Crack-Resistance Behavior of an Encapsulated, Healing Agent Embedded Buffer Layer on Self-Healing Thermal Barrier Coatings
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Healing Agent Powder Encapsulation Process
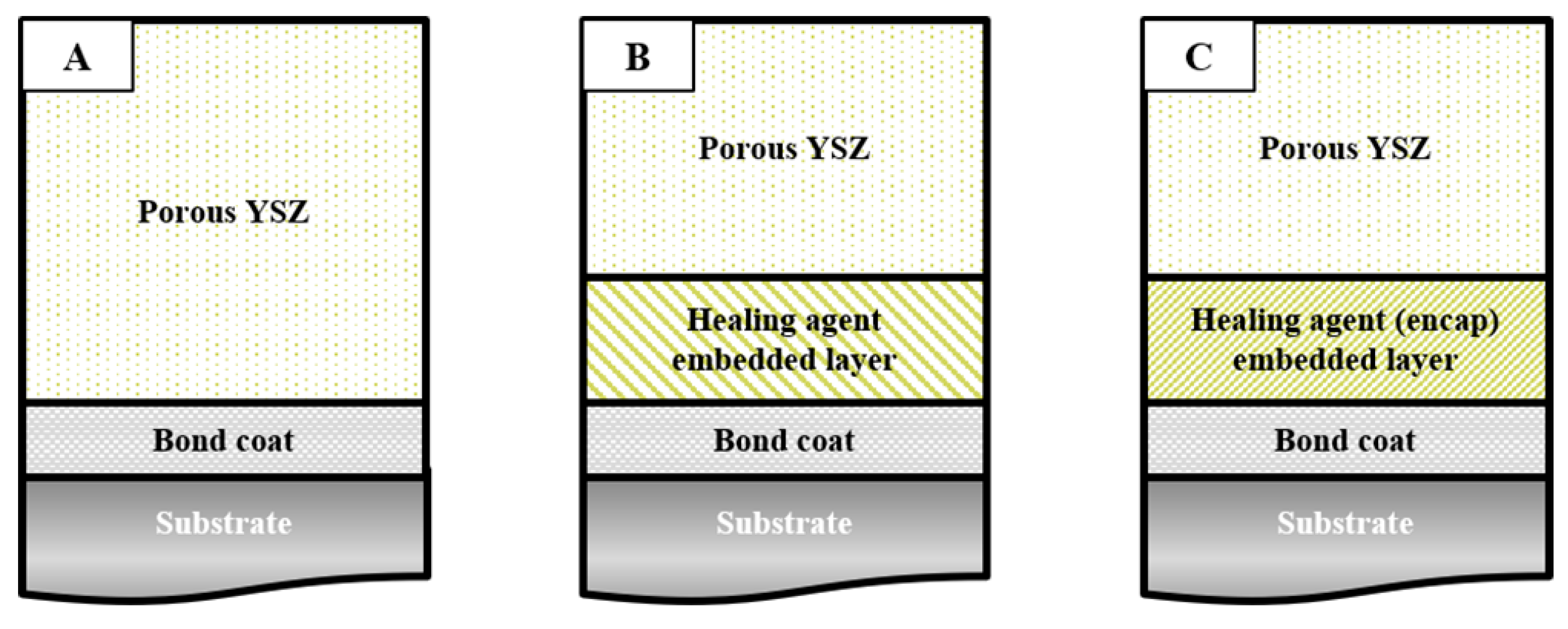
2.2. Coating Fabrication
2.3. Thermal Durability Evaluation and Analysis
3. Results and Discussion
