Preparation and Characterization of Fluoride-Incorporated Plasma Electrolytic Oxidation Coatings on the AZ31 Magnesium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Preparation of Fluoride-Incorporated Coatings on AZ31 Mg Alloys
2.3. Surface Characterization
2.4. Evaluation of Corrosion Behavior In Vitro
2.4.1. Electrochemical Testing
2.4.2. Hydrogen Evolution Tests
2.4.3. Long-Term Immersion Tests
2.5. In Vitro Cytocompatibility Evaluation
3. Results
3.1. Phase Composition
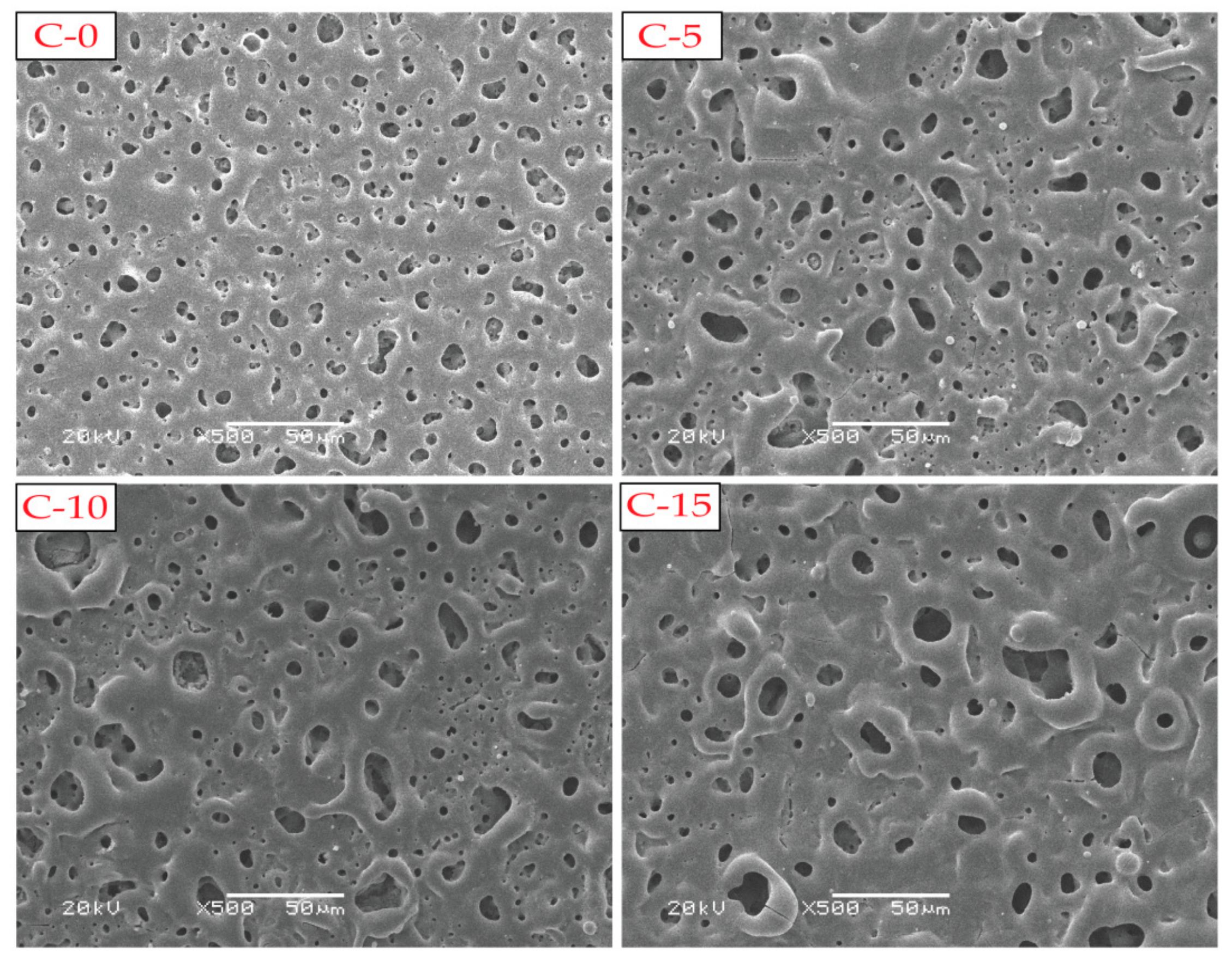
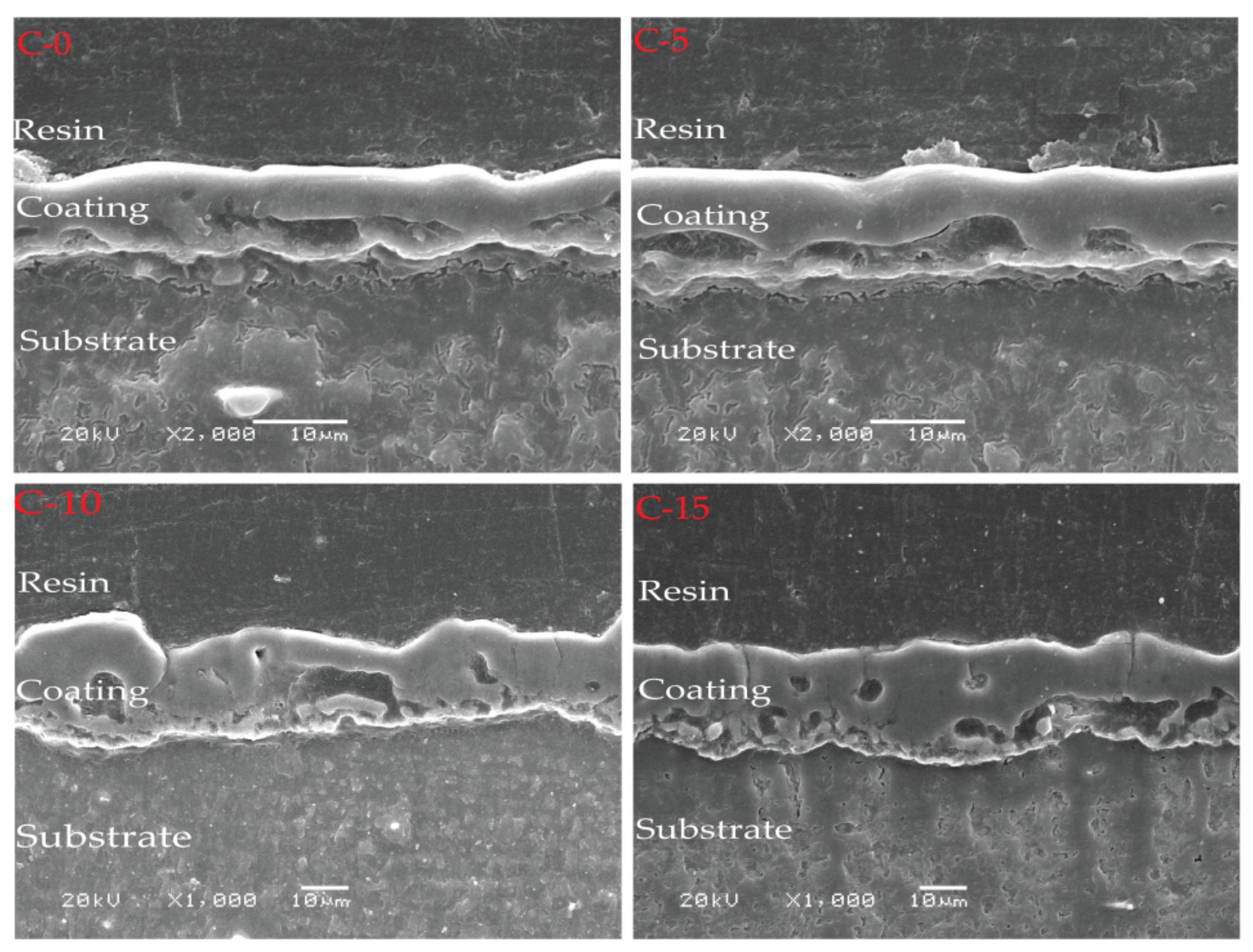
3.2. Surface and Cross-Section Morphologies
