Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials
Abstract
:1. Introduction
2. Experimental Methods
2.1. Material Preparation
2.2. Characterization
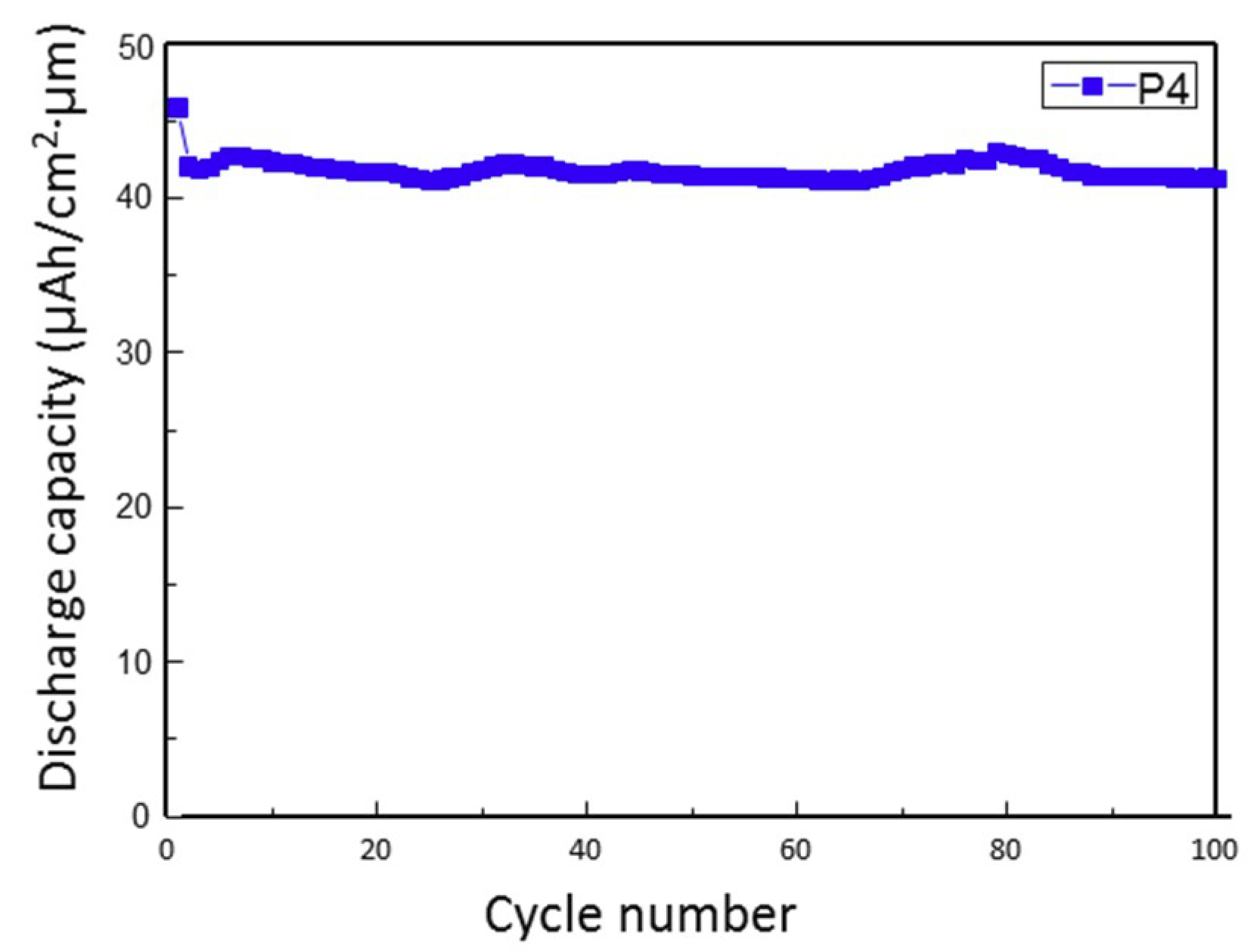
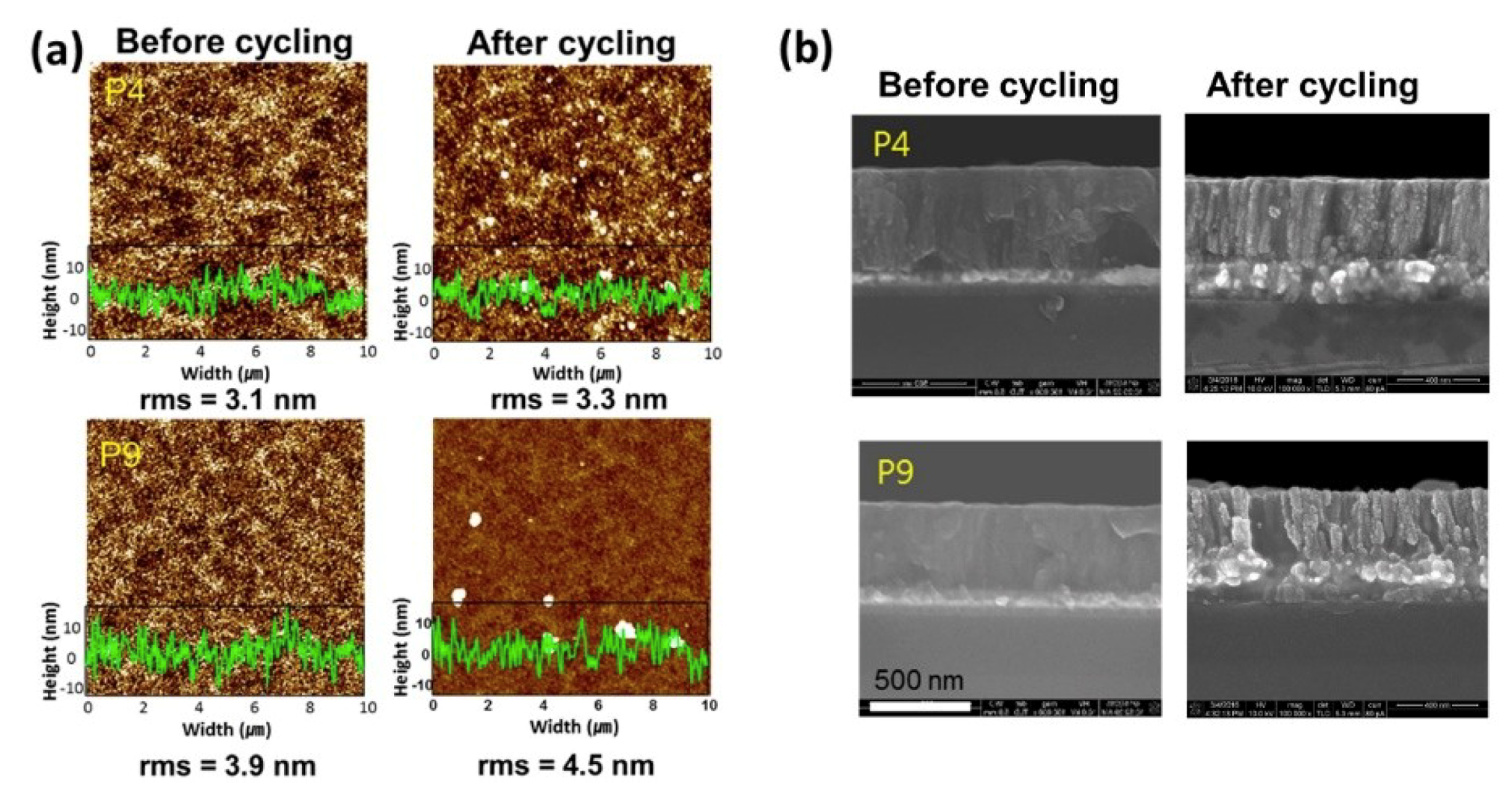
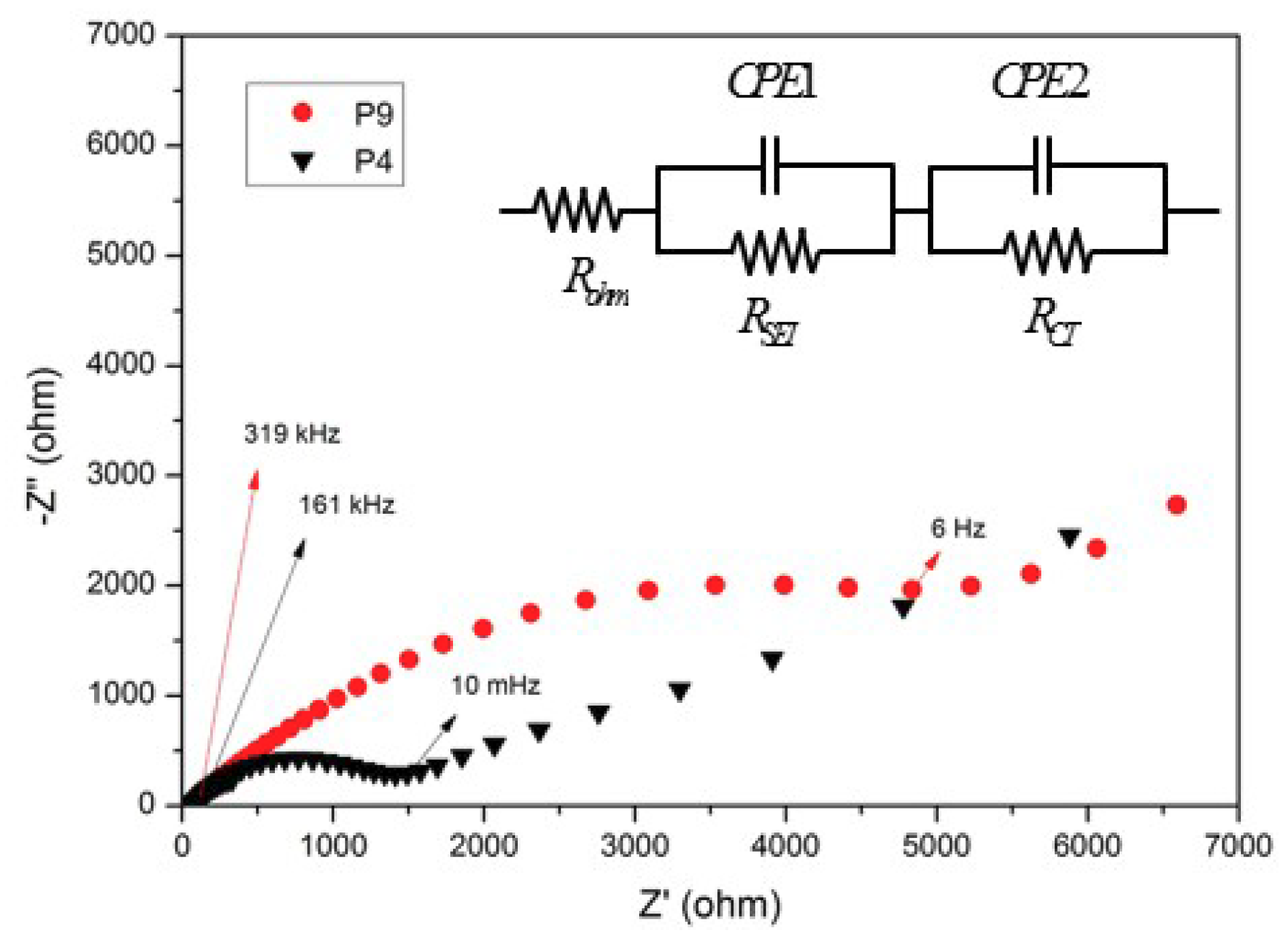
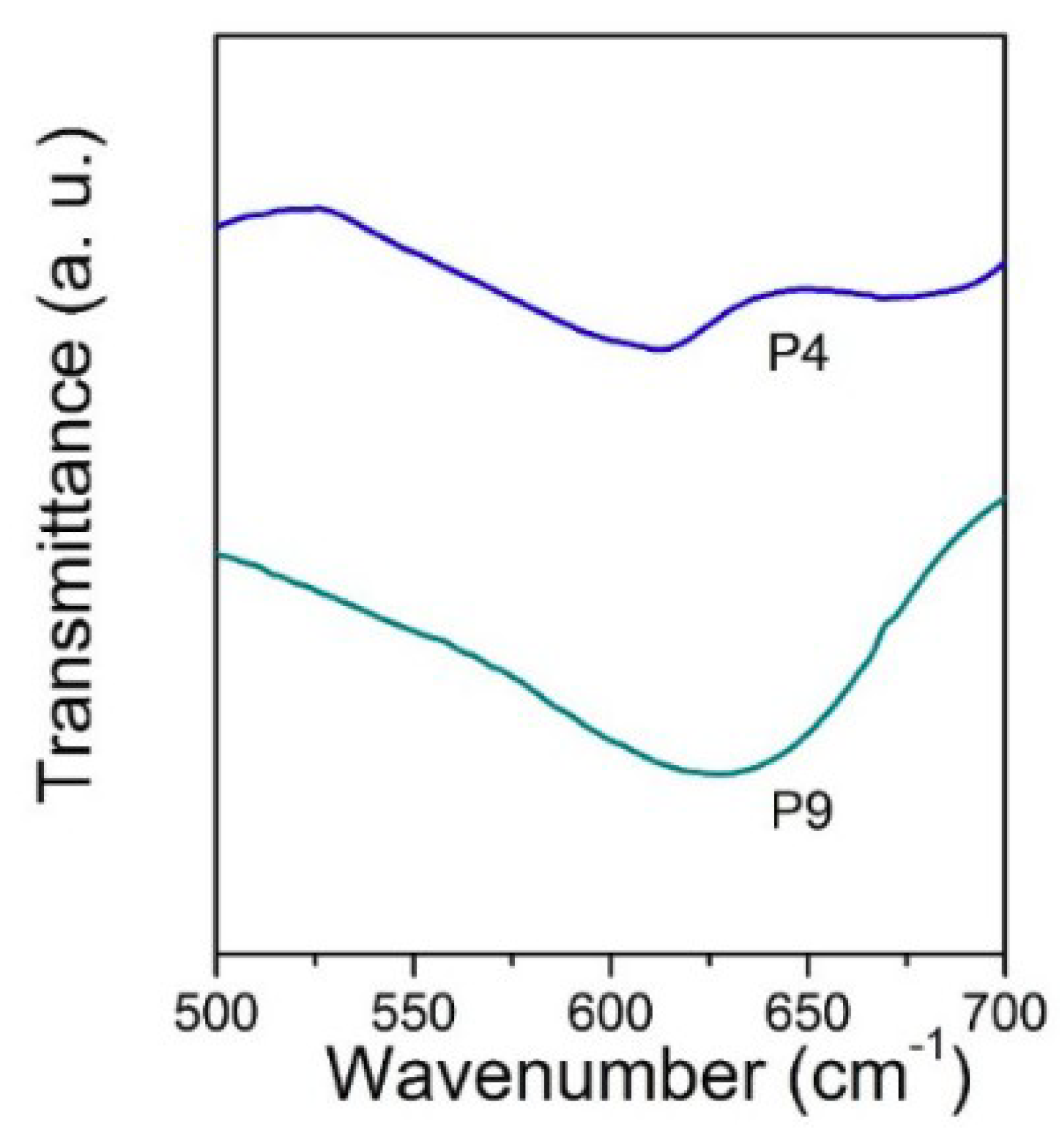
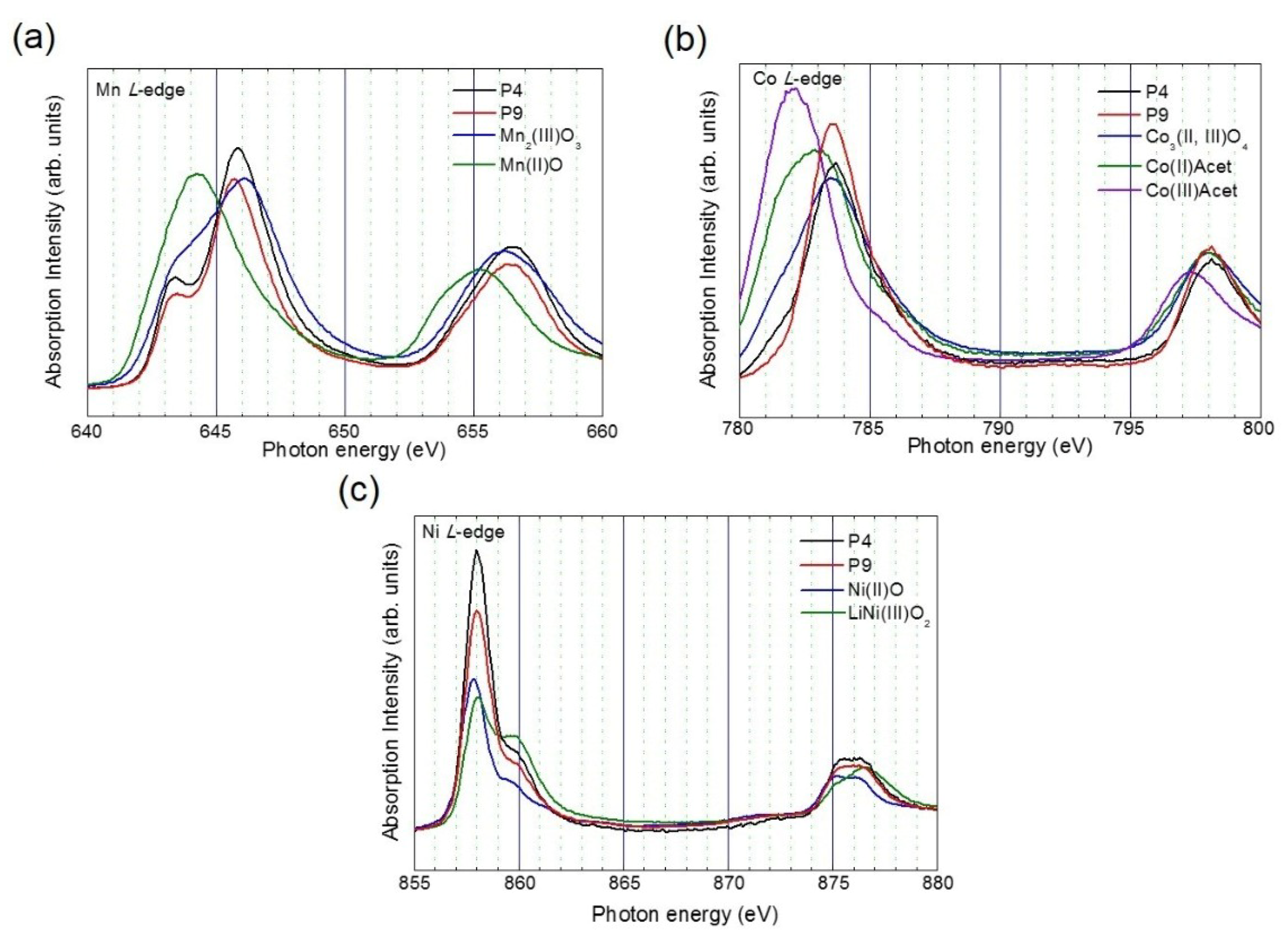
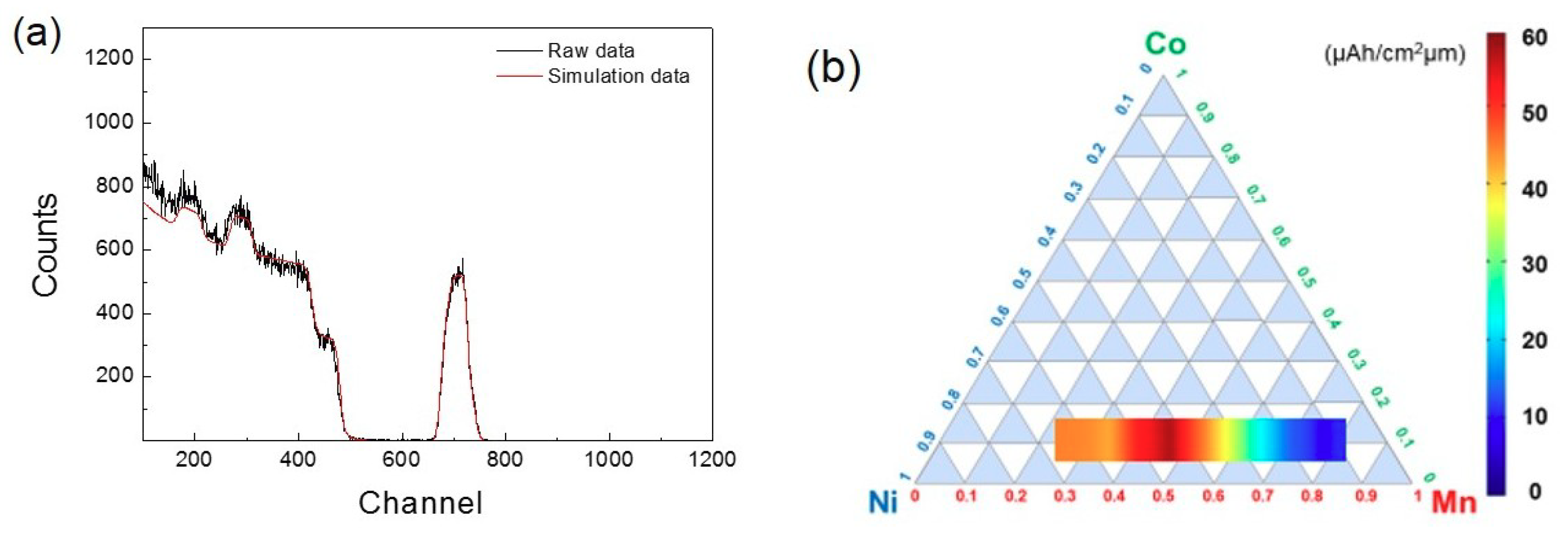
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagaura, T.; Tozawa, K. Lithium ion rechargeable battery. Prog. Batt. Solar Cells 1990, 9, 209–212. [Google Scholar]
- Yoshino, A.; Sanechika, K.; Nakajima, T. Secondary Battery. US Patent 4668595A, 26 May 1987. [Google Scholar]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, R. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Gur, I.; Sawyer, K.; Prasher, R. Searching for a better thermal battery. Science 2012, 335, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kang, K. Surface-modified spinel LiNi0.5Mn1.5O4 for Li-ion batteries. J. Korean Ceram. Soc. 2018, 55, 21–35. [Google Scholar] [CrossRef]
