodium-carbonate-soluble pectin. Food Control. 2015, 53, 195–205. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Amaro, A.L.; Fundo, J.F.; Oliveira, A.; Beaulieu, J.C.; Fernández-Trujillo, J.P.; Almeida, D.P.F. 1-Methylcyclopropene effects on temporal changes of aroma volatiles and phytochemicals of fresh-cut cantaloupe. J. Sci. Food Agric. 2013, 93, 828–837. [Google Scholar] [CrossRef]
- Waqas, A.; Butt, M.S. Application of biodegradable coatings to improve quality and shelf life of minimally processed melon dices. Pakistan J. Food Sci. 2014, 24, 82–90. [Google Scholar]
- Moreira, S.P.; De Carvalho, W.M.; Alexandrino, A.C.; De Paula, H.C.B.; Rodrigues, M.d.C.P.; De Figueiredo, R.W.; Maia, G.A.; De Figueiredo, E.M.A.T.; Brasil, I.M. Freshness retention of minimally processed melon using different packages and multilayered edible coating containing microencapsulated essential oil. Int. J. Food Sci. Technol. 2014, 49, 2192–2203. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-Layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate-chitosan combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martin-Belloso, O. Effect of ripeness on the shelf life of fresh-cut melon preserved by modified atmosphere packaging. Eur. Food Res. Technol. 2007, 225, 301–311. [Google Scholar] [CrossRef]
- Selma, M.V.; Ibáñez, A.M.; Allende, A.; Cantwell, M.; Suslow, T. Effect of gaseous ozone and hot water on microbial and sensory quality of cantaloupe and potential transference of Escherichia coli O157:H7 during cutting. Food Microbiol. 2008, 25, 162–168. [Google Scholar] [CrossRef]
- Lamikanra, O.; Kueneman, D.; Ukuku, D.; Bett-Garber, K.L. Effect of processing under ultraviolet light on the shelf life of fresh-cut cantaloupe melon. J. Food Sci. 2005, 70, 534–539. [Google Scholar] [CrossRef]
- Manzocco, L.; Da Pieve, S.; Maifreni, M. Impact of UV-C light on safety and quality of fresh-cut melon. Innov. Food Sci. Emerg. Technol. 2011, 12, 13–17. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Zhao, G.; Liao, X.; Chen, F.; Wu, J.; Chen, J.; Hu, X. Influence of gamma irradiation on enzyme, microorganism, and flavor of cantaloupe (Cucumis melo L.) juice. J. Food Sci. 2006, 71, 215–220. [Google Scholar] [CrossRef]
- Abdalrazeq, M.; Giosafatto, C.V.L.; Esposito, M.; Fenderico, M.; Di Pierro, P.; Porta, R. Glycerol-plasticized films obtained from whey proteins denatured at alkaline pH. Coatings 2019, 9, 322. [Google Scholar] [CrossRef]
- Sagnelli, D.; Hooshmand, K.; Kemmer, G.C.; Kirkensgaard, J.J.K.; Mortensen, K.; Giosafatto, C.V.L.; Holse, M.; Hebelstrup, K.H.; Bao, J.; Stelte, W.; et al. Cross-linked amylose bio-plastic: A transgenic-based compostable plastic alternative. Int. J. Mol. Sci. 2017, 18, 2075. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melón. LWT-Food Sci. Technol. 2008, 41, 1862–1870. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Sarantopoulos, C.I.G.L.; Carmello-Guerreiro, S.M.; Hubinger, M.D. Effect of osmotic dehydration and pectin edible coatings on quality and shelf life of fresh-cut melon. Food Bioprocess Technol. 2013, 6, 80–91. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Schmid, M.; Muller, K. Effect of dipping and vacuum impregnation coating techniques with alginate based coating on physical quality parameters of Cantaloupe melon. J. Food Sci. 2018, 83, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Haffez, M.M.; Ragab, M.E.; Abou El-Yazied, A.; Emam, M.S. Effect of chitosan, carboxy methyl cellulose and calcium chloride treatments on quality and storability of fresh cut Cantaloupe. Middle East J. Appl. Sci. 2016, 6, 249–268. [Google Scholar]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Brasil, I.M.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.E.; Moreira, R.G. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT-Food Sci. Technol. 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujolá, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruits slices. LWT-Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Fedeniuk, R.W.; Biliaderis, C.G. Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. J. Agric. Food Chem. 1994, 42, 240–247. [Google Scholar] [CrossRef]
- Chen, H.-H.; Xu, S.-Y.; Wang, Z. Gelation properties of flaxseed gum. J. Food Eng. 2006, 77, 295–303. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Amid, B.T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; Heredia, N.; Alanís-Guzmán, M.G.; Arévalo-Niño, K. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Technol. 2017, 128, 63–75. [Google Scholar] [CrossRef]
- Soleimani-Rambod, A.; Zomorodi, S.; Naghizadeh Raeisi, S.; Khosrowshahi Asl, A.; Shahidi, S.-A. The effect of xanthan gum and flaxseed mucilage as edible coatings in cheddar cheese during ripening. Coatings 2018, 8, 80. [Google Scholar] [CrossRef]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT-Food Sci. Technol. 2013, 51, 9–15. [Google Scholar] [CrossRef]
- Official Methods of Analysis, 13th ed.; AOAC International: Washington, DC, USA, 1990.
- Suntornsuk, L.; Gritsanapun, W.; Nilkamhank, S.; Paochom, A. Quantitation of vitamin C content in herbal juice using direct titration. J. Pharm. Biomed. Anal. 2002, 28, 849–855. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; Flores-González, M.d.S.; Arévalo-Niño, K. Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (Fragaria ananassa). J. Food Sci. 2015, 80, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, M.; Grassi, M.; Buccheri, M.; Rizzolo, A. Influence of edible coatings on postharvest physiology and quality of Honeydew melon fruit (Cucumis melo L. inodorus). Adv. Hortic. Sci. 2015, 29, 65–74. [Google Scholar] [CrossRef]
- Aguayo, E.; Escalona, V.H.; Artés, F. Metabolic behavior and quality changes of whole and fresh processed melon. J. Food Sci. 2004, 69, 148–155. [Google Scholar] [CrossRef]