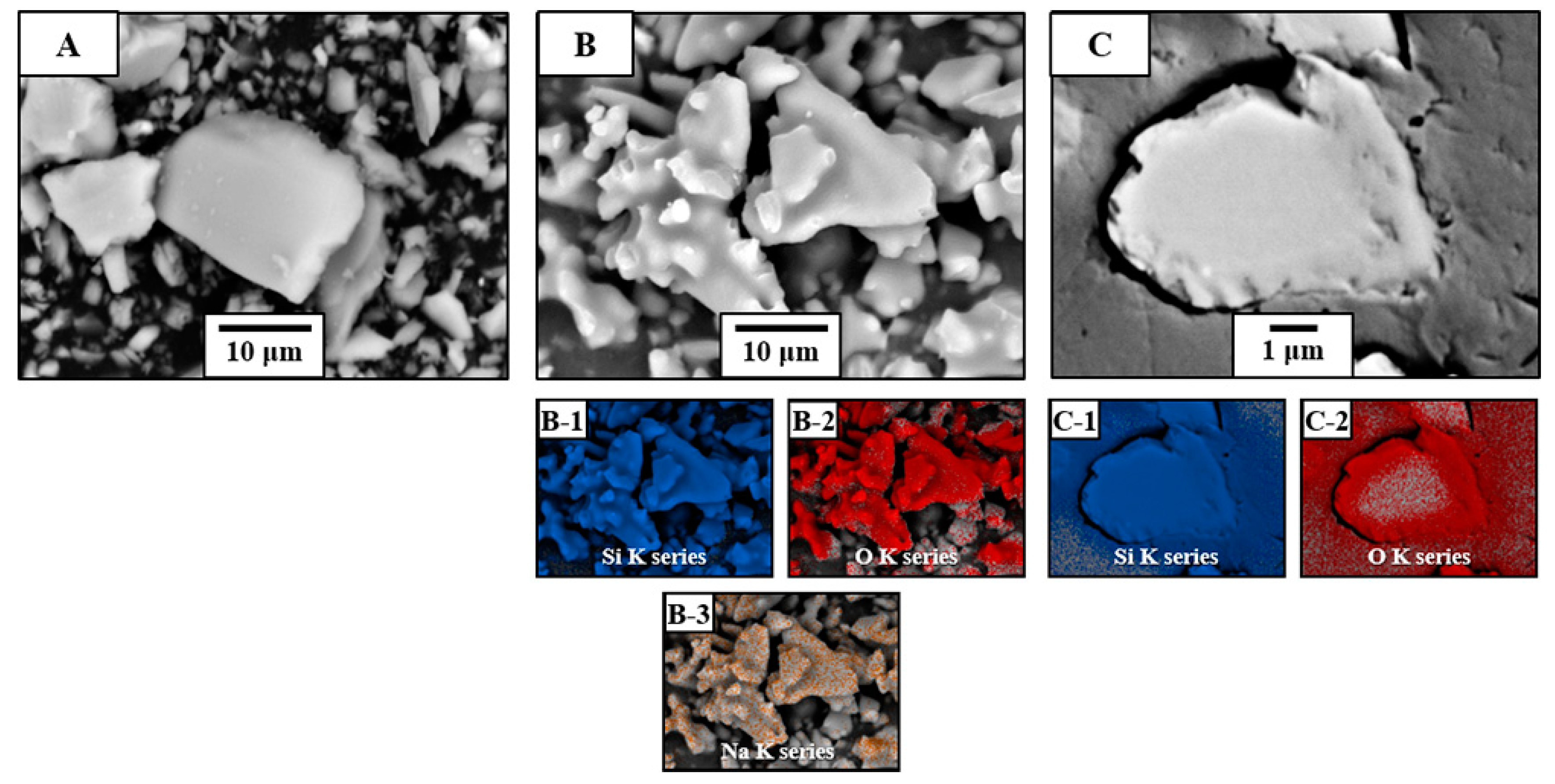
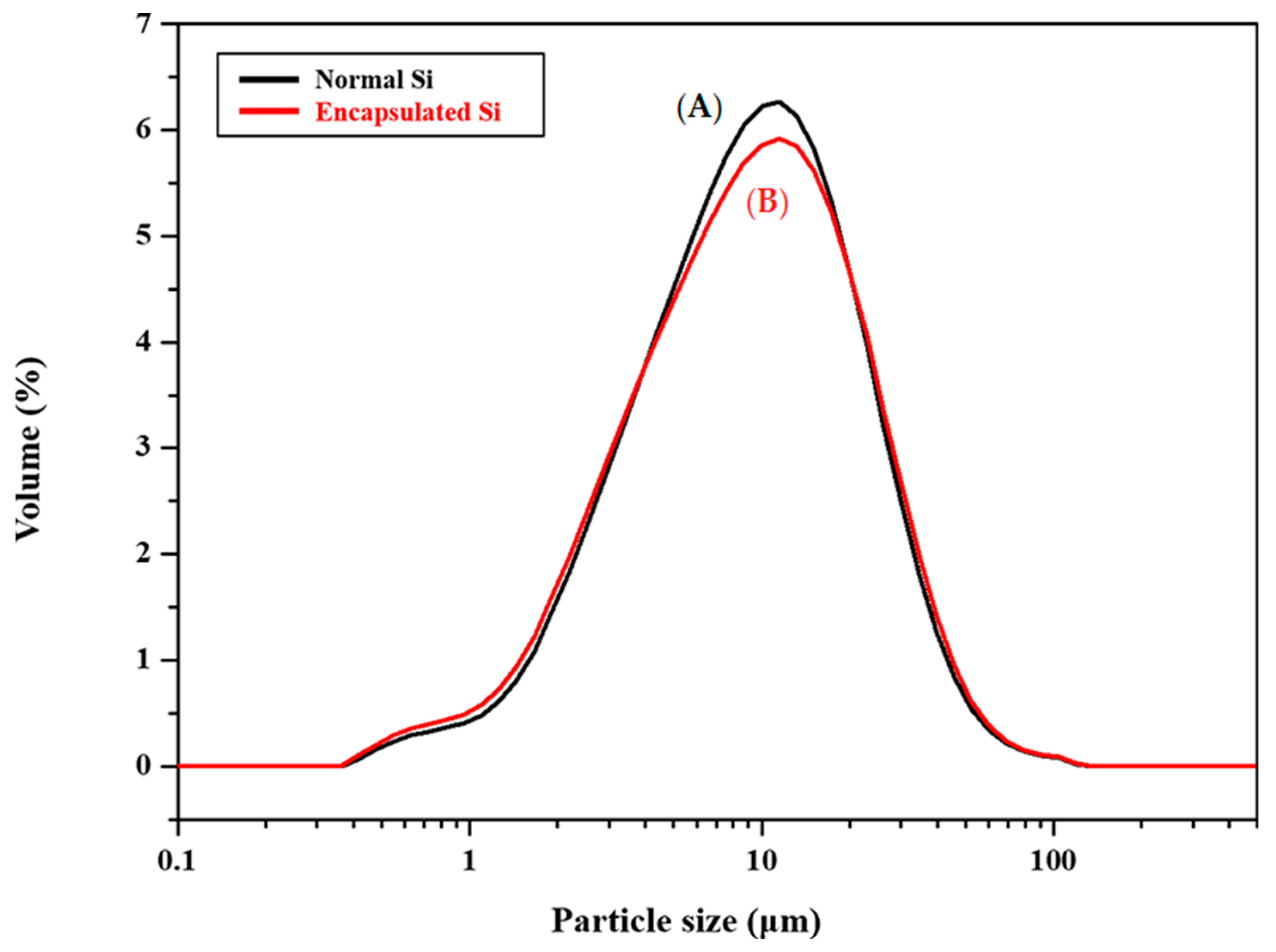
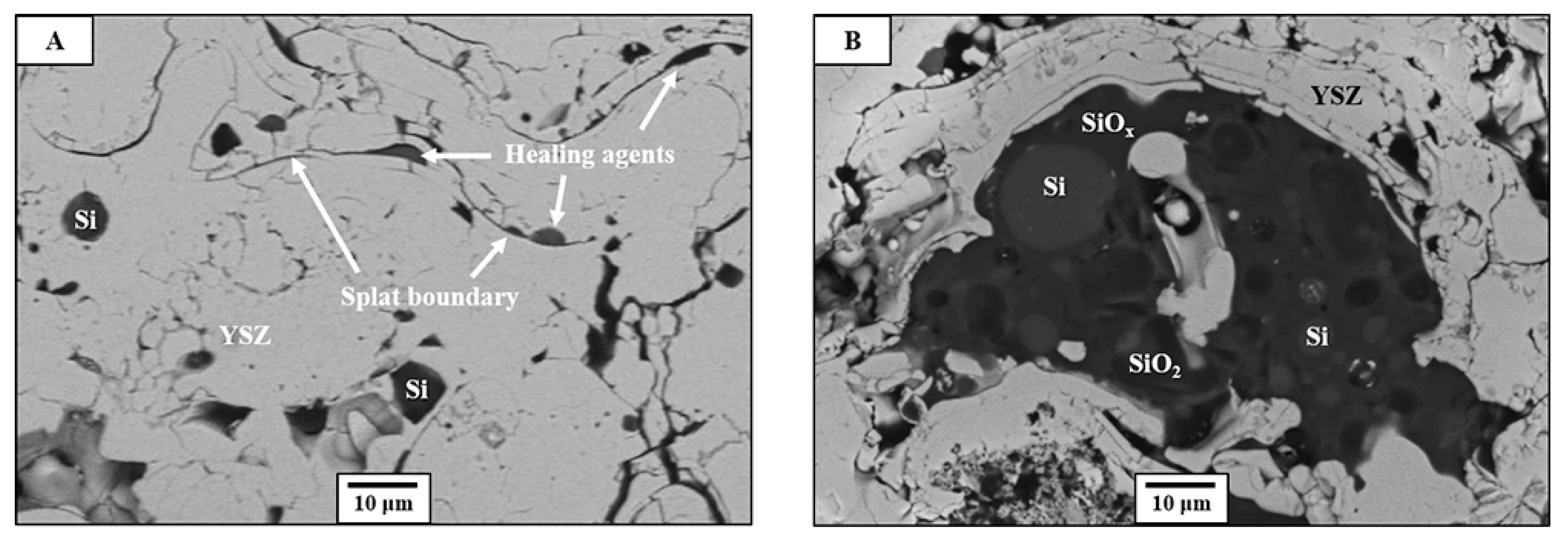
3.1. Protection of Healing Agents and Resistance Against Premature Oxidation
