Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials
Abstract
1. Introduction
2. Experimental Methods
2.1. Material Preparation
2.2. Characterization
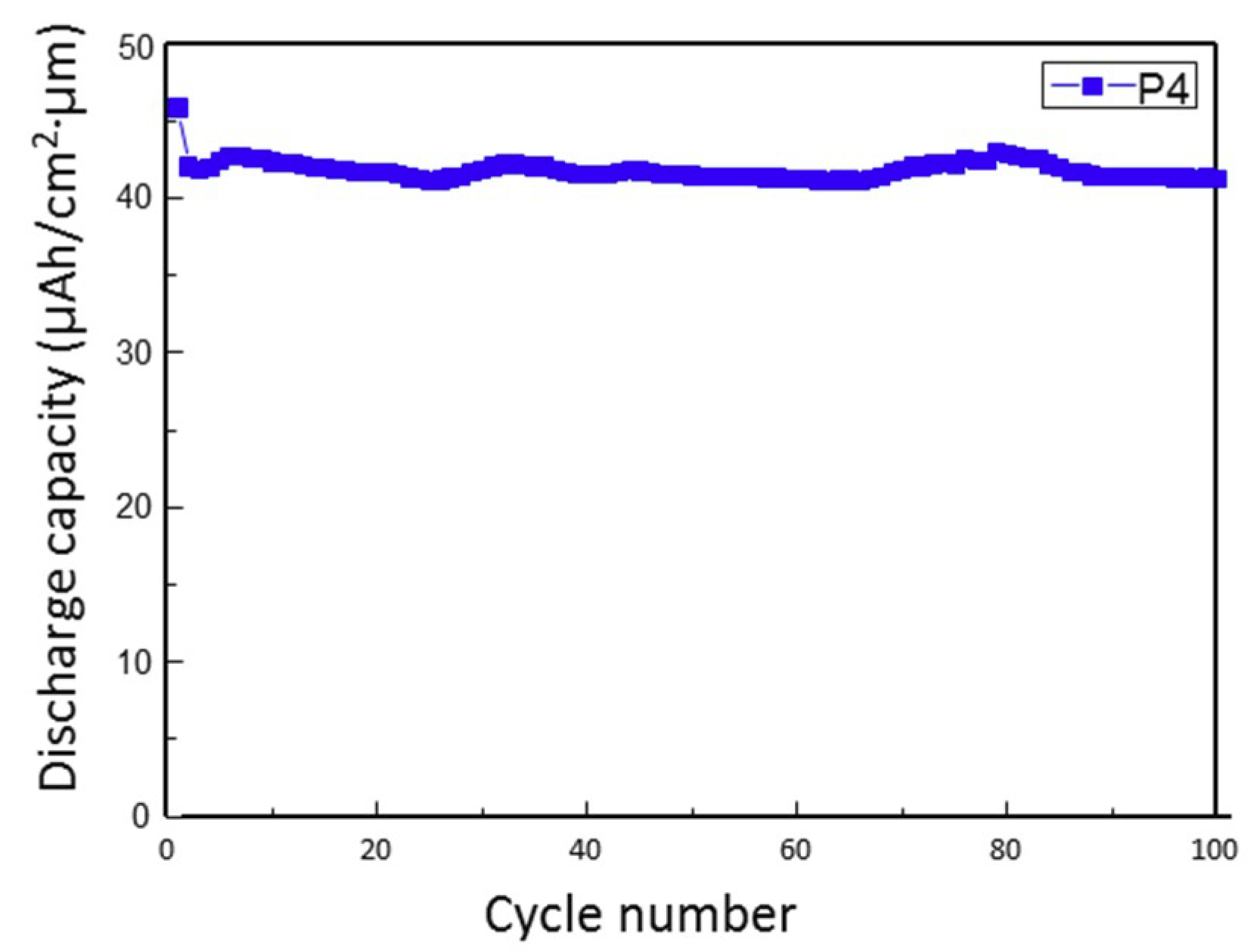
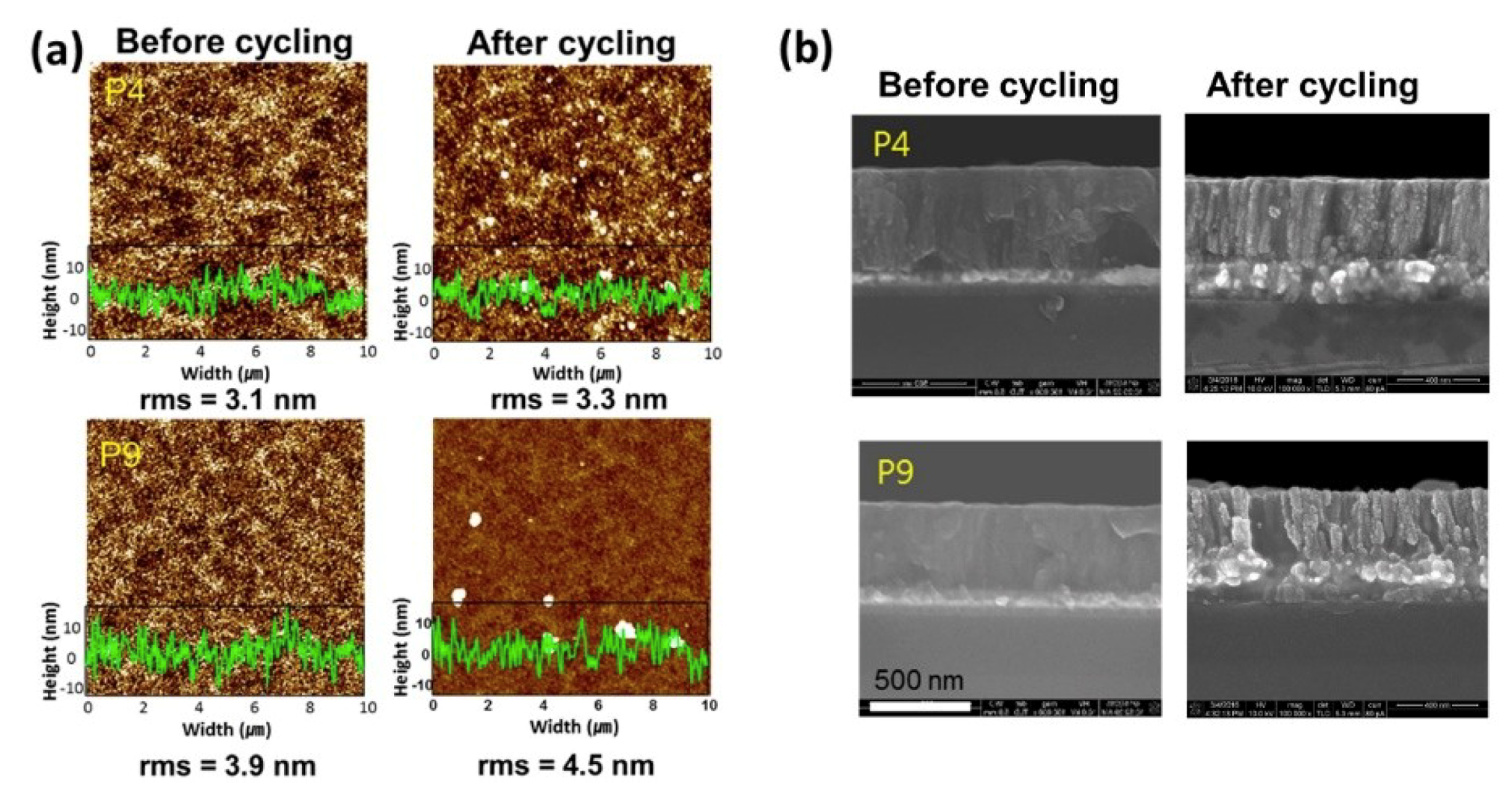
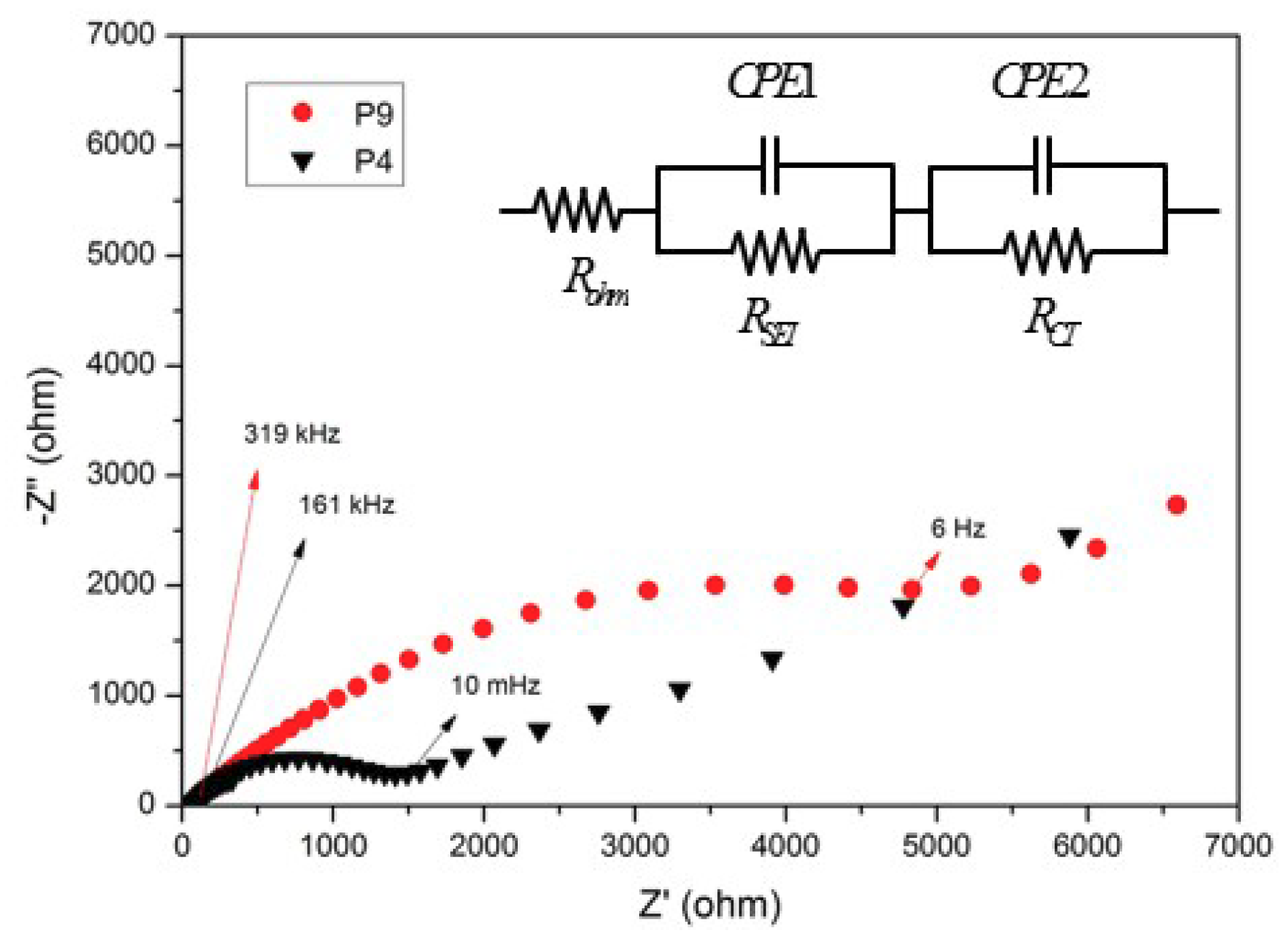
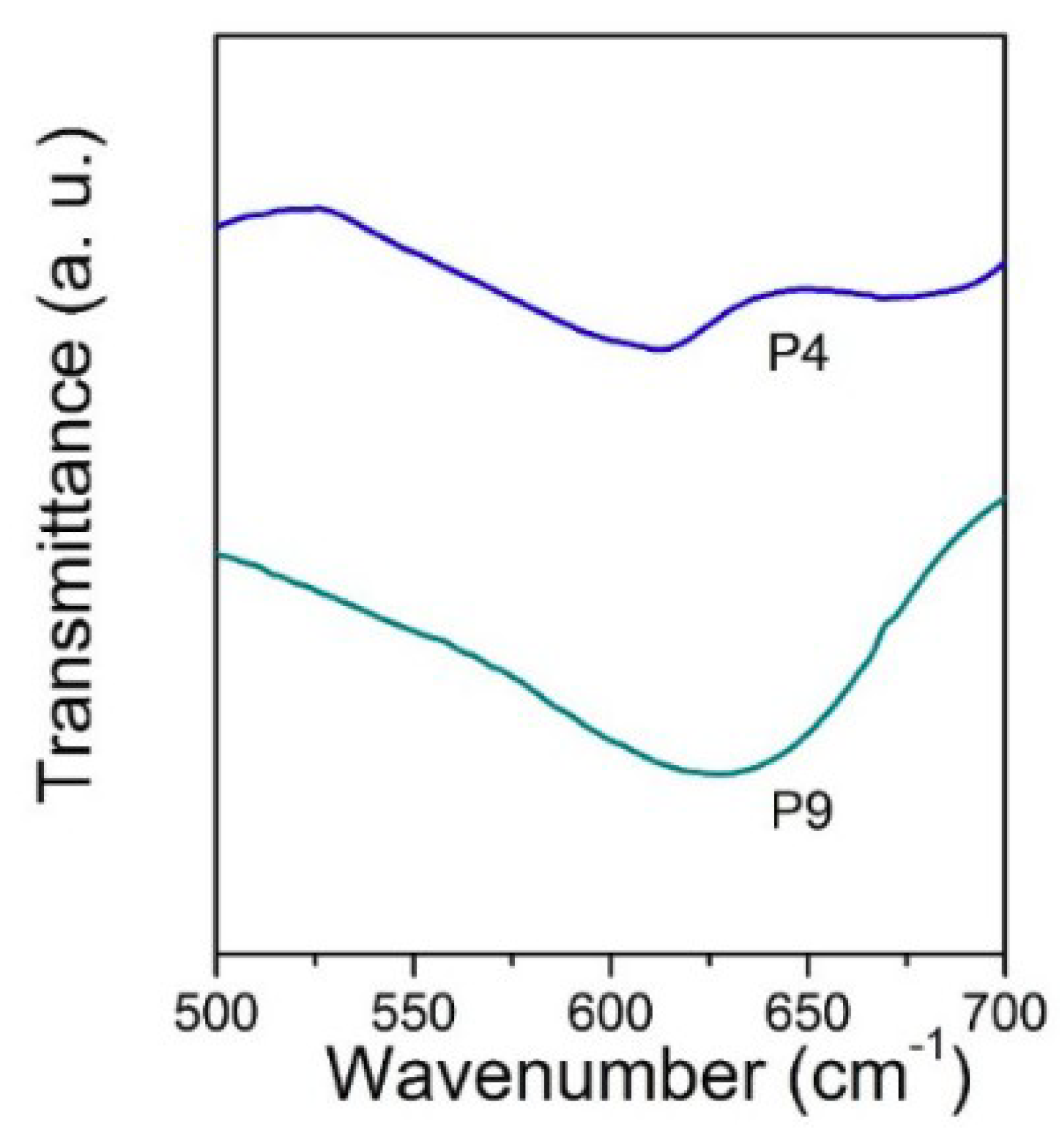
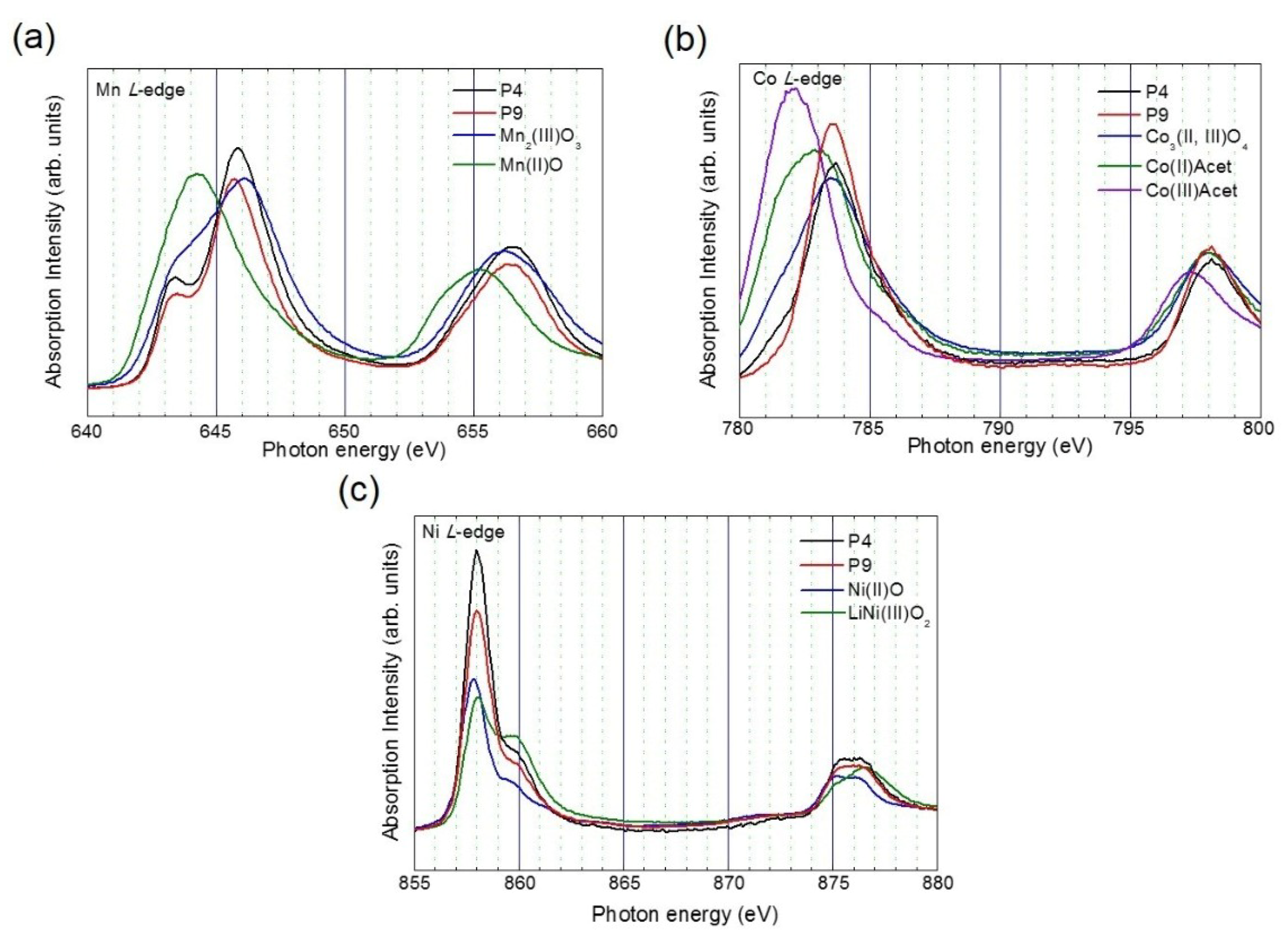
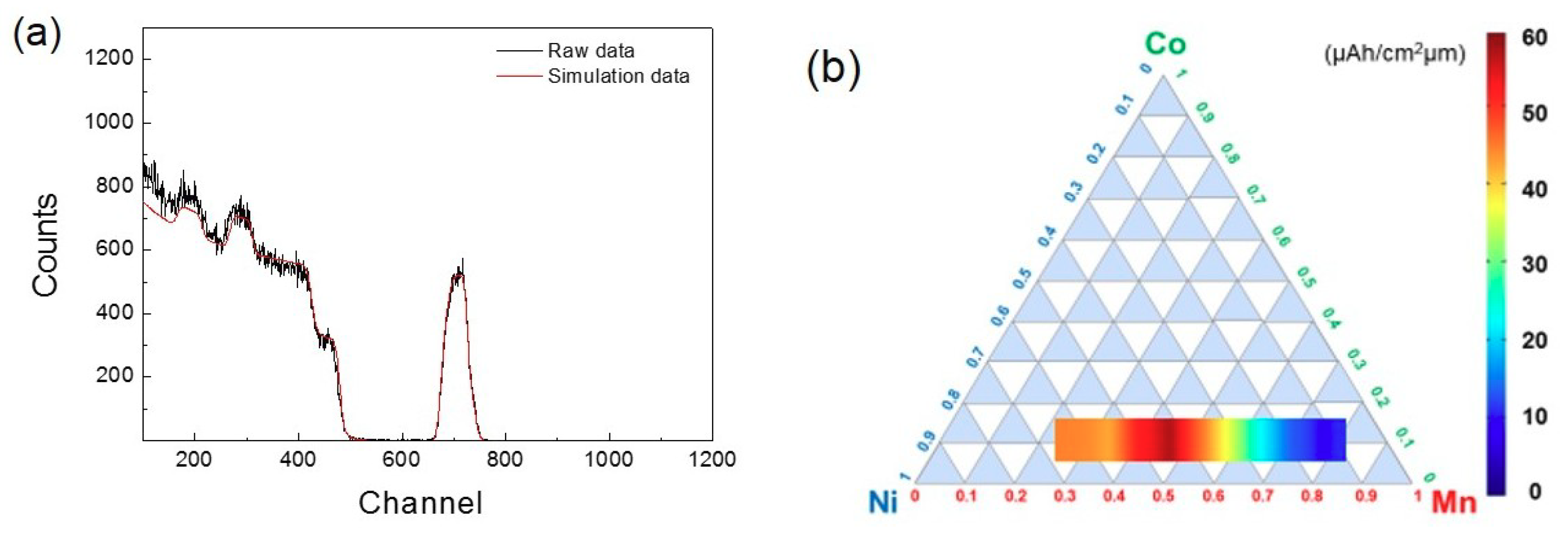
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagaura, T.; Tozawa, K. Lithium ion rechargeable battery. Prog. Batt. Solar Cells 1990, 9, 209–212. [Google Scholar]
- Yoshino, A.; Sanechika, K.; Nakajima, T. Secondary Battery. US Patent 4668595A, 26 May 1987. [Google Scholar]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, R. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Gur, I.; Sawyer, K.; Prasher, R. Searching for a better thermal battery. Science 2012, 335, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kang, K. Surface-modified spinel LiNi0.5Mn1.5O4 for Li-ion batteries. J. Korean Ceram. Soc. 2018, 55, 21–35. [Google Scholar] [CrossRef]
