Effect of Chitosan–Tomato Plant Extract Edible Coating on the Quality, Shelf Life, and Antioxidant Capacity of Pork during Refrigerated Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pork and Plant Material
2.3. Chitosan
2.4. Preparation of Extracts
2.5. Preparation and Application of EC
2.6. Microbiological Analysis
2.7. Physicochemical Analysis
2.8. Antioxidant Capacity
2.9. Sensory Evaluation
2.10. Experimental Design and Statistical Analysis
3. Results and Discussion
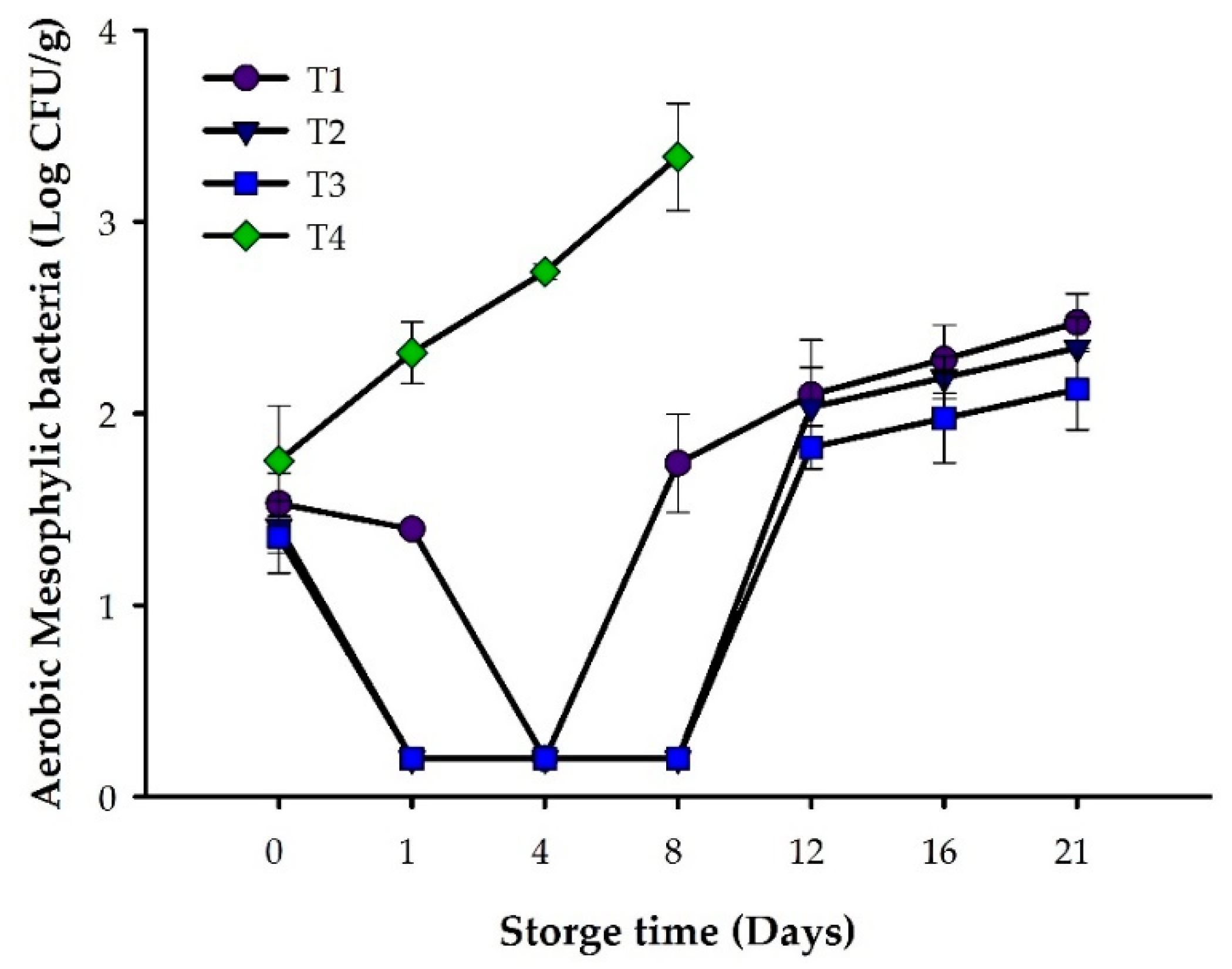