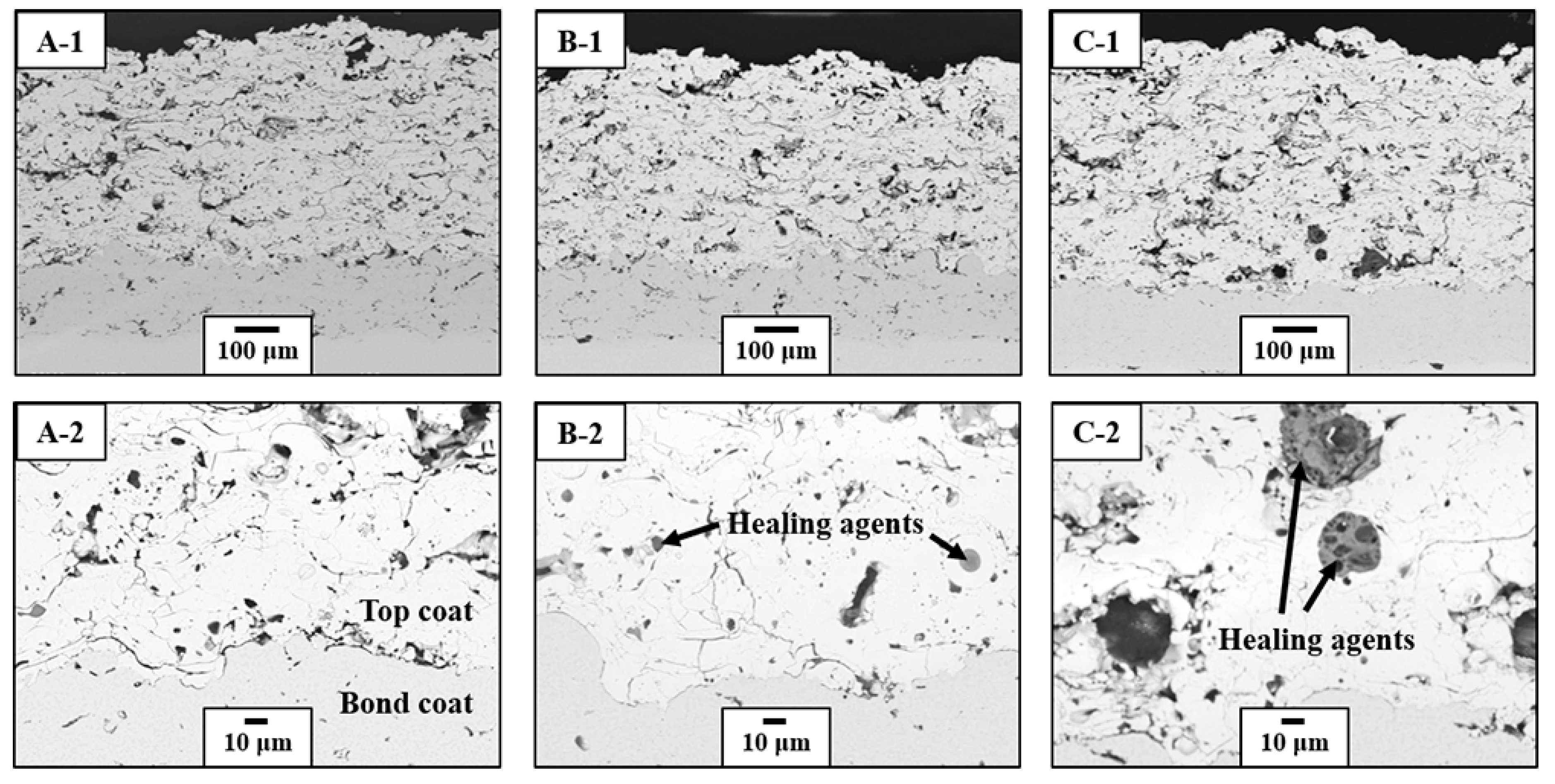
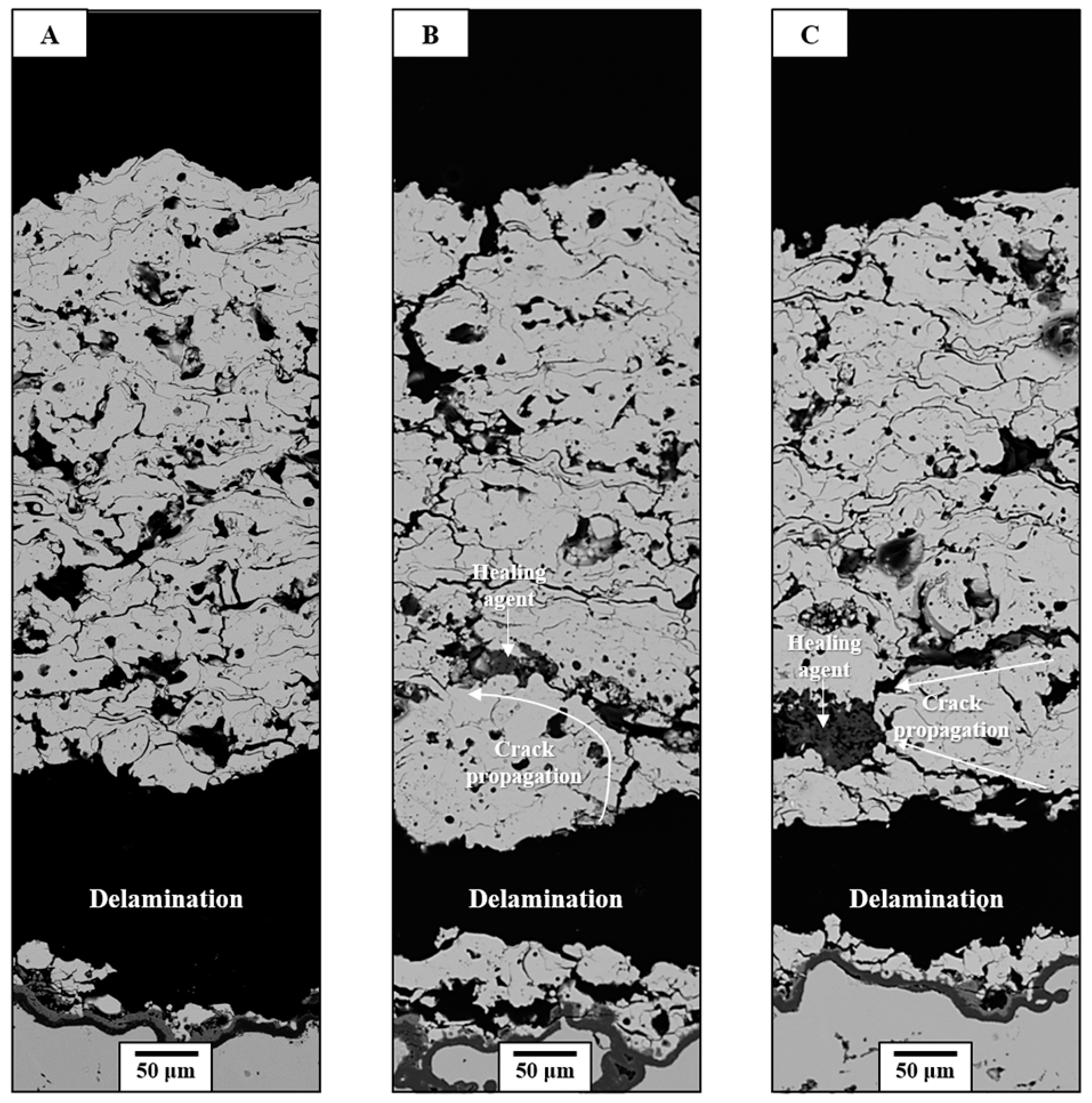
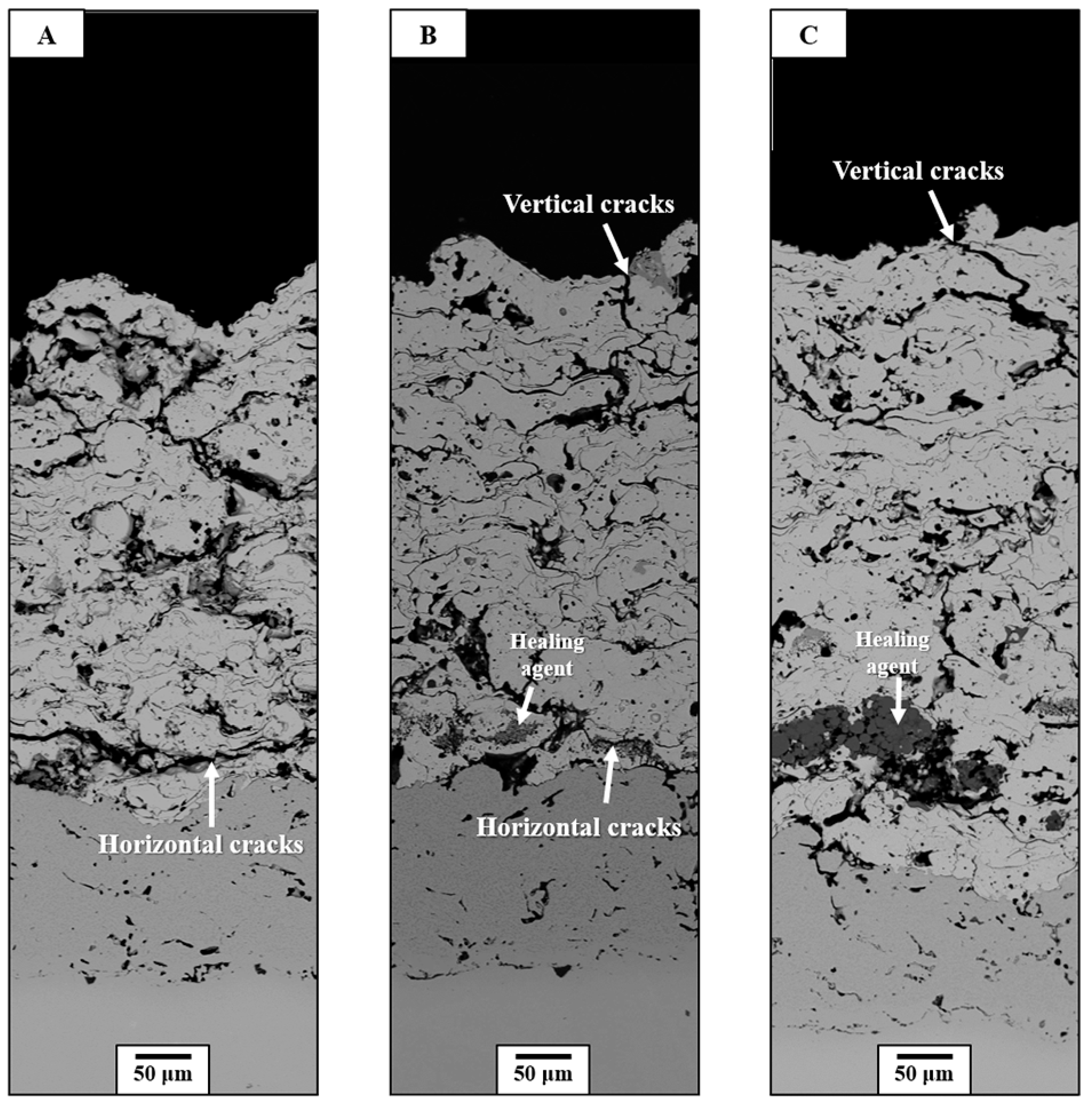
3.2. Thermal Durability Evaluation
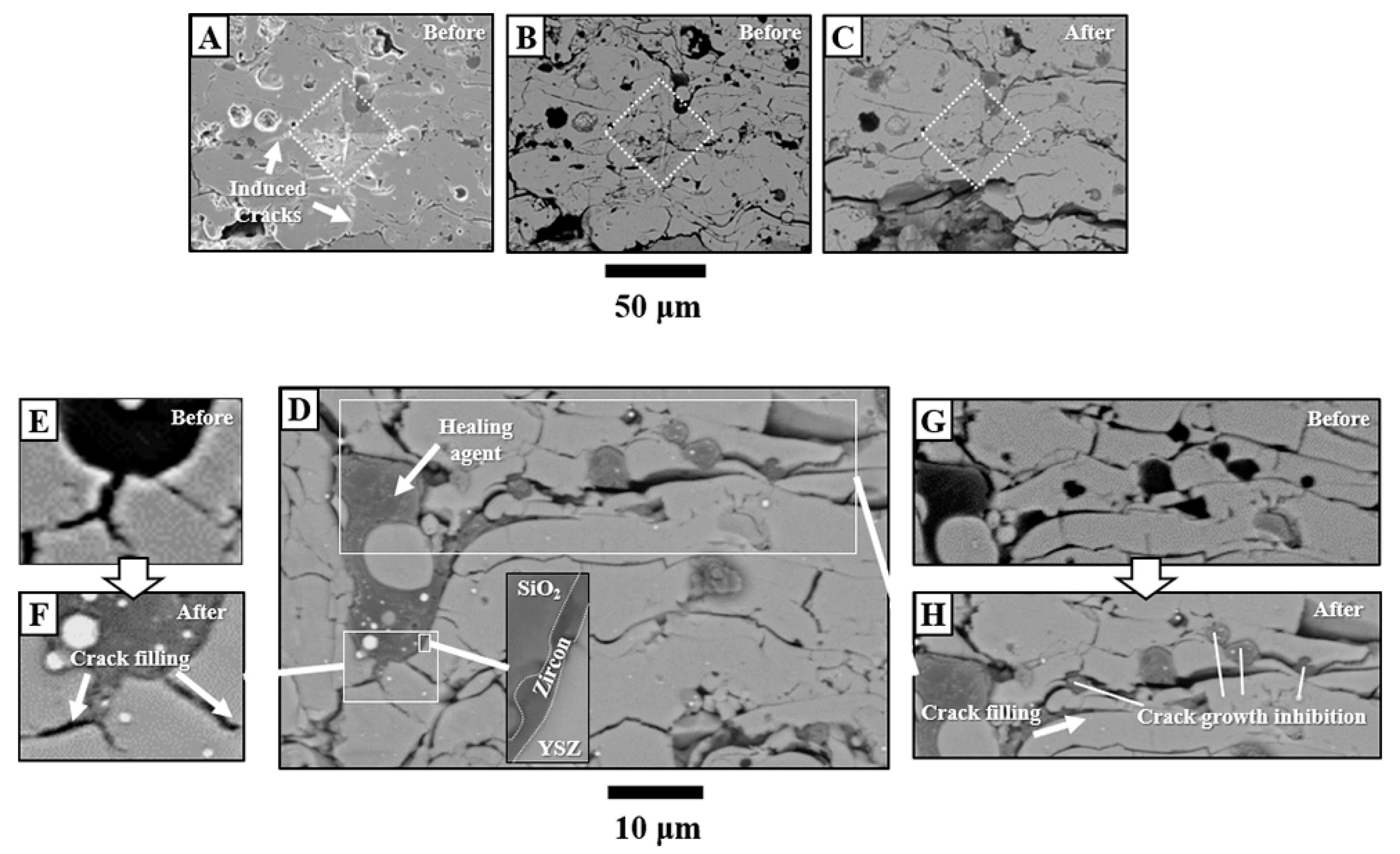