- Choi, M.; Lee, S.-H.; Jung, Y.-I.; Choi, W.-K.; Moon, J.-K.; Choi, J.; Seo, B.-K.; Kim, S.B. The preparation of Fe3O4 thin film and its electrochemical characterization for Li-ion battery. Transit. Electr. Electron. Mater. 2018, 19, 417–422. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0<x≤1): A new cathode material for batteries of high energy density. Solid State Ionics 1981, 3–4, 171–174. [Google Scholar] [CrossRef]
- Bruce, P.G.; Armstrong, A.R.; Gitzendanner, R.L. New intercalation compounds for lithium batteries: Layered LiMnO2. J. Mater. Chem. 1999, 9, 193–198. [Google Scholar] [CrossRef]
- Ramadass, P.; Haran, B.; White, R.; Popov, B.N. Performance study of commercial LiCoO2 and spinel-based Li-ion cells. J. Power Sources 2002, 111, 210–220. [Google Scholar] [CrossRef]
- Aurbach, D.; Gamolsky, K.; Markovsky, B.; Salitra, G.; Gofer, Y.; Heider, U.; Oesten, R.; Schmidt, M. The study of surface phenomena related to electrochemical lithium intercalation into LixMOy host materials (M = Ni, Mn). J. Electrochem. Soc. 2000, 147, 1322–1331. [Google Scholar] [CrossRef]
- Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, D.M.; Yang, F.X. Developments of lithium-ion batteries and challenges of LiFePO4 as one promising cathode material. J. Mater. Sci. 2009, 44, 2435–2443. [Google Scholar] [CrossRef]
- Li, W.; Currie, J.C. Morphology effects on the electrochemical performance of LiNi1−xCoxO2. J. Electrochem. Soc. 1997, 144, 2773–2779. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Lee, J.J.; Ha, J.Y.; Choi, W.K.; Cho, Y.S.; Choi, J.W. Doped SnO2 transparent conductive multilayer thin films explored by continuous composition spread. ACS Comb. Sci. 2015, 17, 247–252. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.X.; Konstantinov, K.; Liu, H.K.; Dou, S.X. Synthesis and characterization of LiCoxMnyNi1−x−yO2 as a cathode material for secondary lithium batteries. J. Power Sources 2003, 119, 184–188. [Google Scholar] [CrossRef]
- Mohammadi, F. Design and electrification of an electric vehicle using lithium-ion batteries. In Proceedings of the 3rd International Conference on Electrical Engineering, Zurich, Switzerland, 2–3 January 2018; pp. 1–13. [Google Scholar]
- Yue, B.; Wang, X.; Wang, J.; Yao, J.; Zhao, X.; Zhang, H.; Yu, W.; Liu, G.; Dong, X. Au-doped Li1.2Ni0.7Co0.1Mn0.2O2 electrospun nanofibers: Synthesis and enhanced capacity retention performance for lithium-ion batteries. RSC Adv. 2018, 8, 4112–4118. [Google Scholar] [CrossRef]
- Kang, H.M.; Baek, S.H.; Song, J.H.; Cho, Y.S.; Choi, J.W. Full range dielectric characteristics of calcium copper titanate thin films prepared by continuous composition-spread sputtering. ACS Comb. Sci. 2014, 16, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Borhani-Haghighi, S.; Kieschnick, M.; Motemani, Y.; Savan, A.; Rogalla, D.; Becker, H.W.; Meijer, J.; Ludwig, A. High-throughput compositional and structural evaluation of a Lia(NixMnyCoz)Or thin film battery materials library. ACS Comb. Sci. 2013, 15, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Yoon, W.S.; Yang, X.Q. Structural changes and thermal stability of charged LiNi1/3Co1/3Mn1/3O2 cathode material for Li-ion batteries studied by time-resolved XRD. J. Power Sources 2009, 189, 515–518. [Google Scholar] [CrossRef]
- Doolittle, L.R. A semiautomatic algorithm for rutherford backscattering analysis. Nucl. Instrum. Methods Phys. Res. Sect. B 1986, 15, 227–231. [Google Scholar] [CrossRef]