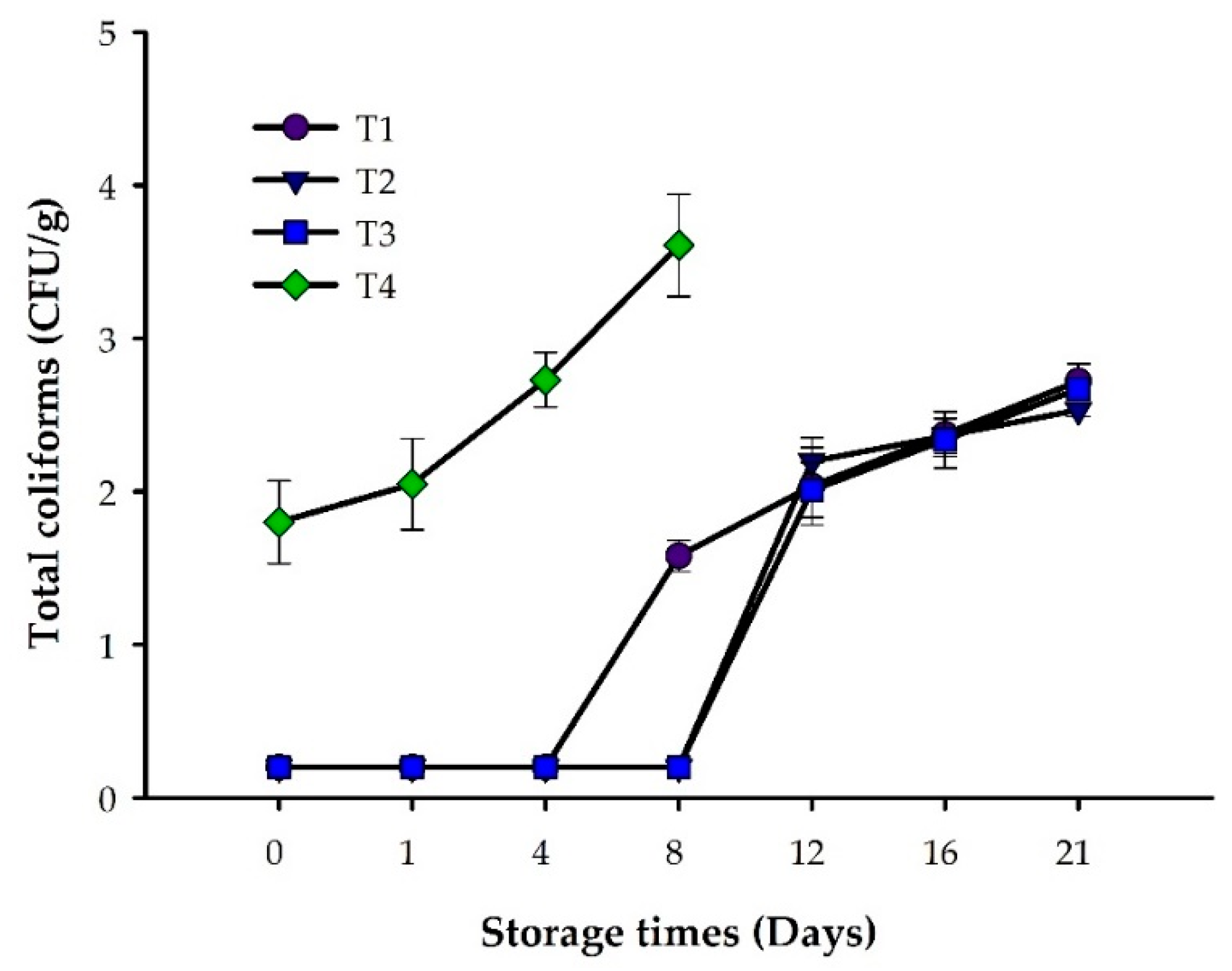
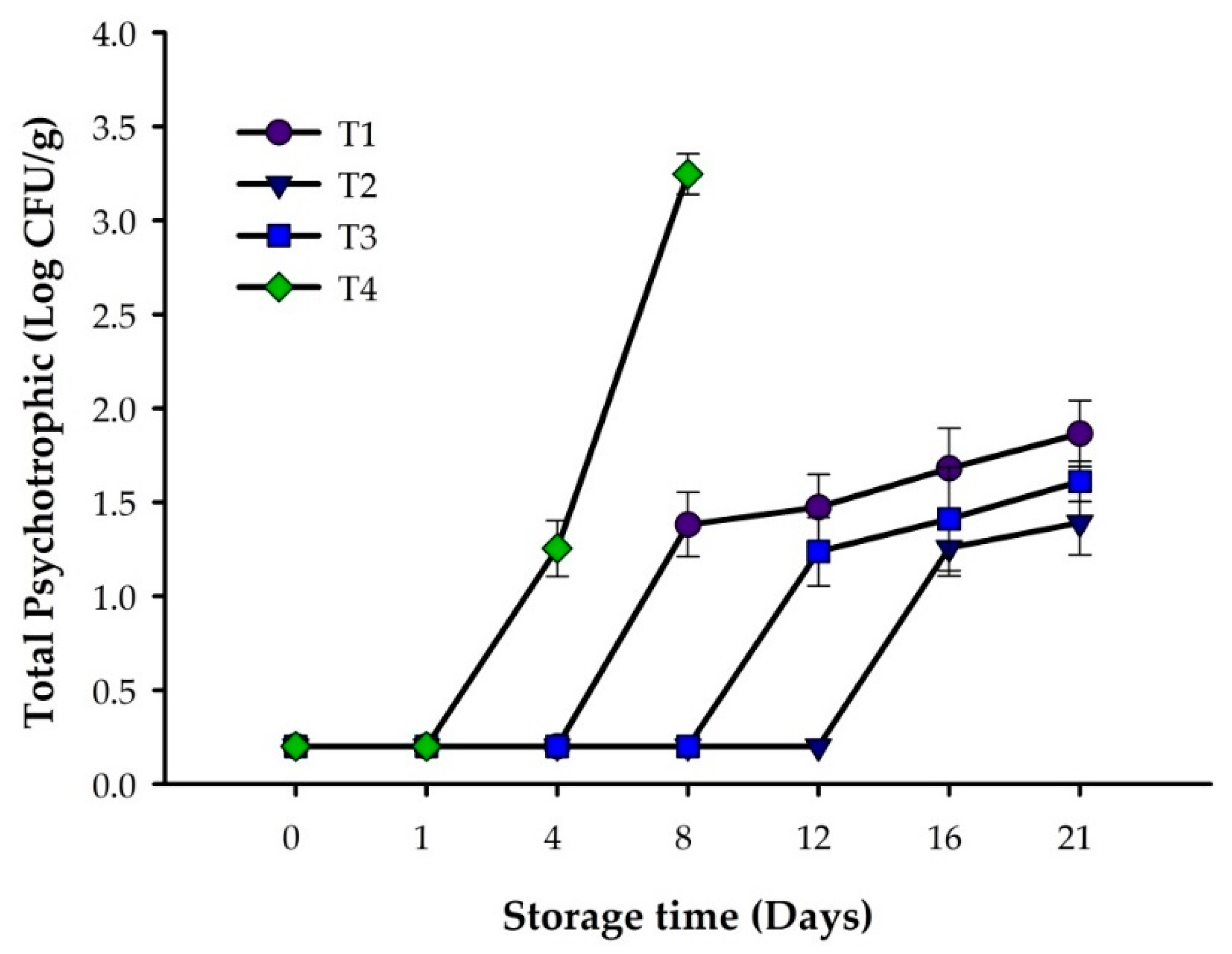
3.1. Microbiological Analysis
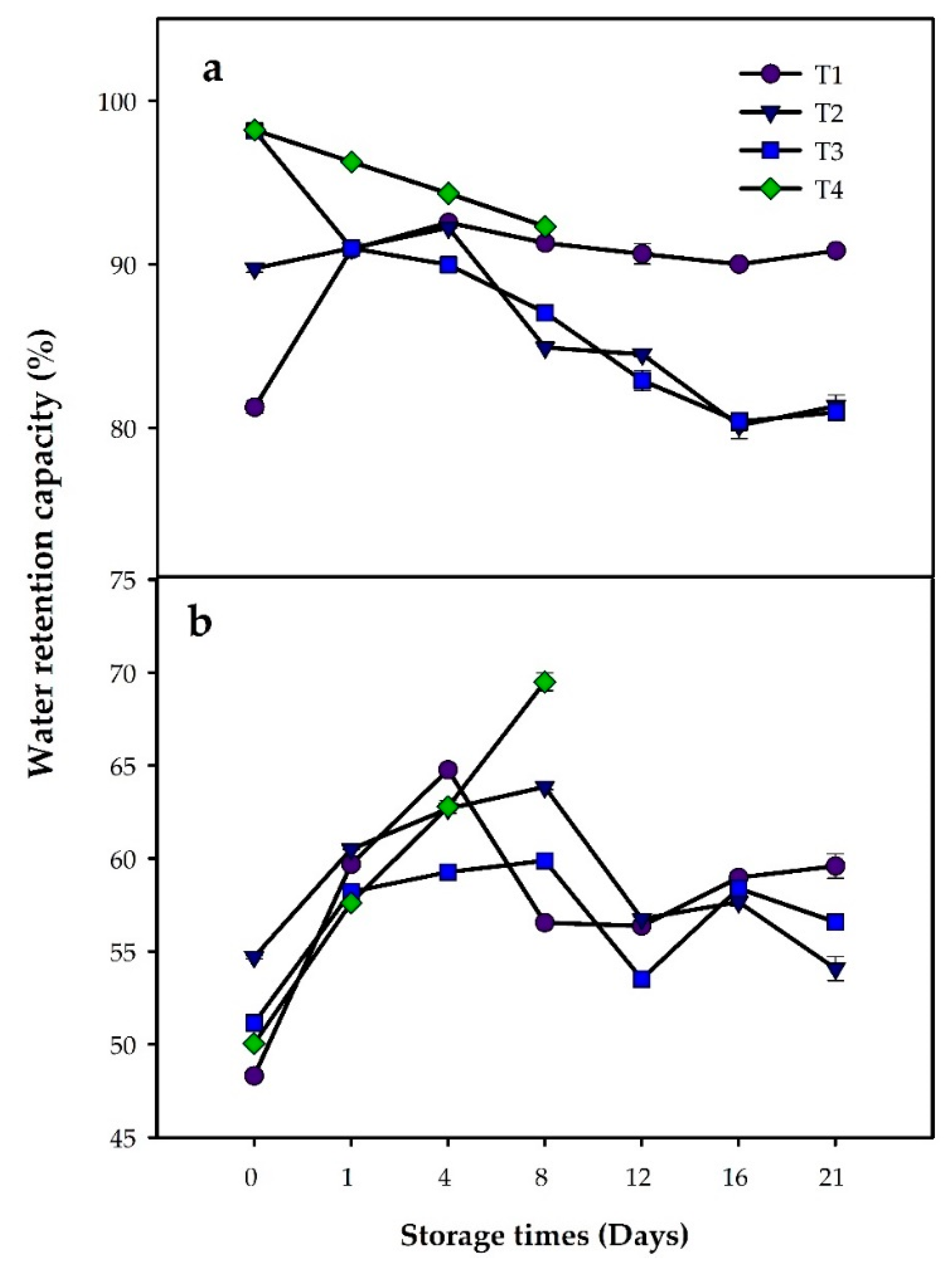
3.2. Physicochemical Analysis
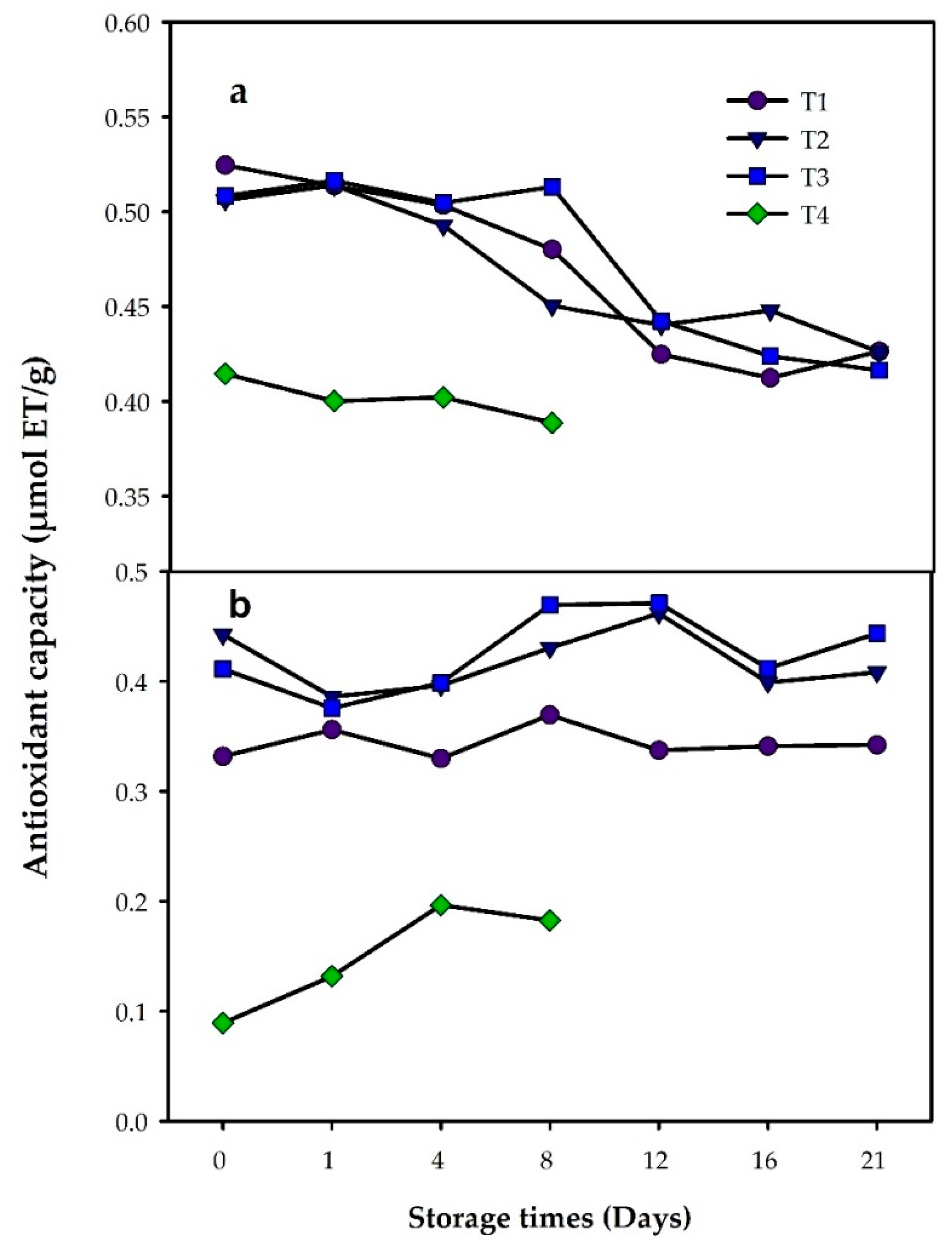
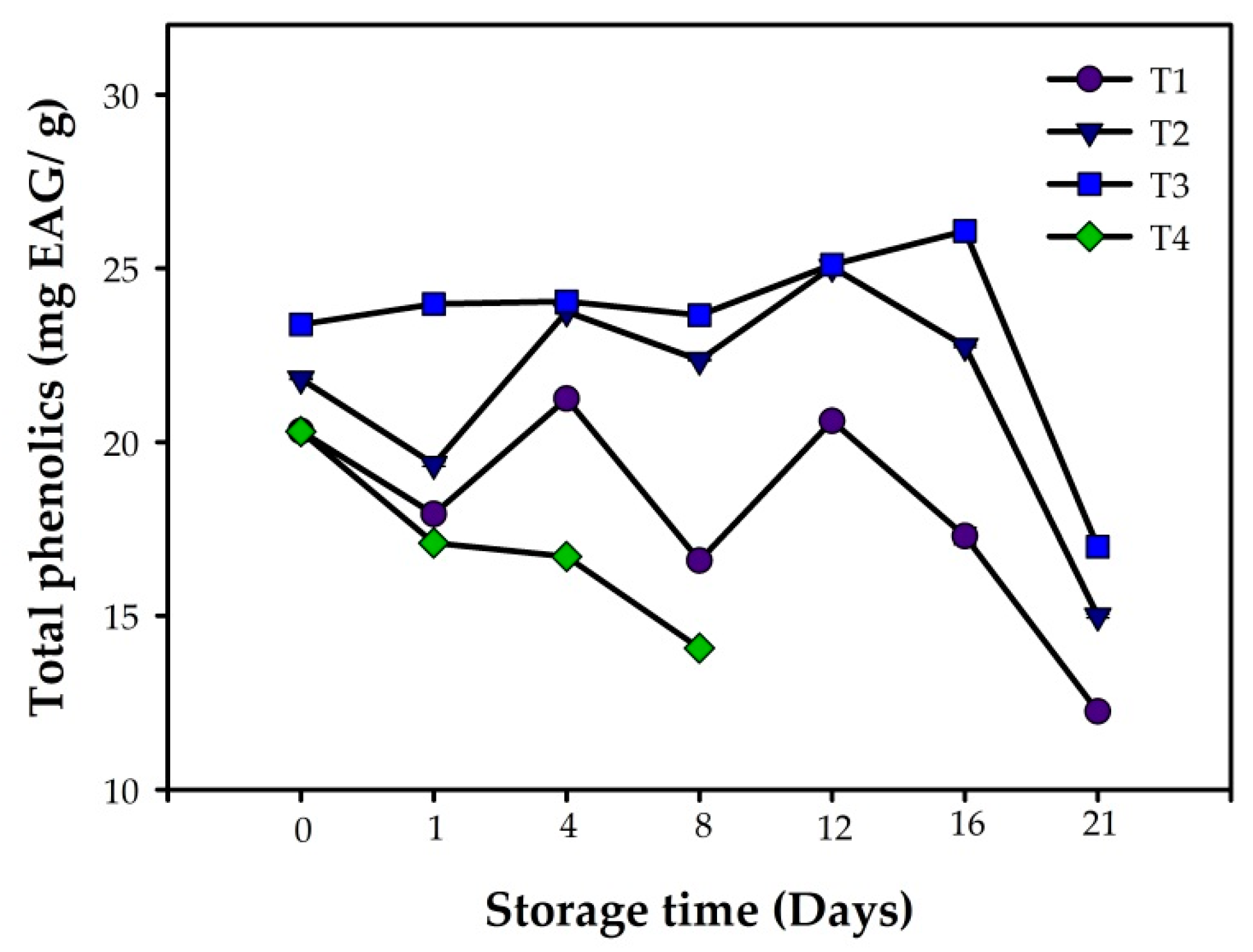
3.3. Antioxidant Capacity and Total Phenolic Compounds
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Agriculture and Consumer Protection Department. Animal Production and Health. Available online: http://www.fao.org/ag/againfo/themes/en/pigs/home.html (accessed on 2 September 2019).