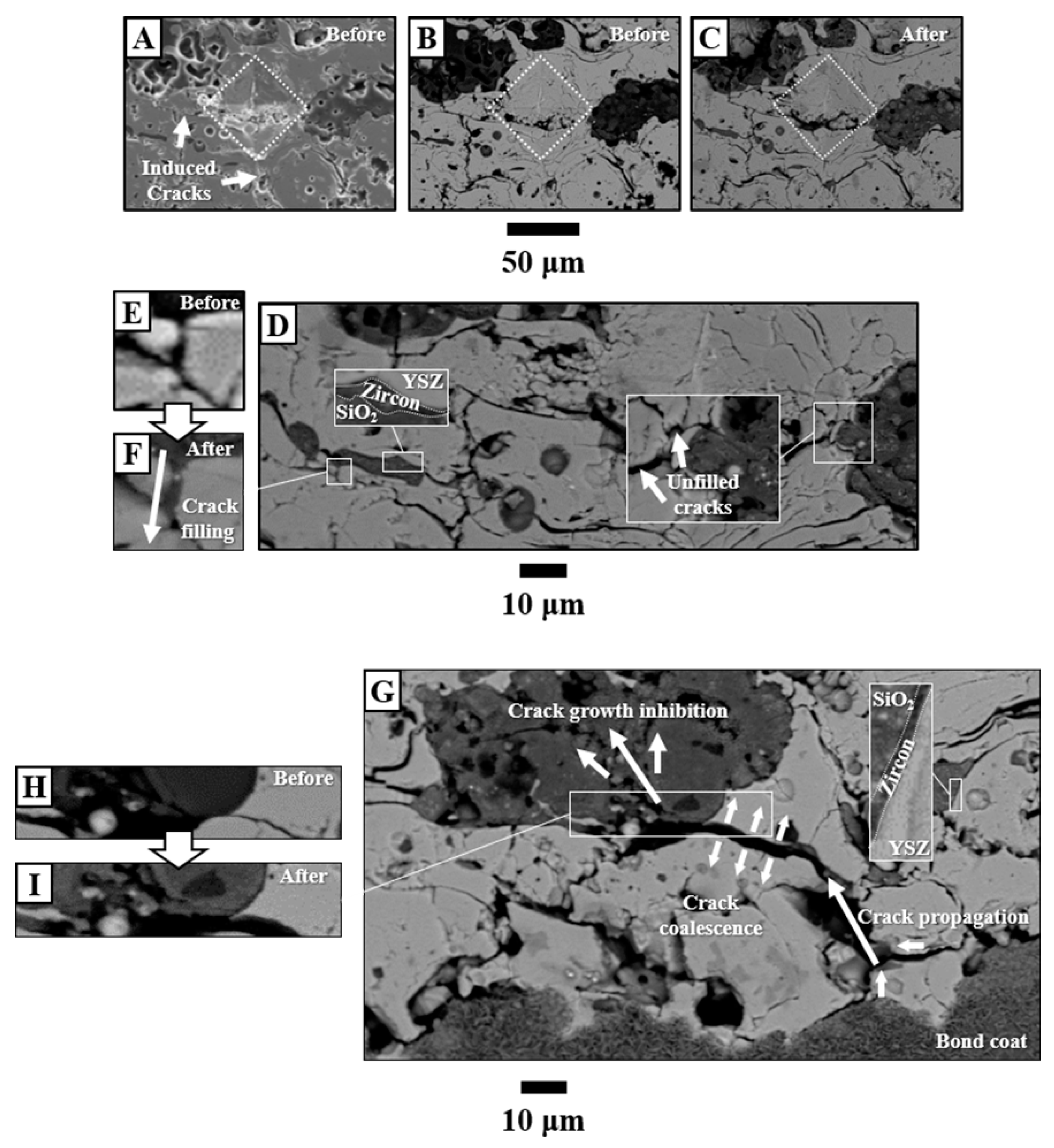
3.3. Crack-Resisting Mechanisms
4. Conclusions
- An encapsulation process is developed to cover the healing agents, preventing them from premature oxidation or evaporation during coating fabrication.
- The TBCs with a crack-healing buffer layer show comparable thermal durability in both the CTF and JETS tests.