- Choi, M.; Lee, S.-H.; Jung, Y.-I.; Choi, W.-K.; Moon, J.-K.; Choi, J.; Seo, B.-K.; Kim, S.B. The preparation of Fe3O4 thin film and its electrochemical characterization for Li-ion battery. Transit. Electr. Electron. Mater. 2018, 19, 417–422. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0<x≤1): A new cathode material for batteries of high energy density. Solid State Ionics 1981, 3–4, 171–174. [Google Scholar] [CrossRef]
- Bruce, P.G.; Armstrong, A.R.; Gitzendanner, R.L. New intercalation compounds for lithium batteries: Layered LiMnO2. J. Mater. Chem. 1999, 9, 193–198. [Google Scholar] [CrossRef]
- Ramadass, P.; Haran, B.; White, R.; Popov, B.N. Performance study of commercial LiCoO2 and spinel-based Li-ion cells. J. Power Sources 2002, 111, 210–220. [Google Scholar] [CrossRef]
- Aurbach, D.; Gamolsky, K.; Markovsky, B.; Salitra, G.; Gofer, Y.; Heider, U.; Oesten, R.; Schmidt, M. The study of surface phenomena related to electrochemical lithium intercalation into LixMOy host materials (M = Ni, Mn). J. Electrochem. Soc. 2000, 147, 1322–1331. [Google Scholar] [CrossRef]
- Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, D.M.; Yang, F.X. Developments of lithium-ion batteries and challenges of LiFePO4 as one promising cathode material. J. Mater. Sci. 2009, 44, 2435–2443. [Google Scholar] [CrossRef]
- Li, W.; Currie, J.C. Morphology effects on the electrochemical performance of LiNi1−xCoxO2. J. Electrochem. Soc. 1997, 144, 2773–2779. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Lee, J.J.; Ha, J.Y.; Choi, W.K.; Cho, Y.S.; Choi, J.W. Doped SnO2 transparent conductive multilayer thin films explored by continuous composition spread. ACS Comb. Sci. 2015, 17, 247–252. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.X.; Konstantinov, K.; Liu, H.K.; Dou, S.X. Synthesis and characterization of LiCoxMnyNi1−x−yO2 as a cathode material for secondary lithium batteries. J. Power Sources 2003, 119, 184–188. [Google Scholar] [CrossRef]
- Mohammadi, F. Design and electrification of an electric vehicle using lithium-ion batteries. In Proceedings of the 3rd International Conference on Electrical Engineering, Zurich, Switzerland, 2–3 January 2018; pp. 1–13. [Google Scholar]
- Yue, B.; Wang, X.; Wang, J.; Yao, J.; Zhao, X.; Zhang, H.; Yu, W.; Liu, G.; Dong, X. Au-doped Li1.2Ni0.7Co0.1Mn0.2O2 electrospun nanofibers: Synthesis and enhanced capacity retention performance for lithium-ion batteries. RSC Adv. 2018, 8, 4112–4118. [Google Scholar] [CrossRef]
- Kang, H.M.; Baek, S.H.; Song, J.H.; Cho, Y.S.; Choi, J.W. Full range dielectric characteristics of calcium copper titanate thin films prepared by continuous composition-spread sputtering. ACS Comb. Sci. 2014, 16, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Borhani-Haghighi, S.; Kieschnick, M.; Motemani, Y.; Savan, A.; Rogalla, D.; Becker, H.W.; Meijer, J.; Ludwig, A. High-throughput compositional and structural evaluation of a Lia(NixMnyCoz)Or thin film battery materials library. ACS Comb. Sci. 2013, 15, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Yoon, W.S.; Yang, X.Q. Structural changes and thermal stability of charged LiNi1/3Co1/3Mn1/3O2 cathode material for Li-ion batteries studied by time-resolved XRD. J. Power Sources 2009, 189, 515–518. [Google Scholar] [CrossRef]
- Doolittle, L.R. A semiautomatic algorithm for rutherford backscattering analysis. Nucl. Instrum. Methods Phys. Res. Sect. B 1986, 15, 227–231. [Google Scholar] [CrossRef]