- Garcia, B.; Barboux, P.; Ribot, F.; Kahn-Harari, A.; Mazerolles, L.; Baffier, N. The structure of low temperature crystallized LiCoO2. Solid State Ionics 1995, 80, 111–118. [Google Scholar] [CrossRef]
- Cho, Y.H.; Jang, D.; Yoon, J.; Kim, H.; Ahn, T.K.; Nam, K.W.; Sung, Y.E.; Kim, W.S.; Lee, Y.S.; Yang, X.Q.; et al. Thermal stability of charged LiNi0.5Co0.2Mn0.3O2 cathode for Li-ion batteries investigated by synchrotron based in situ X-ray diffraction. J. Alloy. Compd. 2013, 562, 219–223. [Google Scholar] [CrossRef]
- Sun, H.H.; Choi, W.; Lee, J.K.; Oh, I.H.; Jung, H.G. Control of Electrochemical properties of nickel-rich layered cathode materials for lithium ion batteries by variation of the manganese to cobalt ratio. J. Power Sources 2015, 275, 877–883. [Google Scholar] [CrossRef]
- Buchberger, I.; Seidlmayer, S.; Pokharel, A.; Piana, M.; Hattendorff, J.; Kudejova, P.; Gilles, R.; Gasteiger, H.A. Aging analysis of graphite/LiNi1/3Mn1/3Co1/3O2 cells using XRD, PGAA, and AC impedance. J. Electrochem. Soc. 2015, 162, A2737–A2746. [Google Scholar] [CrossRef]
