Biopolymer-Based Films Enriched with Stevia rebaudiana Used for the Development of Edible and Soluble Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
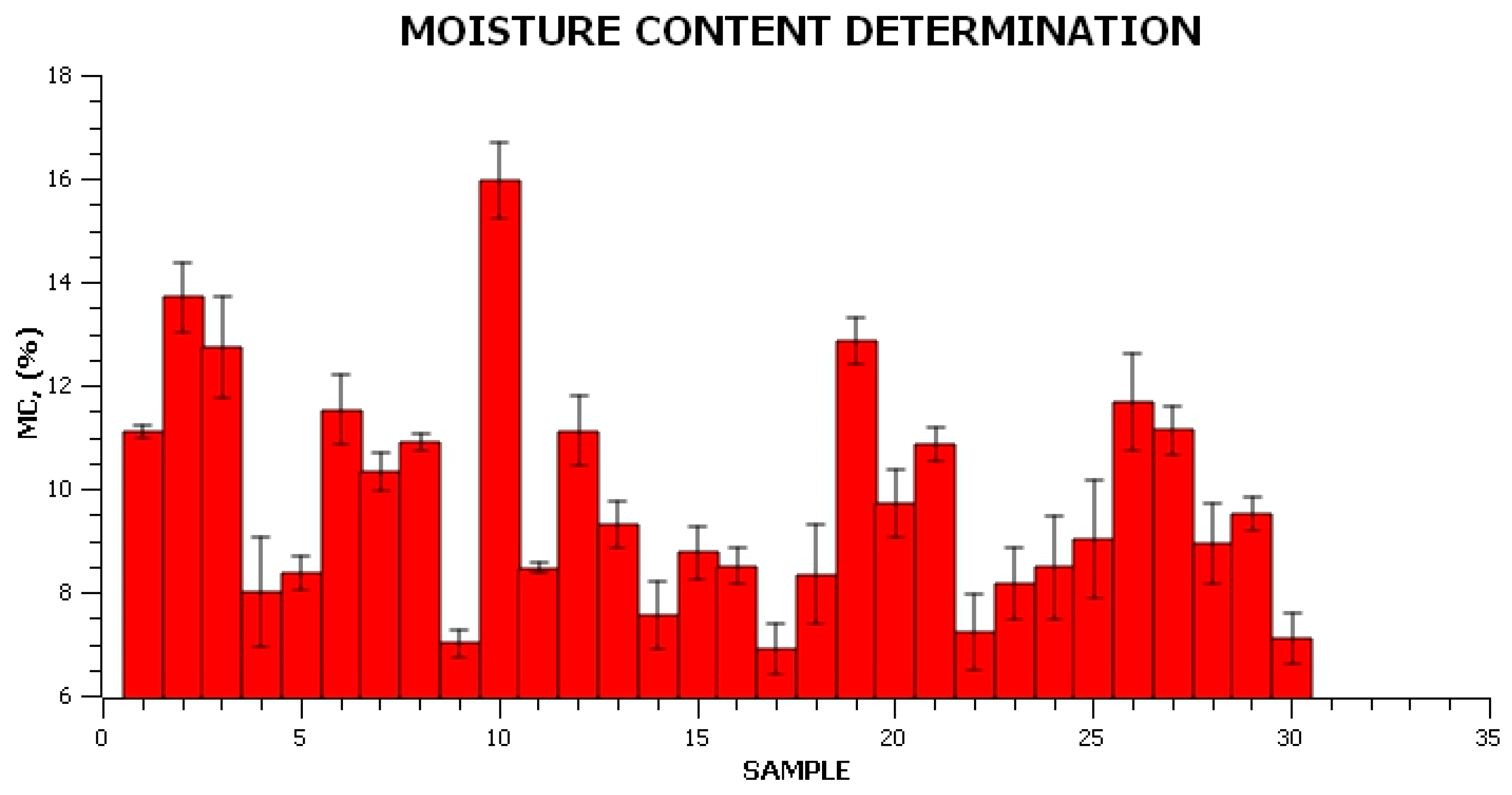
2.2.1. Determination of Physical and Optical Properties
2.2.2. Evaluation of Mechanical Properties
2.2.3. Evaluation of Microbiological Characteristics
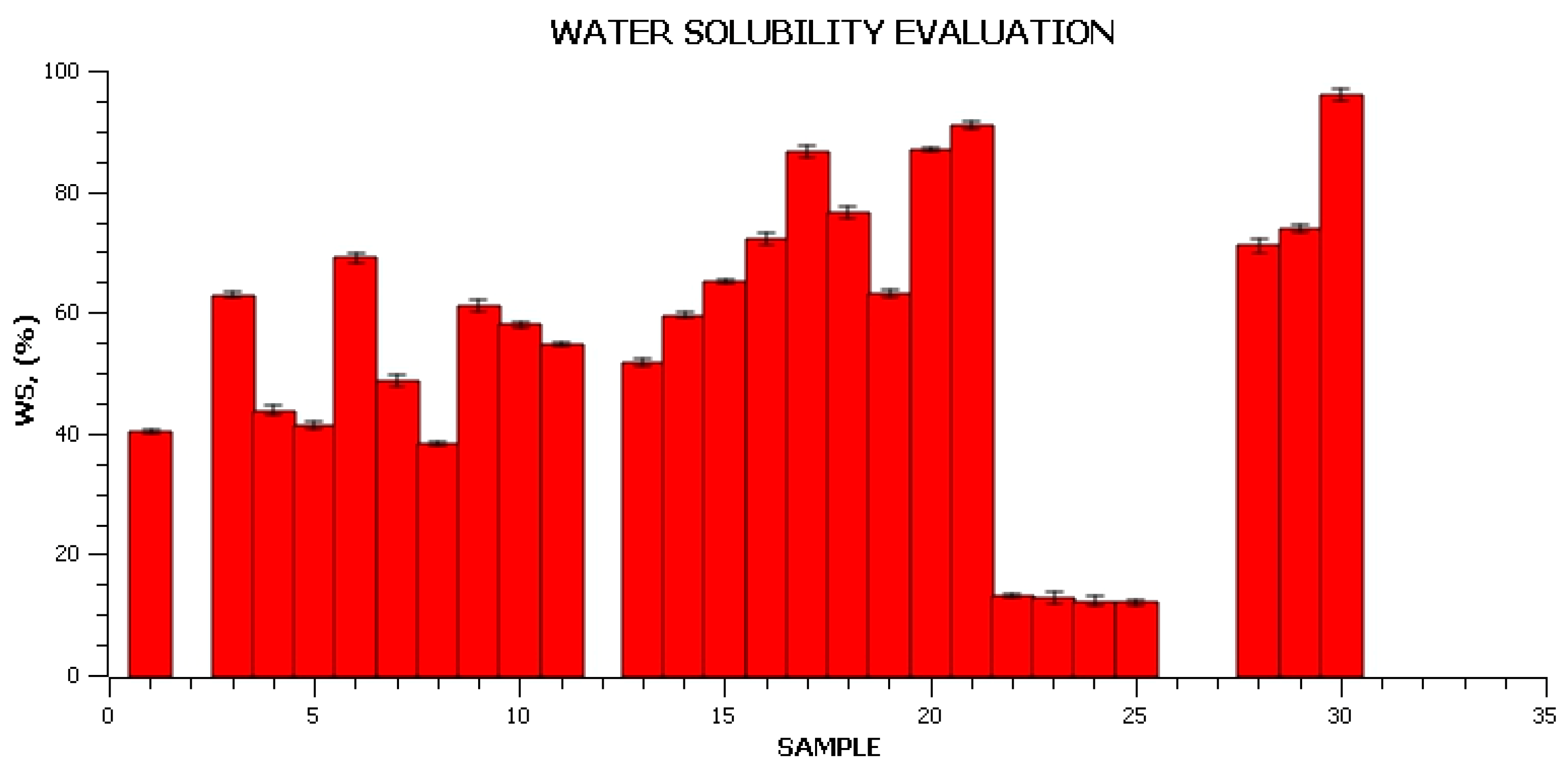
2.2.4. Solubility Assessment
2.3. Statistical Evaluation
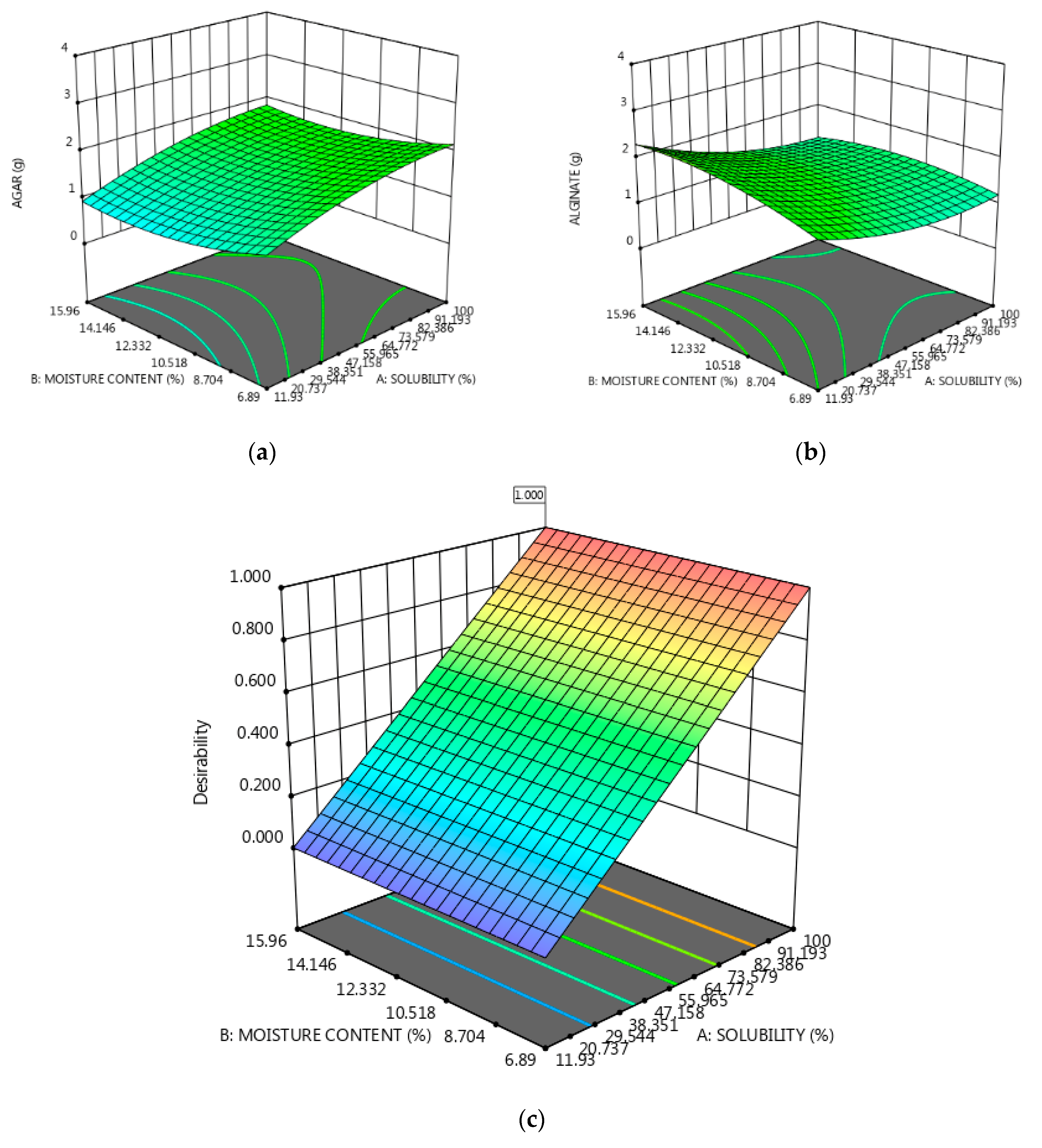
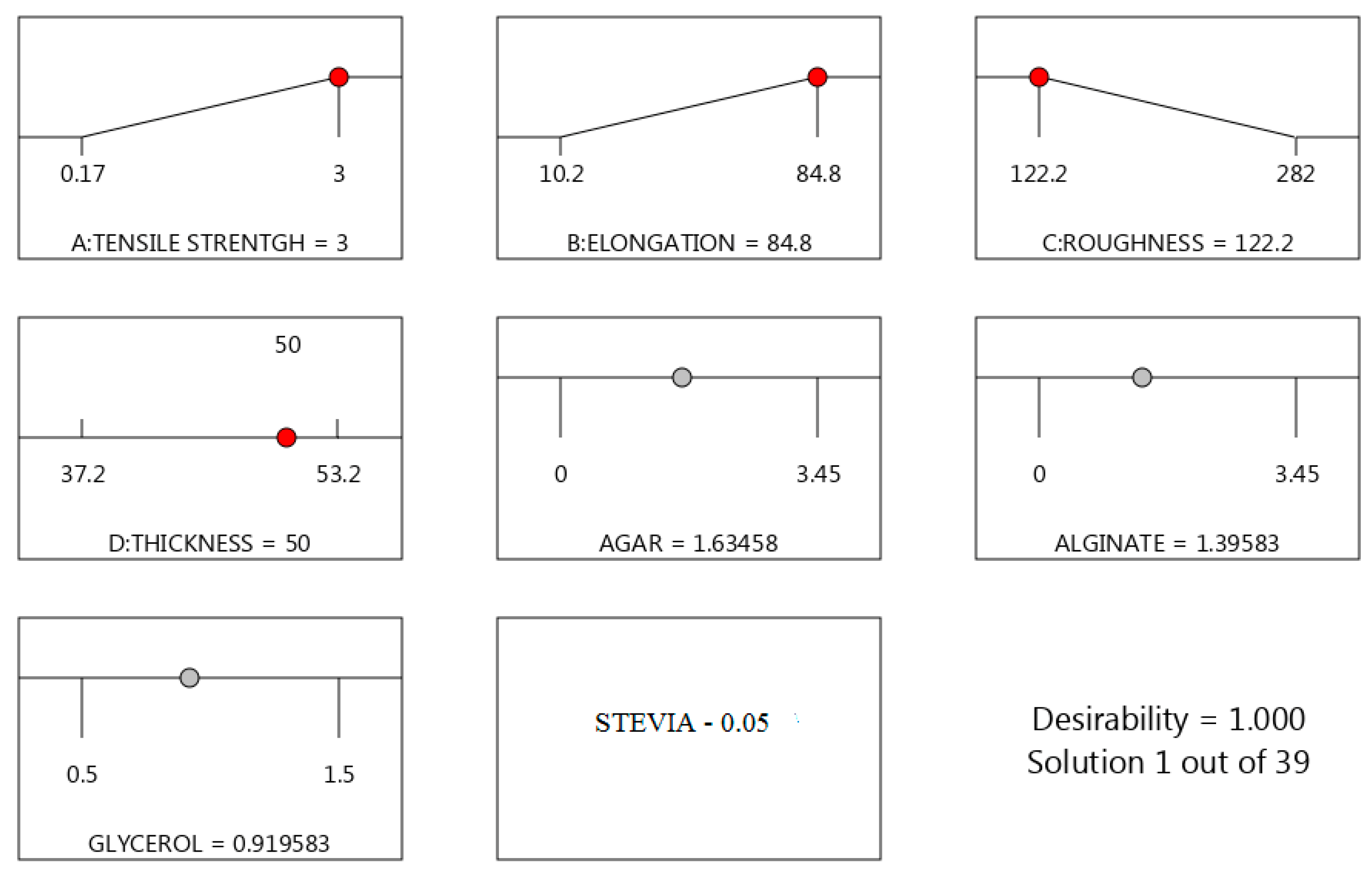
3. Results and Discussion
Characterization of the Obtained Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of Plastic and Plankton in the North Pacific Central Gyre. Mar. Poll. Bull. 2001, 42, 1297–1300. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Sabbah, M.; Al Asmar, A.; Esposito, M.; Sanchez, A.; Villalonga