- Garcia, B.; Barboux, P.; Ribot, F.; Kahn-Harari, A.; Mazerolles, L.; Baffier, N. The structure of low temperature crystallized LiCoO2. Solid State Ionics 1995, 80, 111–118. [Google Scholar] [CrossRef]
- Cho, Y.H.; Jang, D.; Yoon, J.; Kim, H.; Ahn, T.K.; Nam, K.W.; Sung, Y.E.; Kim, W.S.; Lee, Y.S.; Yang, X.Q.; et al. Thermal stability of charged LiNi0.5Co0.2Mn0.3O2 cathode for Li-ion batteries investigated by synchrotron based in situ X-ray diffraction. J. Alloy. Compd. 2013, 562, 219–223. [Google Scholar] [CrossRef]
- Sun, H.H.; Choi, W.; Lee, J.K.; Oh, I.H.; Jung, H.G. Control of Electrochemical properties of nickel-rich layered cathode materials for lithium ion batteries by variation of the manganese to cobalt ratio. J. Power Sources 2015, 275, 877–883. [Google Scholar] [CrossRef]
- Buchberger, I.; Seidlmayer, S.; Pokharel, A.; Piana, M.; Hattendorff, J.; Kudejova, P.; Gilles, R.; Gasteiger, H.A. Aging analysis of graphite/LiNi1/3Mn1/3Co1/3O2 cells using XRD, PGAA, and AC impedance. J. Electrochem. Soc. 2015, 162, A2737–A2746. [Google Scholar] [CrossRef]