- National Nutrient Database for Standard Reference. ARS-USDA. Available online: http://ndb.nal.usda.gov/ndb/foods (accessed on 4 April 2019).
- Barbut, S.; Sosnicki, A.A.; Lonergan, S.M.; Knapp, T.; Ciobanu, D.C.; Gatcliffe, L.J.; Huff-Lonergan, E.; Wilson, E.W. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 2008, 79, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, O.; Panagou, E.Z.; Tassou, C.C.; Nychas, G.J.E. Contribution of Fourier transform infrared (FTIR) spectroscopy data on the quantitative determination of minced pork meat spoilage. Food Res. Int. 2011, 44, 3264–3271. [Google Scholar] [CrossRef]
- Fernández-López, J.; Sevilla, L.; Sayas-Barberá, E.; Navarro, C.; Marín, F.; Pérez-Álvarez, J.A. Evaluation of the antioxidant potential of hyssop (Hyssopus officinalis L.) and Rosemary (Rosmarinus officinalis L.) extracts in cooked pork meat. J. Food Sci. 2003, 68, 660–664. [Google Scholar] [CrossRef]
- Estevéz, M.; Ramírez, R.; Ventanas, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. LWT-Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O‘Sullivan, M.; Dowling, D.P.; Monahan, F.J. PET trays coated with Citrus extract exhibit antioxidant activity with cooked turkey meat. LWT-Food Sci. Technol. 2012, 47, 471–477. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT-Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Rajalakshmi, A.; Krithiga, N.; Jayachitra, A. Antioxidant Activity of the Chitosan Extracted from Shrimp Exoskeleton. Middle-East J. Sci. Res. 2013, 16, 1446–1451. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food Chem. 2013, 141, 1655–1660. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruíz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef]
- Chaparro-Hernández, S.; Ruiz-Cruz, S.; Márquez-Ríos, E.; Ocaño-Higuera, V.E.; Valenzuela-López, C.C.; Ornelas-Paz, J.J.; Del-Toro-Sánchez, C.L. Effect of chitosan-carvacrol edible coatings on the quality and shelf life of tilapia (Oreochromis niloticus) fillets stored in ice. Food Sci. Technol. 2015, 35, 734–741. [Google Scholar] [CrossRef]
- Jafari, N.J.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J. Food Process. Preserv. 2017, 42, e13336. [Google Scholar] [CrossRef]
- Ruiz-Cruz, S.; Valenzuela-López, C.C.; Chaparro-Hernández, S.; Ornelas-Paz, J.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Ocaño-Higuera, V.M.; Valdez-Hurtado, S. Effects of chitosan-tomato plant extract edible coatings on the quality and shelf life of chicken fillets during refrigerated storage. LWT-Food Sci. Technol. 2019, 39, 103–111. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.J.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Cira-Chávez, L.A.; Del-Toro-Sánchez, C.L.; Shirai, K. Physicochemical and Antioxidant Properties of Chitosan Films Incorporated with Cinnamon Oil. Int. J. Polym. Sci. 2015, 2015, 8. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; Ornelas-Paz, J.J.; Cira-Chávez, L.A.; Silva-Beltrán, N.P. Antibacterial and antioxidant properties of edible chitosan coatings incorporated with essential oils. Int. J. Pharma Bio Sci. 2015, 6, 251–264. [Google Scholar]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez-Quiróz, C.; Ornelas-Paz, J.J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada-Alvarado, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 10. [Google Scholar] [CrossRef]
- Ramírez-Guerra, H.; Castillo-Yañez, F.; Montaño-Cota, E.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Canizales-Rodríguez, D.; Torres-Arreola, W.; Montoya-Camacho, N.; Ocaño-Higuera, V.M. Protective effect of an edible tomato plant extract/chitosan coating on the quality and shelf life of sierra fish fillets. J. Chem. 2018, 6, 2436045. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial edible films and coatings for meat and meat products preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef]
- Hajer, A.; Khaoula, K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Yousefi, M.; Azizi, M.; Ehsani, A. Antimicrobial coatings and films on meats: A perspective on the application of antimicrobial edible films or coatings on meats from the past to future. Bali Med. J. 2018, 7, 87–96. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lim, G.O.; Song, K.B. Physical properties of Gelidium corneum-gelatin blend films containing grapefruit seed extract or green tea extract and its application in the packaging of pork loins. J. Food Sci. 2009, 74, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Antoniewski, M.N.; Barringer, S.; Knipe, C.; Zerby, H. Effect of a gelatin coating on the shelf life of fresh meat. J. Food Sci. 2007, 72, E382–E387. [Google Scholar] [CrossRef] [PubMed]
- Chantarasataporn, P.; Tepkasikul, P.; Kingcha, Y.; Yoksan, R.; Pichyangkura, R.; Visessanguan, W.; Chirachanchai, S. Water-based oligochitosan and nanowhisker chitosan as potential food preservatives for shelf-life extensión of minced pork. Food Chem. 2014, 159, 463–470. [Google Scholar] [CrossRef]
- He, S.; Yang, Q.; Ren, X.; Zi, J.; Lu, S.; Wang, S.; Zhang, Y.; Wang, Y. Antimicrobial efficiency of chitosan solutions and coatings incorporated with clove oil and/or ethylenediaminetetraacetate. J. Food Saf. 2014, 34, 345–352. [Google Scholar] [CrossRef]
- Soultos, N.; Tzikas, Z.; Abrahim, A.; Georgantelis, D.; Ambrosiadis, I. Chitosan effects on quality properties of Greek style of fresh pork sausages. Meat Sci. 2008, 80, 1150–1156. [Google Scholar]
- Khanafari, A.; Marandi, R.; Sanaeti, S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran. J. Environ. Health. Sci. Eng. 2008, 5, 19–24. Available online: http://ijehse.tums.ac.ir/index.php/ijehse/article/download/145/144 (accessed on 4 June 2019).