Santana, R.; Cammarota, M.; Marinello, L.; Di Pierro, P.; Porta, R. Effect of mesoporous silica nanoparticles on glycerol-plasticized anionic and cationic polysaccharides edible films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef]
- Yadav, A.; Mangaraj, S.; Singh, R.; Kumar Das, S.; Kumar, N.; Arora, S. Biopolymers as packaging material in food and allied industry. Int. J. Chem. 2018, 6, 2411–2418. [Google Scholar]
- Calabro, P.S.; Grosso, M. Bioplastic and waste management. Waste Manag. 2018, 78. [Google Scholar] [CrossRef]
- Cancino, L. Fruit and vegetable wastes as potential component of biodegradable plastic. AJMS 2018, 1, 1–17. [Google Scholar]
- Karan, H.; Grabert, M.; Funk, C.; Oey, M. Green bioplastics as part of a circular bioeconomy. TRPLSC 2019, 24, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepa, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: opportunities and changes. Curr. Opt. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Parliamentary Questions. 2017. Available online: http://www.europarl.europa.eu/doceo/document/E-8-2017-000379_EN.html (accessed on 10 April 2019).
- Hossain, M.F.; Islam, M.T.; Islam, M.A.; Akhtar, S. Cultivation and uses of Stevia: A review. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12745–12757. [Google Scholar]
- Parker, M.; Lopetcharat, K.; Drake, M.A. Consumer acceptance of natural sweeteners in protein beverages. JDS 2018, 101, 8875–8889. [Google Scholar] [CrossRef]
- Suresh, V.; Preethi Fetricia, J.; Saranaya, V.; Sarithara, S.; Tamilselvan, K. Uses of Stevia. J. Med. Plants 2018, 6, 247–248. [Google Scholar]
- Ciriminna, R.; Meneguzzo, F.; Pecoraino, M.; Pagliaro, M. A bioeconomy perspective for natural sweetener Stevia. Bio Fpr. 2018, 13, 445–452. [Google Scholar] [CrossRef]