- Sa, Q.; Heelan, J.A.; Lu, Y.; Apelian, D.; Wang, Y. Copper impurity effects on LiNi1/3Mn1/3Co1/3O2 cathode material. ACS Appl. Mater. Interfaces 2015, 7, 20585–20590. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.N.; Mukerjee, S.; Abraham, K.M. A high rate Li-rich layered MNC cathode material for lithium-ion batteries. RSC Adv. 2015, 5, 27375–27386. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Li, X.L.; Hu, S.Y.; Zhang, X.W.; Xu, W.; Thevuthasan, S.; Baer, D.R.; Zhang, J.G.; Liu, J.; et al. In situ TEM study of lithiation behavior of silicon nanoparticles attached to and embedded in a carbon matrix. ACS Nano 2012, 6, 8439–8447. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.G.; Liu, J.C.; Song, B.H.; Xiao, P.F.; Lu, L. Li-rich thin film cathode prepared by pulsed laser deposition. Sci. Rep. 2013, 3, 3332. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.W.; Du, K.; Lu, W.; Peng, Z.D.; Cao, Y.B.; Hu, G.R. Synthesis and Characterization of concentration–gradient LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. J. Alloy. Compd. 2014, 613, 296–305. [Google Scholar] [CrossRef]
- Nithya, C.; Kumari, V.S.S.; Gopukumar, S. Synthesis of high voltage (4.9 V) cycling LiNixCoyMn1−x−yO2 cathode materials for lithium rechargeable batteries. Phys. Chem. Chem. Phys. 2011, 13, 6125–6132. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Massot, M. Spectroscopic studies of the local structure in positive electrodes for lithium batteries. Phys. Chem. Chem. Phys. 2002, 4, 4226–4235. [Google Scholar] [CrossRef]
- Hashem, A.M.; El-Taweel, R.S.; Abuzeid, H.M.; Abdel-Ghany, A.E.; Eid, A.E.; Groult, H.; Mauger, A.; Julien, C.M. Structural and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 material prepared by a two-step synthesis via oxalate precursor. Ionics 2012, 18, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shikano, M.; Koike, S.; Sakaebe, H.; Tatsumi, K. Investigation of positive electrodes after cycle testing of high-power Li-ion battery cells: I. An approach to the power fading mechanism using XANES. J. Power Sources 2007, 174, 380–386. [Google Scholar] [CrossRef]
- Shin, D.W.; Choi, J.W.; Cho, Y.S.; Yoon, S.J. Phase evolution and Sn-substitution in LiMn2O4 thin films prepared by pulsed laser deposition. J. Electroceram. 2009, 23, 200–205. [Google Scholar] [CrossRef]