- Norma Oficial Mexicana. NOM-092-SSA1-1994. Bienes y Servicios. Método Para La Cuenta De Bacterias Aerobias En Placa; Diario Oficial de la Federación: México, México, 1994; Available online: http://www.salud.gob.mx/unidades/cdi/nom/092ssa14.html (accessed on 24 January 2018).
- Norma Oficial Mexicana. NOM-113-SSA1-1994. Bienes Y Servicios. Método Para La Cuenta De Organismos Coliformes Totales En Placa; Diario Oficial de la Federación: México, México, 1994; Available online: http://www.salud.gob.mx/unidades/cdi/nom/112ssa14.html (accessed on 24 January 2018).
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Zhang, M.; Mittal, G.S.; Barbut, S. Effects of tests conditions on the water holding capacity of meat by a centrifugal method. LWT-Food Sci. Technol. 1995, 28, 50–55. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Qwele, K.; Hugo, A.; Oyedemi, S.O.; Moyo, B.; Masika, P.J.; Muchenge, V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 2013, 93, 455–462. [Google Scholar] [CrossRef]
- Lee, K.; Choi, W.; Yoon, C. Effects of micro-perforated film on the quality and shelf life improvements of pork loins during chilled storage. Meat Sci. 2003, 66, 77–82. [Google Scholar] [CrossRef]
- Chang, H.L.; Chen, Y.C.; Tan, F.J. Antioxidative properties of a chitosan–glucose Maillard reaction product and its effect on pork qualities during refrigerated storage. Food Chem. 2011, 124, 589–595. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan glucose complex–A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Chmiel, M.; Słowiński, M.; Dasiewicz, K. Lightness of the color measured by computer image analysis as a factor for assessing the quality of pork meat. Meat Sci. 2011, 88, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Lee, S.; Yoon, J.; Hong, K.; Kang, Y.; Park, S.; Park, S.K.; Ha, S.; Kim, G.; Bae, D. Inhibition of pork and fish oxidation by a novel plastic film coated with horseradish extract. LWT-Food Sci. Technol. 2009, 42, 856–861. [Google Scholar] [CrossRef]
- Juncher, D.; Ronn, B.; Mortensen, E.; Henckel, P.; Karlsson, A.; Skibsted, L.; Bertelsen, G. Effect of pre-slaughter physiological conditions on the oxidative stability of colour and lipid during chill storage of pork. Meat Sci. 2001, 58, 347–357. [Google Scholar] [CrossRef]
- Lahucky, R.; Nuernberg, K.; Kovac, L.; Bucko, O.; Nuernberg, G. Assessment of the antioxidant potential of selected plant extracts–In vitro and in vivo experiments on pork. Meat Sci. 2010, 85, 779–784. [Google Scholar] [CrossRef]
- Lindahl, G.; Henckel, P.; Karlson, A.; Andersen, H. Significance of early postmortem temperature and pH decline on colour characteristics of pork loin from different crossbreeds. Meat Sci. 2006, 72, 613–623. [Google Scholar] [CrossRef]
- Kristensen, L.; Purslow, P.P. The effect of ageing on the water-holding capacity of pork: Role of cytoeskeletal proteins. Meat Sci. 2001, 58, 17–23. [Google Scholar] [CrossRef]
- Bertram, H.C.; Andersen, H.J.; Karlsson, A.H.; Horn, P.; Hedegaard, J.; Norgaard, L.; Engelsen, S.B. Prediction of technological quality (cooking loss and Napole Yield) of pork based on fresh meat characteristics. Meat Sci. 2003, 65, 707–712. [Google Scholar] [CrossRef]
- Straadt, I.K.; Rasmussen, M.; Andersen, H.J.; Bertram, H.C. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss–A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007, 75, 687–695. [Google Scholar] [CrossRef]
- Kılıç, B.; Şimşek, A.; Claus, J.R.; Atilgan, E. Encapsulated phosphates reduce lipid oxidation in both ground chicken and ground beef during raw and cooked meat storage with some influence on color, pH, and cooking loss. Meat Sci. 2014, 97, 93–103. [Google Scholar] [CrossRef]