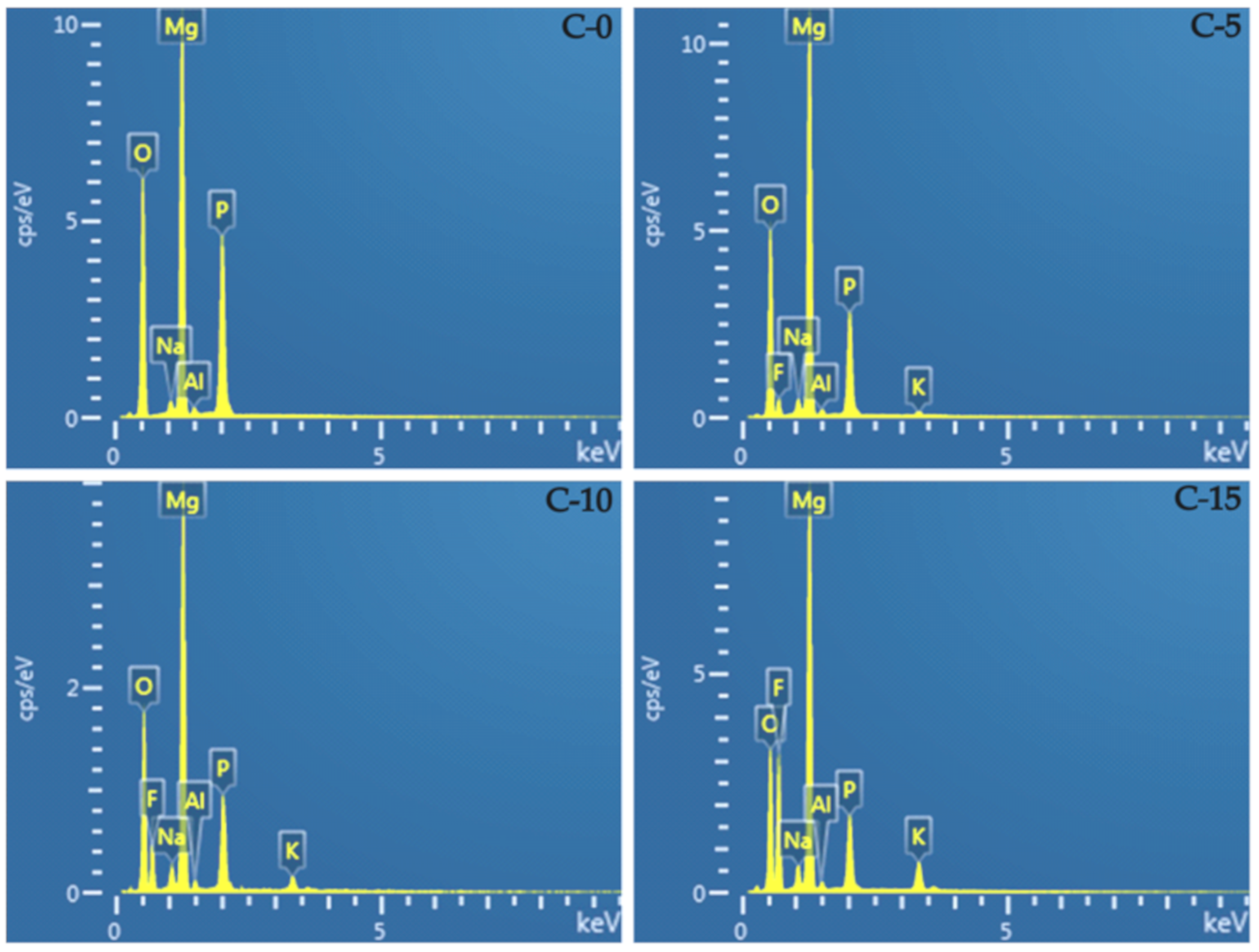
3.3. Elemental Composition
3.4. Corrosion Resistance
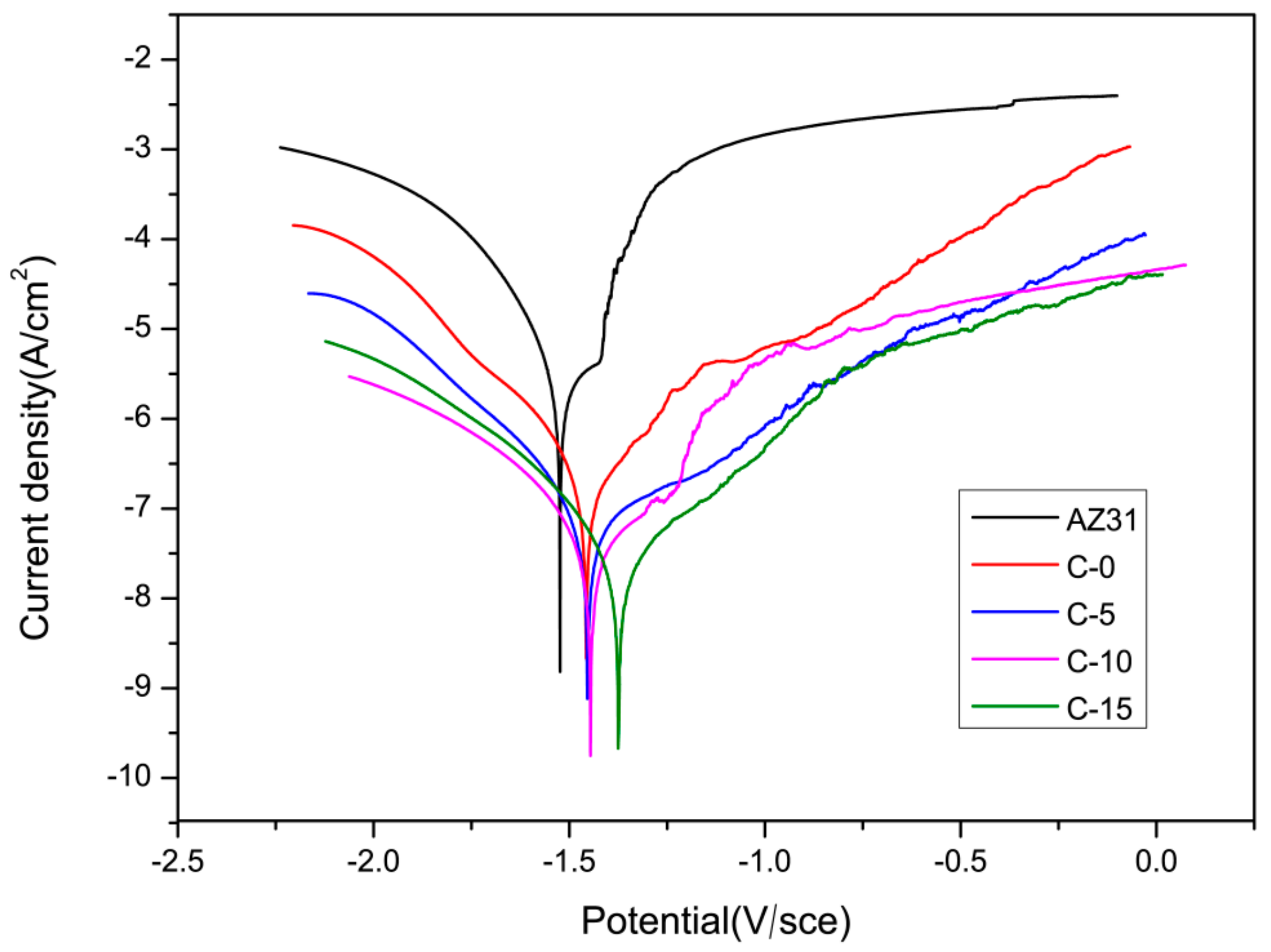