- Crack-resisting mechanisms are elaborated on depending on the distribution and shape of healing agents through the observation of crack-healing behaviors.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miller, R.A. Current status of thermal barrier coatings—An overview. Surf. Coat. Technol. 1987, 30, 1–11. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas—Turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.; Hutchinson, J.; Meier, G.; Pettit, F. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Clarke, D.; Levi, C. Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Strangman, T.E. Thermal barrier coatings for turbine airfoils. Thin Solid Films 1985, 127, 93–106. [Google Scholar] [CrossRef]
- Haynes, J.A.; Ferber, M.; Porter, W. Thermal cycling behavior of plasma—Sprayed thermal barrier coatings with various MCrAlX bond coats. J. Therm. Spray Technol. 2000, 9, 38. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, N.; Wang, Z.; Gong, S.; Xu, H. Thermal cycling life and thermal diffusivity of a plasma—Sprayed nanostructured thermal barrier coating. Scr. Materialia 2004, 51, 945–948. [Google Scholar] [CrossRef]
- Cao, X.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Vaßen, R.; Kerkhoff, G.; Stöver, D. Development of a micromechanical life prediction model for plasma sprayed thermal barrier coatings. Mater. Sci. Eng. A 2001, 303, 100–109. [Google Scholar] [CrossRef]
- Hasselman, D.; Johnson, L.F.; Bentsen, L.D.; SYED, R.; LEE, H.L.; SWAIN, M.V. Thermal diffusivity and conductivity of dense polycrystalline Zr0. Am. Ceram. Soc. Bull 1987, 66, 799–806. [Google Scholar]
- Hayashi, H.; Saitou, T.; Maruyama, N.; Inaba, H.; Kawamura, K.; Mori, M. Thermal expansion coefficient of yttria stabilized zirconia for various yttria contents. Solid State Ion. 2005, 176, 613–619. [Google Scholar] [CrossRef]
- Cheng, J.; Jordan, E.; Barber, B.; Gell, M. Thermal/residual stress in an electron beam physical vapor deposited thermal barrier coating system. Acta Mater. 1998, 46, 5839–5850. [Google Scholar] [CrossRef]
- Pan, D.; Chen, M.; Wright, P.; Hemker, K. Evolution of a diffusion aluminide bond coat for thermal barrier coatings during thermal cycling. Acta Mater. 2003, 51, 2205–2217. [Google Scholar] [CrossRef]
- Czech, N.; Esser, W.; Schmitz, F. Effect of environment on mechanical properties of coated superalloys and gas turbine blades. Mater. Sci. Technol. 1986, 2, 244–249. [Google Scholar] [CrossRef]
- Tamura, M.; Takahashi, M.; Ishii, J.; Suzuki, K.; Sato, M.; Shimomura, K. Multilayered thermal barrier coating for land—Based gas turbines. J. Therm. Spray Technol. 1999, 8, 68–72. [Google Scholar] [CrossRef]
- Sumner, I.; Ruckle, D. Development of improved—Durability plasma sprayed ceramic coatings for gas turbine engines. In Proceedings of the 16th Joint Propulsion Conference, Hartford, CT, USA, 30 June–2 July 1980. [Google Scholar]
- Chang, G.C.; Phucharoen, W.; Miller, R.A. Behavior of thermal barrier coatings for advanced gas turbine blades. Surf. Coat. Technol. 1987, 30, 13–28. [Google Scholar] [CrossRef]
- Chang, G.; Phucharoen, W.; Miller, R. Finite element thermal stress solutions for thermal barrier coatings. Surf. Coat. Technol. 1987, 32, 307–325. [Google Scholar] [CrossRef]
- Taylor, T.A. Thermal barrier coating for substrates and process for producing it. U.S. Patent 5,073,433, 17 December 1991. [Google Scholar]
- Duvall, D.; Ruckle, D. Ceramic thermal barrier coatings for turbine engine components. In Proceedings of the ASME 1982 International Gas Turbine Conference and Exhibit, London, UK, 18–22 April 1982. [Google Scholar]
- Musil, J.; Fiala, J. Plasma spray deposition of graded metal—Ceramic coatings. Surf. Coat. Technol. 1992, 52, 211–220. [Google Scholar] [CrossRef]
- Schulz, U.; Leyens, C.; Fritscher, K.; Peters, M.; Saruhan-Brings, B.; Lavigne, O.; Dorvaux, J.-M.; Poulain, M.; Mévrel, R.; Caliez, M. Some recent trends in research and technology of advanced thermal barrier coatings. Aerosp. Sci. Technol. 2003, 7, 73–80. [Google Scholar] [CrossRef]
- Johnson, C.; Ruud, J.; Bruce, R.; Wortman, D. Relationships between residual stress, microstructure and mechanical properties of electron beam—Physical vapor deposition thermal barrier coatings. Surf. Coat. Technol. 1998, 108, 80–85. [Google Scholar] [CrossRef]
- Padture, N.; Schlichting, K.; Bhatia, T.; Ozturk, A.; Cetegen, B.; Jordan, E.; Gell, M.; Jiang, S.; Xiao, T.; Strutt, P. Towards durable thermal barrier coatings with novel microstructures deposited by solution—Precursor plasma spray. Acta Mater. 2001, 49, 2251–2257. [Google Scholar] [CrossRef]
- Kassner, H.; Siegert, R.; Hathiramani, D.; Vassen, R.; Stoever, D. Application of suspension plasma spraying (SPS) for manufacture of ceramic coatings. J. Therm. Spray Technol. 2008, 17, 115–123. [Google Scholar] [CrossRef]
- Guignard, A.; Mauer, G.; Vaßen, R.; Stöver, D. Deposition and characteristics of submicrometer—Structured thermal barrier coatings by suspension plasma spraying. J. Therm. Spray Technol. 2012, 21, 416–424. [Google Scholar] [CrossRef]
- Derelioglu, Z.; Carabat, A.; Song, G.; van der Zwaag, S.; Sloof, W. On the use of B-alloyed MoSi2 particles as crack healing agents in yttria stabilized zirconia thermal barrier coatings. J. Eur. Ceram. Soc. 2015, 35, 4507–4511. [Google Scholar] [CrossRef]
- Carabat, A.; Meijerink, M.; Brouwer, J.; Kelder, E.; van Ommen, J.; van der Zwaag, S.; Sloof, W. Protecting the MoSi2 healing particles for thermal barrier coatings using a sol-gel produced Al2O3 coating. J. Eur. Ceram. Soc. 2018, 38, 2728–2734. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; van der Zwaag, S.; Sloof, W.G.; Xiao, P. Damage evolution in a self-healing air plasma sprayed thermal barrier coating containing self-shielding MoSi2 particles. J. Am. Ceram. Soc. 2019, 102, 16313. [Google Scholar] [CrossRef]