- Yingyuad, S.; Ruamsin, S.; Reekprkhon, D.; Douglas, S.; Pongamphai, S.; Siripatrawan, U. Effect of chitosan coating and vacuum packaging on the quality of refrigerated grilled pork. Packag. Technol. Sci. 2006, 19, 149–157. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xu, H.; Xue, Y.; Huang, R.; Deng, H.; Pan, S. Layer-by-layer inmobilization of lysozyme-chitosan-organic rectorite composites on electrospun nanofibrous mats of pork preservation. Food Res. Int. 2012, 48, 784–791. [Google Scholar] [CrossRef]
- Biswas, A.K.; Chatli, M.K.; Sahoo, J. Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem. 2012, 133, 467–472. [Google Scholar] [CrossRef]
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.; Montes-Villanueva, N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011, 88, 397–403. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef]
- Qi, S.; Huang, H.; Huang, J.; Wang, Q.; Wei, Q. Lychee (Litchi sinensis Sonn.) seed water extract as potential antioxidant and anti-obese natural additive in meat products. Food Control. 2015, 50, 195–201. [Google Scholar] [CrossRef]

| Parameter | Treatment | Storage Time (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 8 | 12 | 16 | 21 | ||
| pH | T1 | 5.34 ± 0.02 a | 5.36 ± 0.01 a | 5.41 ± 0.00 a | 5.54 ± 0.02 a | 5.54 ± 0.02 a | 5.63 ± 0.07 a | 5.74 ± 0.02 a |
| T2 | 5.23 ± 0.04 b | 5.29 ± 0.00 b | 5.34 ± 0.01 b | 5.41 ± 0.00 b | 5.47 ± 0.03 b | 5.47 ± 0.07 b | 5.57 ± 0.02 b | |
| T3 | 5.23 ± 0.01 b | 5.30 ± 0.01 b | 5.32 ± 0.02 b | 5.33 ± 0.01 c | 5.36 ± 0.01 c | 5.41 ± 0.02 c | 5.46 ± 0.00 c | |
| T4 | 5.49 ± 0.03 c | 5.52 ± 0.05 c | 5.70 ± 0.01 c | 6.64 ± 0.02 d | NE | NE | NE | |
| L* | T1 | 55.88 ± 1.93 a | 56.08 ± 1.29 a,c,d | 55.09 ± 1.58 a,c | 54.15 ± 1.34 a | 57.20 ± 1.36 a | 57.96 ± 1.45 a | 58.34 ± 1.24 a,c |
| T2 | 59.68 ± 1.23 b | 58.84 ± 2.48 b | 59.45 ± 2.00 b | 53.01 ± 1.43 a | 59.81 ± 2.79 b | 60.40 ± 2.07 b | 59.69 ± 1.81 b,c | |
| T3 | 55.94 ± 2.04 a | 56.64 ± 1.18 c | 56.18 ± 1.10 c | 53.00 ± 0.61 a | 58.86 ± 1.20 b | 58.73 ± 1.24 a | 58.89 ± 0.94 c | |
| T4 | 58.19 ± 0.63 c | 55.37 ± 2.00 d | 54.92 ± 1.41 c | 51.59 ± 1.40 b | NE | NE | NE | |
| a* | T1 | −0.93 ± 0.34 a | 0.60 ± 0.38 a | 1.35 ± 0.25 a | 3.55 ± 1.87 a | 1.65 ± 0.25 a | 1.47 ± 0.35 a | 0.89 ± 0.31 a |
| T2 | −1.39 ± 0.64 b | 0.29 ± 0.79 a | 0.37 ± 0.80 b | 4.12 ± 0.89 a,b | 1.61 ± 0.77 a | 1.03 ± 0.75 a | 1.01 ± 0.78 a | |
| T3 | −0.19 ± 0.54 c | 1.04 ± 0.56 a | 1.57 ± 0.70 b,c | 1.86 ± 0.50 b | 1.18 ± 0.70 a | 1.03 ± 0.48 a | 1.88 ± 1.81 a | |
| T4 | −0.58 ± 0.68 d | 1.72 ± 0.51 b | 2.06 ± 0.44 c | 3.1 ± 1.33 b | NE | NE | NE | |
| b* | T1 | 5.30 ± 0.51 a | 7.83 ± 0.67 a | 8.55 ± 0.53 a | 12.36 ± 1.33 a | 9.05 ± 0.38 a | 9.46 ± 0.38 a | 9.39 ± 0.45 a |
| T2 | 5.95 ± 1.25 a | 5.72 ± 1.21 a | 8.36 ± 0.80 a | 16.41 ± 1.35 b | 9.42 ± 0.97 a | 8.93 ± 0.68 a | 9.22 ± 0.88 a | |
| T3 | 7.10 ± 0.61 b | 10.30 ± 0.82 b | 10.68 ± 0.90 b | 12.28 ± 0.78 a | 11.12 ± 0.92 b | 10.87 ± 0.91 b | 11.24 ± 0.98 b | |
| T4 | 7.77 ± 1.19 b | 8.77 ± 0.77 c | 9.32 ± 0.49 c | 13.86 ± 1.40 a | NE | NE | NE | |
| Exudate loss (%) | T1 | 4.65 ± 0.45 a | 4.14 ± 0.59 a | 4.41 ± 0.53 a | 4.16 ± 0.28 a | 4.11 ± 0.59 a | 4.38 ± 0.57 a | 3.68 ± 0.07 a |
| T2 | 3.52 ± 0.15 b | 4.57 ± 0.70 b | 5.70 ± 0.18 b | 4.47 ± 0.71 b | 4.42 ± 0.40 b | 3.79 ± 0.15 b | 3.76 ± 0.75 b | |
| T3 | 3.92 ± 0.34 c | 4.20 ± 0.42 c | 5.03 ± 0.35 c | 3.54 ± 0.58 c | 3.34 ± 0.46 c | 4.41 ± 0.32 c | 4.91 ± 0.22 c | |
| T4 | 4.94 ± 0.51 d | 3.70 ± 0.39 d | 4.46 ± 0.34 d | 1.90 ± 0.15 d | NE | NE | NE | |
| Parameter | Treatment | Storage Time (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 8 | 12 | 16 | 21 | ||
| Odor | T1 | 8.4 ± 0.51 a | 8.5 ± 0.52 a | 8.3 ± 0.48 a | 8.0 ± 0.0 a | 6.9 ± 0.31 a | 6.8 ± 0.63 a | 6.0 ± 0.81 a |