- Yim, H.; Kong, W.Y.; Yoon, S.J.; Nahm, S.; Jang, H.W.; Sung, Y.E.; Ha, J.Y.; Davydov, A.V.; Choi, J.W. Three-dimensional hemisphere-structured LiSn0.0125Mn1.975O4 thin-film cathodes. Electrochem. Commun. 2014, 43, 36–39. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Yim, H.; Parmar, N.S.; Lee, J.-S.; Choi, J.-W. Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials. Coatings 2019, 9, 366. https://doi.org/10.3390/coatings9060366
Jung J, Yim H, Parmar NS, Lee J-S, Choi J-W. Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials. Coatings. 2019; 9(6):366. https://doi.org/10.3390/coatings9060366
Chicago/Turabian StyleJung, JongSeok, Haena Yim, Narendra Singh Parmar, Jae-Seung Lee, and Ji-Won Choi. 2019. "Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials" Coatings 9, no. 6: 366. https://doi.org/10.3390/coatings9060366
APA StyleJung, J., Yim, H., Parmar, N. S., Lee, J.-S., & Choi, J.-W. (2019). Continuous Composition Spread and Electrochemical Studies of Low Cobalt Content Li(Ni,Mn,Co)O2 Cathode Materials. Coatings, 9(6), 366. https://doi.org/10.3390/coatings9060366