- Amchra, F.; Chaouqui, S.; Al Faiz, C.; Khiraoiu, A. Effect of Stevia rebaudiana, sucrose and aspartame on human health: A comprehensive review. JMPS 2018, 6, 102–108. [Google Scholar]
- Ghandi, S.; Gat, Y.; Arya, S.; Kumar, V.; Panghal, A.; Kumar, A. Natural sweeteners: health benefits of Stevia. JFRM 2018, 6, 392–402. [Google Scholar] [CrossRef]
- Rizwan, F.; Hu, R.; Yesmine, S.; Monjur, F.; Chatterjee, T. Preliminary analysis of the effect of Stevia (Stevia rebaudiana) in patients with chronic kidney disease (stage I to stage III). Contemp. Clin. Trials Commun. 2018, 12, 17–25. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.; Mangnuson, B.; Wolker-Riev, U.; Jeppesen, P.; Rogers, P.; Rowland, I.; Mathews, R. Stevia Leaf to Stevia sweetener: exploring its science, benefits, and future potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.; Ylmaz, S.; Yilmaz, S.; Sefa Kilis, M. A research on the genotoxicity of Stevia in human lymphocytes. Drug Chem. Toxicol. 2018, 41, 221–224. [Google Scholar] [CrossRef]
- Marquez, G.R.; Mariniello, L.; Di Pierro, P.; Esposito, M. Fresh-cut fruit and vegetables coatings by transglutaminase – crosslinked whey protein/pectin edible films. LWT – Food Sci. Technol. 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Phan, T.; Debeaufort, D.; Voilley, A.; Luu, D. Biopolymer interaction affects the functional properties of edible films based on agar, cassava starch and arabynoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar] [CrossRef]
- ASTM D882—Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Available online: http://mahshahr.aut.ac.ir/lib/exe/fetch.php?media=labs:astm_d882.pdf (accessed on 21 January 2019).