| T2 | 8.4 ± 0.51 a | 8.6 ± 0.51 a | 8.3 ± 0.48 a | 8.0 ± 0.0 a | 7.6 ± 0.51 b | 7.5 ± 0.52 b | 6.4 ± 0.69 a | |
| T3 | 8.2 ± 0.78 a | 8.2 ± 0.63 a | 8.3 ± 0.48 a | 8.0 ± 0.0 a | 7.4 ± 0.51 b | 7.1 ± 0.31 ab | 6.3 ± 0.82 a | |
| T4 | 8.3 ± 0.67 a | 8.3 ± 0.67 a | 8.2 ± 0.42 a | 4.2 ± 0.22 b | NE | NE | NE | |
| Flavor* | T1 | 7.8 ± 0.78 a | 7.9 ± 0.87 a | 8.1 ± 0.56 a | 7.5 ± 0.52 a | 6.7 ± 0.48 a | 6.6 ± 0.51 a | 4.6 ± 1.07 a |
| T2 | 8 ± 0.66 a | 8.2 ± 0.42 a | 8.2 ± 0.42 a | 7.7 ± 0.48 a | 7.5 ± 0.52 b | 7.1 ± 0.31 b | 5.9 ± 0.89 b | |
| T3 | 7.7 ± 0.48 a | 7.7 ± 0.48 a | 7.7 ± 0.48 a | 7.5 ± 0.52 a | 6.5 ± 0.52 a | 6.2 ± 0.63 a | 5.2 ± 0.63 a,b | |
| T4 | 7.8 ± 0.78 a | 7.8 ± 0.78 a | 7.7 ± 0.82 a | NE | NE | NE | NE | |
| Color | T1 | 8.9 ± 0.31 a | 9.0 ± 0.0 a | 8.8 ± 0.42 a | 8.0 ± 0.0 a | 7.6 ± 0.51 a | 7.1 ± 0.31 a | 6.0 ± 0.66 a |
| T2 | 8.9 ± 0.31 a | 9.0 ± 0.0 a | 8.8 ± 0.42 a | 8.0 ± 0.0 a | 7.7 ± 0.48 a | 7.5 ± 0.52 a | 6.3 ± 0.67 a | |
| T3 | 8.9 ± 0.31 a | 9.0 ± 0.0 a | 8.8 ± 0.42 a | 8.0 ± 0.0 a | 7.5 ± 0.52 a | 7.4 ± 0.69 a | 6.0 ± 0.81 a | |
| T4 | 8.8 ± 0.42 a | 8.8 ± 0.42 b | 8.8 ± 0.42 a | 5.9 ± 0.37 b | NE | NE | NE | |
| Texture | T1 | 8.3 ± 0.67 a | 8.6 ± 0.51 a | 8.3 ± 0.48 a | 6.9 ± 1.8 a | 6.5 ± 0.84 a | 6.4 ± 0.51 a | 4.5 ± 0.84 a |
| T2 | 8.4 ± 0.31 ª | 8.4 ± 0.51 a,b | 8.5 ± 0.52 a | 7.3 ± 1.05 a | 6.9 ± 0.73 a | 6.3 ± 0.48 a | 5.8 ± 0.63 b | |
| T3 | 8.4 ± 0.51 ª | 8.9 ± 0.31 ac | 8.4 ± 0.69 a | 7.3 ± 1.15 a | 6.8 ± 0.78 a | 6.2 ± 0.78 a | 5.8 ± 0.78 b | |
| T4 | 8.3 ± 0.67 a | 8.1 ± 0.73 b | 8.2 ± 0.63 a | NE | NE | NE | NE | |
| Overall acceptability | T1 | 8.2 ± 0.42 a | 8.3 ± 0.48 a | 8.1 ± 0.31 a | 7.5 ± 0.97 a | 6.7 ± 0.48 a | 6.5 ± 0.52 a | 5.3 ± 0.67 a |
| T2 | 8.3 ± 0.48 a | 8.3 ± 0.48 a | 8.2 ± 0.42 a,c | 7.9 ± 0.31 a | 7.5 ± 0.52 b | 7.0 ± 0.47 b | 6.3 ± 0.67 b | |
| T3 | 7.8 ± 0.42 b | 7.8 ± 0.42 b | 7.9 ± 0.31 a | 7.4 ± 0.69 a | 6.9 ± 0.56 a | 6.7 ± 0.48 a,b | 5.9 ± 0.73 a,b | |
| T4 | 8.2 ± 0.42 a | 8.0 ± 0.0 b,c | 7.8 ± 0.42 a,b | 4.52 ± 0.42 b | NE | NE | NE | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaparro-Hernández, S.; Ruiz-Cruz, S.; Márquez-Ríos, E.; Ornelas-Paz, J.d.J.; Del-Toro-Sánchez, C.L.; Gassos-Ortega, L.E.; Ocaño-Higuera, V.M.; López-Mata, M.A.; Devora-Isiordia, G.E. Effect of Chitosan–Tomato Plant Extract Edible Coating on the Quality, Shelf Life, and Antioxidant Capacity of Pork during Refrigerated Storage. Coatings 2019, 9, 827. https://doi.org/10.3390/coatings9120827
Chaparro-Hernández S, Ruiz-Cruz S, Márquez-Ríos E, Ornelas-Paz JdJ, Del-Toro-Sánchez CL, Gassos-Ortega LE, Ocaño-Higuera VM, López-Mata MA, Devora-Isiordia GE. Effect of Chitosan–Tomato Plant Extract Edible Coating on the Quality, Shelf Life, and Antioxidant Capacity of Pork during Refrigerated Storage. Coatings. 2019; 9(12):827. https://doi.org/10.3390/coatings9120827
Chicago/Turabian StyleChaparro-Hernández, Saraí, Saul Ruiz-Cruz, Enrique Márquez-Ríos, José de Jesús Ornelas-Paz, Carmen L. Del-Toro-Sánchez, Laura E. Gassos-Ortega, Víctor M. Ocaño-Higuera, Marco A. López-Mata, and Germán E. Devora-Isiordia. 2019. "Effect of Chitosan–Tomato Plant Extract Edible Coating on the Quality, Shelf Life, and Antioxidant Capacity of Pork during Refrigerated Storage" Coatings 9, no. 12: 827. https://doi.org/10.3390/coatings9120827
APA StyleChaparro-Hernández, S., Ruiz-Cruz, S., Márquez-Ríos, E., Ornelas-Paz, J. d. J., Del-Toro-Sánchez, C. L., Gassos-Ortega, L. E., Ocaño-Higuera, V. M., López-Mata, M. A., & Devora-Isiordia, G. E. (2019). Effect of Chitosan–Tomato Plant Extract Edible Coating on the Quality, Shelf Life, and Antioxidant Capacity of Pork during Refrigerated Storage. Coatings, 9(12), 827. https://doi.org/10.3390/coatings9120827