- Syahidah, K.; Rosnah, S.; Noranizan, M.A.; Zaulia, O.; Anvarjon, A. Quality changes of fresh cut cantaloupe (Cucumis melo L. var Reticulatus cv. Glamour) in different types of polypropylene packaging. Int. Food Res. J. 2015, 22, 753–760. [Google Scholar]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, J.F. The release kinetics of β-carotene nanocapsules/xanthan gum coatings and quality changes in fresh-cut melon (cantaloupe). Carbohydr. Polym. 2017, 157, 1874–1882. [Google Scholar] [CrossRef]
- Lamikanra, O.; Chen, J.C.; Banks, D.; Hunter, P.A. Biochemical and microbial changes during the storage of minimally processed Cantaloupe. J. Agric. Food Chem. 2000, 48, 5955–5961. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Cheng, L.; Wang, J.; Che, F.; Zhang, P.; Wu, B. Quality characteristics of fresh-cut ‘Hami’ melon treated with 1-methylcyclopropene. Afr. J. Biotechnol. 2011, 10, 18200–18209. [Google Scholar] [CrossRef]

| Storage (Days) | Control | LM | CH | LMCH |
|---|---|---|---|---|
| Juice Leakage (%) | ||||
| 3 | A0.56 (0.07) f | A0.45 (0.13) f | A0.28 (0.04) d,e | A0.34 (0.16) d,e |
| 6 | C2.64 (0.07) e | B1.44 (0.27) e | A0.83 (0.13) d | AB1.05 (0.33) c,d |
| 9 | C3.98 (0.12) d | B2.83 (0.24) d | A2.14 (0.38) c | A1.97 (0.13) b,c |
| 12 | B4.33 (0.22) c | A3.13 (0.22) c | A2.59 (0.23) b,c | A2.72 (0.25) a,b |
| 15 | B4.74 (0.11) b | A3.43 (0.34) b | A3.05 (0.22) a,b | A3.33 (0.33) a |
| 18 | B5.12 (0.15) a | A3.78 (0.29) a | A3.32 (0.25) a | A3.53 (0.69) a |
| Firmness (N) | ||||
| 0 | A19.20 (0.13) b,c | B28.32 (1.70) c | A,B21.97 (2.00) a | A20.67 (1.42) a,b |
| 3 | A23.57 (2.24) c | A25.80 (0.72) b,c | A27.85 (3.51) a | A22.42 (2.35) b |
| 6 | A15.15 (1.02) a,b | A16.63 (1.77) a | A19.73 (1.21) a | A18.42 (1.44) a,b |
| 9 | A10.90 (0.96) a | AB13.92 (1.58) a | B21.23 (2.69) a | A,B14.20 (1.25) a,b |
| 12 | A14.23 (1.13) a,b | A18.91 (2.71) a,b | A21.13 (1.08) a | A12.72 (2.11) a |
| 15 | A10.40 (0.19) a | B17.32 (0.59) a,b | A,B16.20 (2.02) a | A,B14.48 (1.04) a,b |
| 18 | A14.05 (0.79) a,b | A15.12 (0.65) a | B23.09 (1.31) a | A13.78 (1.67) a,b |
| Storage (Days) | Control | LM | CH | LMCH |
|---|---|---|---|---|
| pH | ||||
| 0 | D6.79 (0.02) d | C6.68 (0.03) d | B5.99 (0.01) d,e | A5.72 (0.01) f |
| 3 | A6.12 (0.02) c | B6.34 (0.09) c | A6.10 (0.01) e | A6.00 (0.01) e |
| 6 | B5.68 (0.03) b | A5.53 (0.01) b | C5.78 (0.00) c,d | A5.56 (0.00) d |
| 9 | D5.52 (0.03) a,b | C5.47 (0.01) a,b | B5.65 (0.01) b,c | A4.98 (0.00) c |
| 12 | B5.53 (0.04) a,b | B5.53 (0.03) b | B5.40 (0.19) a | A4.53 (0.02) b |
| 15 | C5.58 (0.03) b | B5.40 (0.04) a | B5.42 (0.01) a,b | A4.35 (0.04) a |
| 18 | C5.38 (0.14) a | B5.53 (0.05) b | B5.57 (0.10) a,b,c | A4.33 (0.02) a |
| TA (Malic Acid, g 100 g−1) | ||||
| 0 | A0.026 (0.00) a | A0.034 (0.01) a | C0.103 (0.00) b,c | B0.073 (0.00) a |
| 3 | A0.064 (0.01) a | A0.073 (0.00) a | B0.107(0.00) b,c | B0.115 (0.01) b |
| 6 | B0.265 (0.00) d | B0.282 (0.12) d | A0.188 (0.00) d | A0.158 (0.01) c |