- Puscaselu, R.; Amariei, S. The application of the Peleg model in order to obtain completely soluble materials for food product packaging. JAPR 2018, 10, 98–106. [Google Scholar]
- Wang, L.F.; Rhim, J.W. Preparation and application of agar/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Orsuwan, A.; Shankar, S.; Wang, L.F.; Sothornvit, R.; Rhim, J.W. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocol. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena Bustillos, R.J.; Azeredo, H.; Lorevice, M.; Moura, M.; Mattoso, L.; McHugh, L. Recent advances on edible films based on fruits and vegetables – A review. Compr. Rev. Food Sci. Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Saifullah, M.; Yusof, Y.A.; Chin, L.; Aziz, M.; Mohammed, M.; Aziz, N. Tableting and dissolution characteristics on mixed fruit powder. Agric. Agric. Sci. Procedia 2014, 2, 18–25. [Google Scholar] [CrossRef][Green Version]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Controll. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Kopacic, S.; Walzi, A.; Zankel, A.; Leitner, E.; Bauer, W. Alginate and chitosan as a functional barrier for paper-based packaging materials. Coatings 2018, 8, 235. [Google Scholar] [CrossRef]
- Murrieta-Martinez, C.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodriguez-Felix, F.; Ramirez-Wong, B.; Santacruz-Ortega, H.; Santos-Sauceda, I.; Olibarria-Rodriguez, G.; Marquez-Rios, E. Effect of different polyalchols as plasticizers on the functional properties of squid protein films. Coatings 2019, 9, 77. [Google Scholar] [CrossRef]
- Guttierez, T. Active and intelligent films made from starchy sources /blackberry pulp. J. Polym. Environ. 2018, 26, 2374–2391. [Google Scholar] [CrossRef]