- Ouyang, T.; Fang, X.; Zhang, Y.; Liu, D.; Wang, Y.; Feng, S.; Zhou, T.; Cai, S.; Suo, J. Enhancement of high temperature oxidation resistance and spallation resistance of SiC-self-healing thermal barrier coatings. Surf. Coat. Technol. 2016, 286, 365–375. [Google Scholar] [CrossRef]
- Scott, H. Phase relationships in the zirconia-yttria system. J. Mater. Sci. 1975, 10, 1527–1535. [Google Scholar] [CrossRef]
- Veytizou, C.; Quinson, J.-F.; Valfort, O.; Thomas, G. Zircon formation from amorphous silica and tetragonal zirconia: Kinetic study and modelling. Solid State Ion. 2001, 139, 315–323. [Google Scholar] [CrossRef]
- Nozahic, F.; Monceau, D.; Estournès, C. Thermal cycling and reactivity of a MoSi2/ZrO2 composite designed for self-healing thermal barrier coatings. Mater. Des. 2016, 94, 444–448. [Google Scholar] [CrossRef]
- Kim, E.-H.; Lee, W.-R.; Jung, Y.-G.; Lee, C.-S. A new binder system for preparing high strength inorganic molds in precision casting. Mater. Chem. Phys. 2011, 126, 344–351. [Google Scholar] [CrossRef]
- Kim, E.-H.; Cho, G.-H.; Jung, Y.-G.; Kim, I.-S.; Jo, C.-Y.; Lee, J.-S. Adhesion phenomena between particles according to the content of organic binder in core for thin-wall casting. J. Nanosci. Nanotechnol. 2014, 14, 8048–8052. [Google Scholar] [CrossRef]
- Jung, Y.-G.; Tumenbayar, E.; Choi, H.-H.; Park, H.-Y.; Kim, E.-H.; Zhang, J. Effects of alumina precursor species in a ternary—Phase binder system on the strength of sand mold. Ceram. Int. 2018, 44, 2223–2230. [Google Scholar] [CrossRef]
- Park, H.-M.; Jun, S.-H.; Lyu, G.; Jung, Y.-G.; Yan, B.-I.; Park, K.-Y. Thermal durability of thermal barrier coatings in furnace cyclic thermal fatigue test: Effects of purity and monoclinic phase in feedstock powder. J. Korean Ceram. Soc. 2018, 55, 608–617. [Google Scholar] [CrossRef]
- Park, K.-Y.; Yang, B.-I.; Jeon, S.-H.; Park, H.-M.; Jung, Y.-G. Variation of thermal barrier coating lifetime characteristics with thermal durability evaluation methods. J. Therm. Spray Technol. 2018, 27, 1436–1446. [Google Scholar] [CrossRef]
- Laha, T.; Agarwal, A.; McKechnie, T.; Seal, S. Synthesis and characterization of plasma spray formed carbon nanotube reinforced aluminum composite. Mater. Sci. Eng. A 2004, 381, 249–258. [Google Scholar] [CrossRef]
- Koch, D.; Mauer, G.; Vaßen, R. Manufacturing of composite coatings by atmospheric plasma spraying using different feed-stock materials as YSZ and mosi 2. J. Therm. Spray Technol. 2017, 26, 708–716. [Google Scholar] [CrossRef]
- Ritchie, R.O. Mechanisms of fatigue—Crack propagation in ductile and brittle solids. Int. J. Fract. 1999, 100, 55–83. [Google Scholar] [CrossRef]
- Ponnusami, S.A.; Turteltaub, S.; van der Zwaag, S. Cohesive-zone modelling of crack nucleation and propagation in particulate composites. Eng. Fract. Mech. 2015, 149, 170–190. [Google Scholar] [CrossRef]
- Ojovan, M.I. Glass formation in amorphous SiO2 as a percolation phase transition in a system of network defects. J. Exp. Theor. Phys. Lett. 2004, 79, 632–634. [Google Scholar] [CrossRef]
- Song, D.; Paik, U.; Guo, X.; Zhang, J.; Woo, T.-K.; Lu, Z.; Jung, S.-H.; Lee, J.-H.; Jung, Y.-G. Microstructure design for blended feedstock and its thermal durability in lanthanum zirconate based thermal barrier coatings. Surf. Coat. Technol. 2016, 308, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Nozahic, F.; Estournès, C.; Carabat, A.L.; Sloof, W.G.; Van Der Zwaag, S.; Monceau, D. Self-healing thermal barrier coating systems fabricated by spark plasma sintering. Mater. Des. 2018, 143, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Seward, T.P., III; Vascott, T. High Temperature Glass Melt Property Database for Process Modeling; Wiley-American Ceramic Society: Westerville, OH, USA, 2005. [Google Scholar]
- Fluegel, A. Glass viscosity calculation based on a global statistical modelling approach. Glass Technol.-Eur. J. Glass Sci. Technol. Part A 2007, 48, 13–30. [Google Scholar]


| Parameter | Gun Type | Current (A) | Primary Gas, Ar (L/min) | Secondary Gas, H2 (L/min) | Powder Feed Rate (g/min) | Spray Distance (mm) | Gun Speed (mm/s) |
|---|---|---|---|---|---|---|---|
| Top coat | METCO-3MB | 480 | 23.596 | 5.663 | 40 | 80 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Song, T.; Paik, U.; Lyu, G.; Jung, Y.-G.; Choi, B.-G.; Kim, I.-S.; Zhang, J. Crack-Resistance Behavior of an Encapsulated, Healing Agent Embedded Buffer Layer on Self-Healing Thermal Barrier Coatings. Coatings 2019, 9, 358. https://doi.org/10.3390/coatings9060358
Song D, Song T, Paik U, Lyu G, Jung Y-G, Choi B-G, Kim I-S, Zhang J. Crack-Resistance Behavior of an Encapsulated, Healing Agent Embedded Buffer Layer on Self-Healing Thermal Barrier Coatings. Coatings. 2019; 9(6):358. https://doi.org/10.3390/coatings9060358
Chicago/Turabian StyleSong, Dowon, Taeseup Song, Ungyu Paik, Guanlin Lyu, Yeon-Gil Jung, Baig-Gyu Choi, In-Soo Kim, and Jing Zhang. 2019. "Crack-Resistance Behavior of an Encapsulated, Healing Agent Embedded Buffer Layer on Self-Healing Thermal Barrier Coatings" Coatings 9, no. 6: 358. https://doi.org/10.3390/coatings9060358
APA StyleSong, D., Song, T., Paik, U., Lyu, G., Jung, Y.-G., Choi, B.-G., Kim, I.-S., & Zhang, J. (2019). Crack-Resistance Behavior of an Encapsulated, Healing Agent Embedded Buffer Layer on Self-Healing Thermal Barrier Coatings. Coatings, 9(6), 358. https://doi.org/10.3390/coatings9060358