- Sa, Q.; Heelan, J.A.; Lu, Y.; Apelian, D.; Wang, Y. Copper impurity effects on LiNi1/3Mn1/3Co1/3O2 cathode material. ACS Appl. Mater. Interfaces 2015, 7, 20585–20590. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.N.; Mukerjee, S.; Abraham, K.M. A high rate Li-rich layered MNC cathode material for lithium-ion batteries. RSC Adv. 2015, 5, 27375–27386. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Li, X.L.; Hu, S.Y.; Zhang, X.W.; Xu, W.; Thevuthasan, S.; Baer, D.R.; Zhang, J.G.; Liu, J.; et al. In situ TEM study of lithiation behavior of silicon nanoparticles attached to and embedded in a carbon matrix. ACS Nano 2012, 6, 8439–8447. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.G.; Liu, J.C.; Song, B.H.; Xiao, P.F.; Lu, L. Li-rich thin film cathode prepared by pulsed laser deposition. Sci. Rep. 2013, 3, 3332. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.W.; Du, K.; Lu, W.; Peng, Z.D.; Cao, Y.B.; Hu, G.R. Synthesis and Characterization of concentration–gradient LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. J. Alloy. Compd. 2014, 613, 296–305. [Google Scholar] [CrossRef]
- Nithya, C.; Kumari, V.S.S.; Gopukumar, S. Synthesis of high voltage (4.9 V) cycling LiNixCoyMn1−x−yO2 cathode materials for lithium rechargeable batteries. Phys. Chem. Chem. Phys. 2011, 13, 6125–6132. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Massot, M. Spectroscopic studies of the local structure in positive electrodes for lithium batteries. Phys. Chem. Chem. Phys. 2002, 4, 4226–4235. [Google Scholar] [CrossRef]
- Hashem, A.M.; El-Taweel, R.S.; Abuzeid, H.M.; Abdel-Ghany, A.E.; Eid, A.E.; Groult, H.; Mauger, A.; Julien, C.M. Structural and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 material prepared by a two-step synthesis via oxalate precursor. Ionics 2012, 18, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shikano, M.; Koike, S.; Sakaebe, H.; Tatsumi, K. Investigation of positive electrodes after cycle testing of high-power Li-ion battery cells: I. An approach to the power fading mechanism using XANES. J. Power Sources 2007, 174, 380–386. [Google Scholar] [CrossRef]
- Shin, D.W.; Choi, J.W.; Cho, Y.S.; Yoon, S.J. Phase evolution and Sn-substitution in LiMn2O4 thin films prepared by pulsed laser deposition. J. Electroceram. 2009, 23, 200–205. [Google Scholar] [CrossRef]
- Yim, H.; Kong, W.Y.; Yoon, S.J.; Nahm, S.; Jang, H.W.; Sung, Y.E.; Ha, J.Y.; Davydov, A.V.; Choi, J.W. Three-dimensional hemisphere-structured LiSn0.0125Mn1.975O4 thin-film cathodes. Electrochem. Commun. 2014, 43, 36–39. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Yim, H.; Parmar, N.S.; Lee, J.-S.; Choi, J.-W. Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials. Coatings 2019, 9, 366. https://doi.org/10.3390/coatings9060366
Jung J, Yim H, Parmar NS, Lee J-S, Choi J-W. Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials. Coatings. 2019; 9(6):366. https://doi.org/10.3390/coatings9060366
Chicago/Turabian StyleJung, JongSeok, Haena Yim, Narendra Singh Parmar, Jae-Seung Lee, and Ji-Won Choi. 2019. "Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials" Coatings 9, no. 6: 366. https://doi.org/10.3390/coatings9060366
APA StyleJung, J., Yim, H., Parmar, N. S., Lee, J.-S., & Choi, J.-W. (2019). Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials. Coatings, 9(6), 366. https://doi.org/10.3390/coatings9060366