- Puscaselu, R.; Amariei, S. The antibacterial properties of seaweed biopolymer-based films incorporated with essential oils. JAPT 2017, 27, 157–163. [Google Scholar]

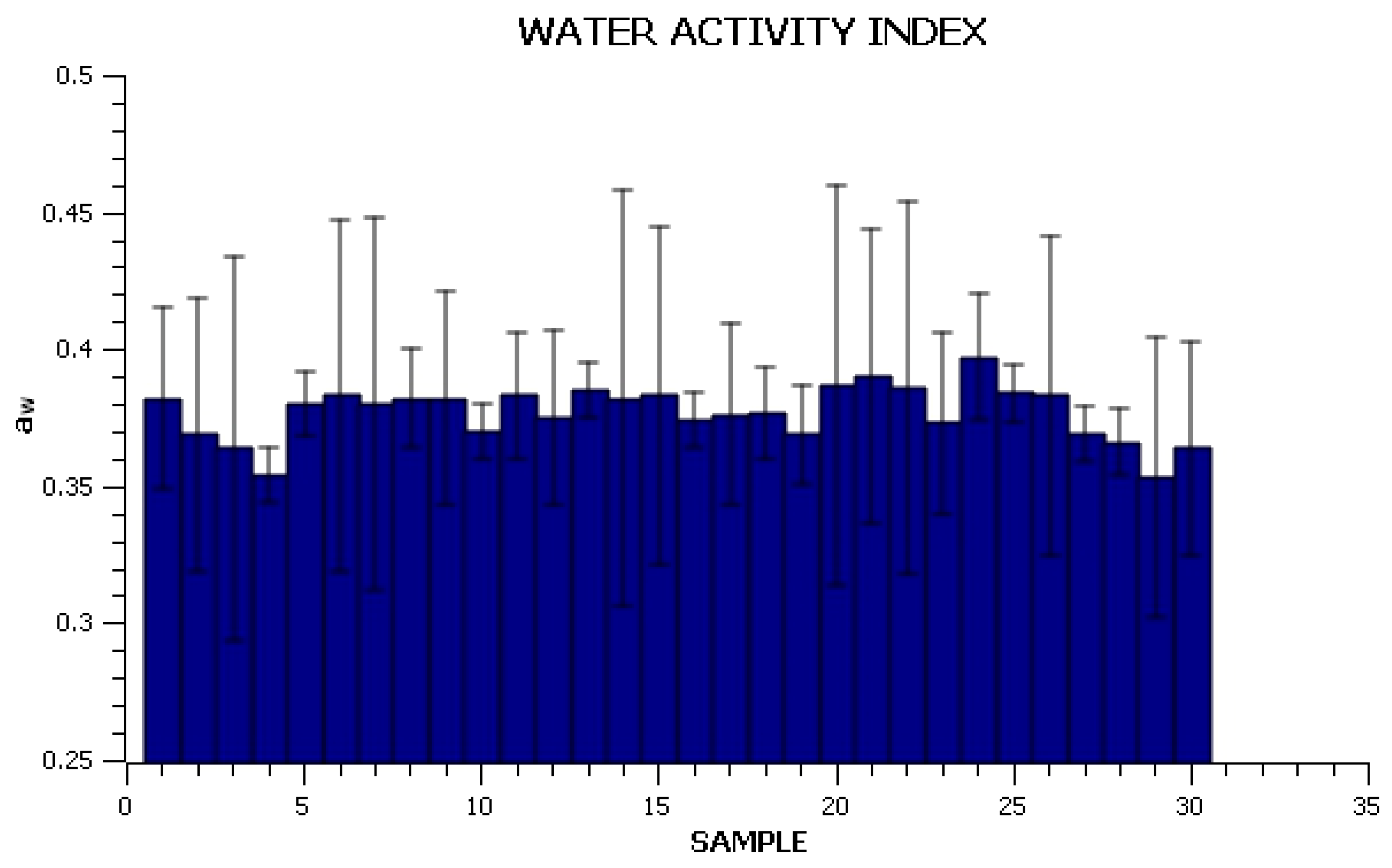
| SAMPLE | mAGAR, (g) | mALGINATE, (g) | mSTEVIA, (g) | mGLYCEROL, (g) | VAPĂ, (mL) |
|---|---|---|---|---|---|
| S1 | 2.95 | 0.00 | 0.05 | 1.00 | 150.00 |
| S2 | 0.00 | 2.95 | 1.00 | ||
| S3 | 1.00 | 1.95 | 1.00 | ||
| S4 | 1.95 | 1.00 | 1.00 | ||
| S5 | 2.00 | 0.95 | 1.00 | ||
| S6 | 0.95 | 2.00 | 1.00 | ||
| S7 | 0.50 | 2.45 | 1.00 | ||
| S8 | 2.45 | 0.50 | 1.00 | ||
| S9 | 1.25 | 1.70 | 1.00 | ||
| S10 | 1.70 | 1.25 | 1.00 | ||
| S11 | 3.45 | 0.00 | 0.50 | ||
| S12 | 0.00 | 3.45 | 0.50 | ||
| S13 | 1.00 | 2.45 | 0.50 | ||
| S14 | 2.45 | 1.00 | 0.50 | ||
| S15 | 2.00 | 1.45 | 0.50 | ||
| S16 | 1.45 | 2.00 | 0.50 | ||
| S17 | 1.50 | 1.95 | 0.50 | ||
| S18 | 1.95 | 1.50 | 0.50 | ||
| S19 | 1.25 | 2.20 | 0.50 | ||
| S20 | 2.20 | 1.25 | 0.50 | ||
| S21 | 1.00 | 2.20 | 0.75 | ||
| S22 | 2.20 | 1.00 | 0.75 | ||
| S23 | 1.50 | 1.70 | 0.75 | ||
| S24 | 1.70 | 1.50 | 0.75 | ||
| S25 | 2.00 | 1.20 | 0.75 | ||
| S26 | 1.20 | 2.00 | 0.75 | ||
| S27 | 1.25 | 1.95 | 0.75 | ||
| S28 | 1.95 | 1.25 | 0.75 | ||
| S29 | 1.60 | 1.60 | 0.75 | ||
| S30 | 1.225 | 1.225 | 1.50 |
| T | Rr | TS | E | Rz | Tr | L* | a* | b* | |
|---|---|---|---|---|---|---|---|---|---|
| T | 1 | 0.905(**) | 0.850(**) | 0.853(**) | 0.351 | −0.600(*) | −0.657(*) | 0.067 | 0.589(*) |
| Rr | 1 | 0.999(**) | 0.343 | −0.245 | 0.127 | 0.013 | −0.352 | 0.066 | |
| TS | 1 | 0.340 | −0.249 | 0.132 | 0.127 | −0.354 | 0.077 | ||
| E | 1 | −0.265 | 0.267 | 0.234 | −0.102 | −0.250 | |||
| Rz | 1 | −0.347 | −0.317 | 0.101 | 0.358 | ||||
| Tr | 1 | 0.743(*) | −0.081 | −0.322 | |||||
| L* | 1 | −0.245 | −0.751(**) | ||||||