| 9 | B0.213 (0.00) c | B0.218 (0.01) c | A0.179 (0.01) d | C0.269 (0.01) e |
| 12 | B0.162 (0.01) b | B0.171 (0.00) b | A0.128 (0.01) c | C0.265 (0.00) e |
| 15 | A0.068 (0.00) a | B0.145 (0.00) b | A0.077(0.01) a,b | C0.205 (0.01) d |
| 18 | A0.055 (0.00) a | A0.043(0.01) a | A0.059 (0.01) a | B0.205 (0.01) d |
| TSS (°Bx) | ||||
| 0 | C9.00 (0.00) b | A8.00 (0.00) c | D10.0 (0.00) d | B8.30 (0.17) b |
| 3 | B9.00 (0.00) b | A8.10 (0.05) c | B9.33 (0.06) c | B9.00 (0.00) c |
| 6 | B8.90 (0.10) b | A8.00 (0.00) c | A8.10 (0.10) b | A8.07 (0.06) b |
| 9 | A8.10 (0.06) a | A8.03 (0.05) c | A8.17 (0.06) b | B9.13 (0.06) c |
| 12 | C8.90 (0.11) b | A8.03 (0.06) c | B8.27 (0.10) b | D9.17 (0.06) c |
| 15 | B8.00 (0.00) a | A7.03 (0.06) b | B8.03 (0.06) b | A7.20 (0.17) a |
| 18 | C8.00 (0.00) a | A6.47 (0.25) a | B7.10 (0.06) a | B7.27 (0.06) a |
| AA (mg 100 g−1) | ||||
| 0 | A10.09 (1.03) c | A10.68 (1.78) b | A,B13.05 (1.03) c | B14.83 (1.03) b |
| 3 | A,B9.49 (1.03) c | B10.68 (0.10) b | B11.27 (1.03) b,c | A7.71 (1.03) a |
| 6 | A8.31 (1.03) b,c | A6.53 (1.03) a | A7.12 (1.03) a | A8.31 (1.03) a |
| 9 | A6.53 (1.03) a,b | A7.12 (1.09) a | A8.31 (1.03) b | A6.53 (1.03) a |
| 12 | A,B6.53 (1.03) a,b | B5.34 (0.10) a | B7.71 (1.03) a | A,B7.12 (1.03) a |
| 15 | A6.53 (1.03) a,b | A6.53 (1.03) a | A6.53 (1.03) a | A5.93 (1.03) a |
| 18 | A4.75 (1.03) a | A5.93 (1.03) a | A5.93 (1.03) a | A5.93 (1.03) a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treviño-Garza, M.Z.; Correa-Cerón, R.C.; Ortiz-Lechuga, E.G.; Solís-Arévalo, K.K.; Castillo-Hernández, S.L.; Gallardo-Rivera, C.T.; Arévalo Niño, K. Effect of Linseed (Linum usitatissimum) Mucilage and Chitosan Edible Coatings on Quality and Shelf-Life of Fresh-Cut Cantaloupe (Cucumis melo). Coatings 2019, 9, 368. https://doi.org/10.3390/coatings9060368
Treviño-Garza MZ, Correa-Cerón RC, Ortiz-Lechuga EG, Solís-Arévalo KK, Castillo-Hernández SL, Gallardo-Rivera CT, Arévalo Niño K. Effect of Linseed (Linum usitatissimum) Mucilage and Chitosan Edible Coatings on Quality and Shelf-Life of Fresh-Cut Cantaloupe (Cucumis melo). Coatings. 2019; 9(6):368. https://doi.org/10.3390/coatings9060368
Chicago/Turabian StyleTreviño-Garza, Mayra Z., Ruth C. Correa-Cerón, Eugenia G. Ortiz-Lechuga, Karla K. Solís-Arévalo, Sandra L. Castillo-Hernández, Claudia T. Gallardo-Rivera, and Katiushka Arévalo Niño. 2019. "Effect of Linseed (Linum usitatissimum) Mucilage and Chitosan Edible Coatings on Quality and Shelf-Life of Fresh-Cut Cantaloupe (Cucumis melo)" Coatings 9, no. 6: 368. https://doi.org/10.3390/coatings9060368
APA StyleTreviño-Garza, M. Z., Correa-Cerón, R. C., Ortiz-Lechuga, E. G., Solís-Arévalo, K. K., Castillo-Hernández, S. L., Gallardo-Rivera, C. T., & Arévalo Niño, K. (2019). Effect of Linseed (Linum usitatissimum) Mucilage and Chitosan Edible Coatings on Quality and Shelf-Life of Fresh-Cut Cantaloupe (Cucumis melo). Coatings, 9(6), 368. https://doi.org/10.3390/coatings9060368