| a* | 1 | −0.016 | |||||||
| b* | 1 |
| Sample | Appearance | Microstructure | ||
|---|---|---|---|---|
| S2 With very small pores; no cracks, high homogeneity and full solubility of the biopolymers. |  |  | 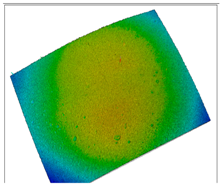 |  |
| S17 Homogeneous membrane, without pores, cracks, and non-solubilized ingredients. |  |  | 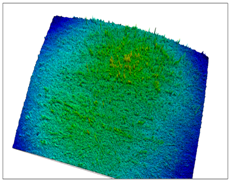 |  |
| S22 High porosity area (pore diameter 8.52–13.3 nm and depth 142–305 nm) and irregular appearance. Negative particles are deposited on the film structure. |  | 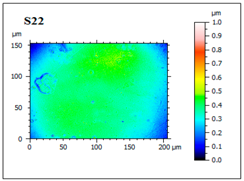 |  | 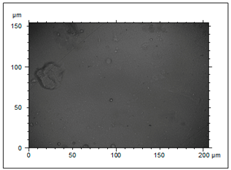 |
| S24 Membrane with superior characteristics; although it has asperities on the surface, the structure is more homogeneous. |  | 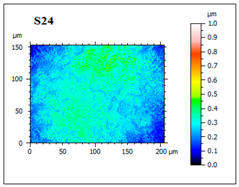 |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puscaselu, R.; Gutt, G.; Amariei, S. Biopolymer-Based Films Enriched with Stevia rebaudiana Used for the Development of Edible and Soluble Packaging. Coatings 2019, 9, 360. https://doi.org/10.3390/coatings9060360
Puscaselu R, Gutt G, Amariei S. Biopolymer-Based Films Enriched with Stevia rebaudiana Used for the Development of Edible and Soluble Packaging. Coatings. 2019; 9(6):360. https://doi.org/10.3390/coatings9060360
Chicago/Turabian StylePuscaselu, Roxana, Gheorghe Gutt, and Sonia Amariei. 2019. "Biopolymer-Based Films Enriched with Stevia rebaudiana Used for the Development of Edible and Soluble Packaging" Coatings 9, no. 6: 360. https://doi.org/10.3390/coatings9060360
APA StylePuscaselu, R., Gutt, G., & Amariei, S. (2019). Biopolymer-Based Films Enriched with Stevia rebaudiana Used for the Development of Edible and Soluble Packaging. Coatings, 9(6), 360. https://doi.org/10.3390/coatings9060360