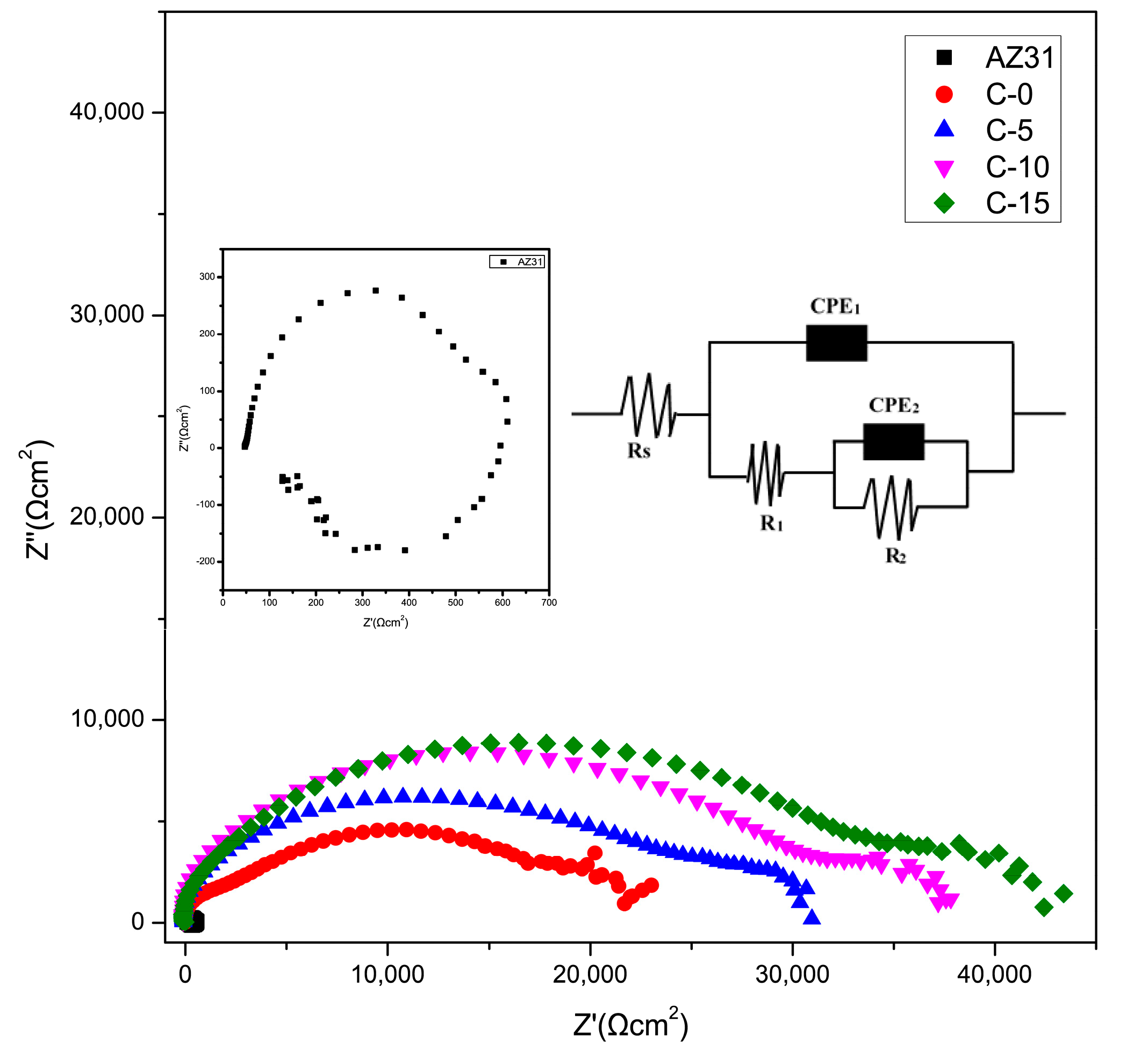
3.4.1. Electrochemical Measurements
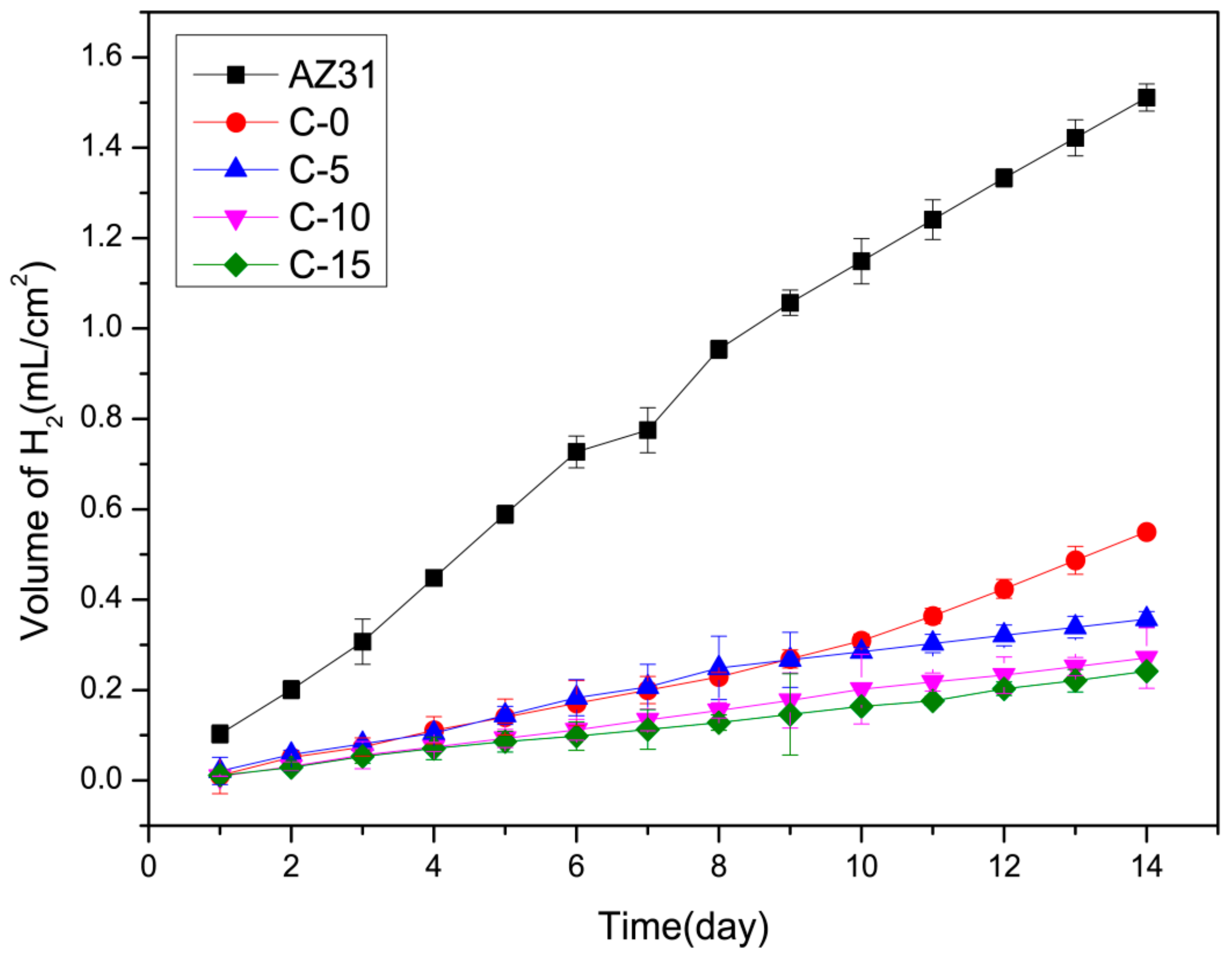
3.4.2. Hydrogen Evolution Tests
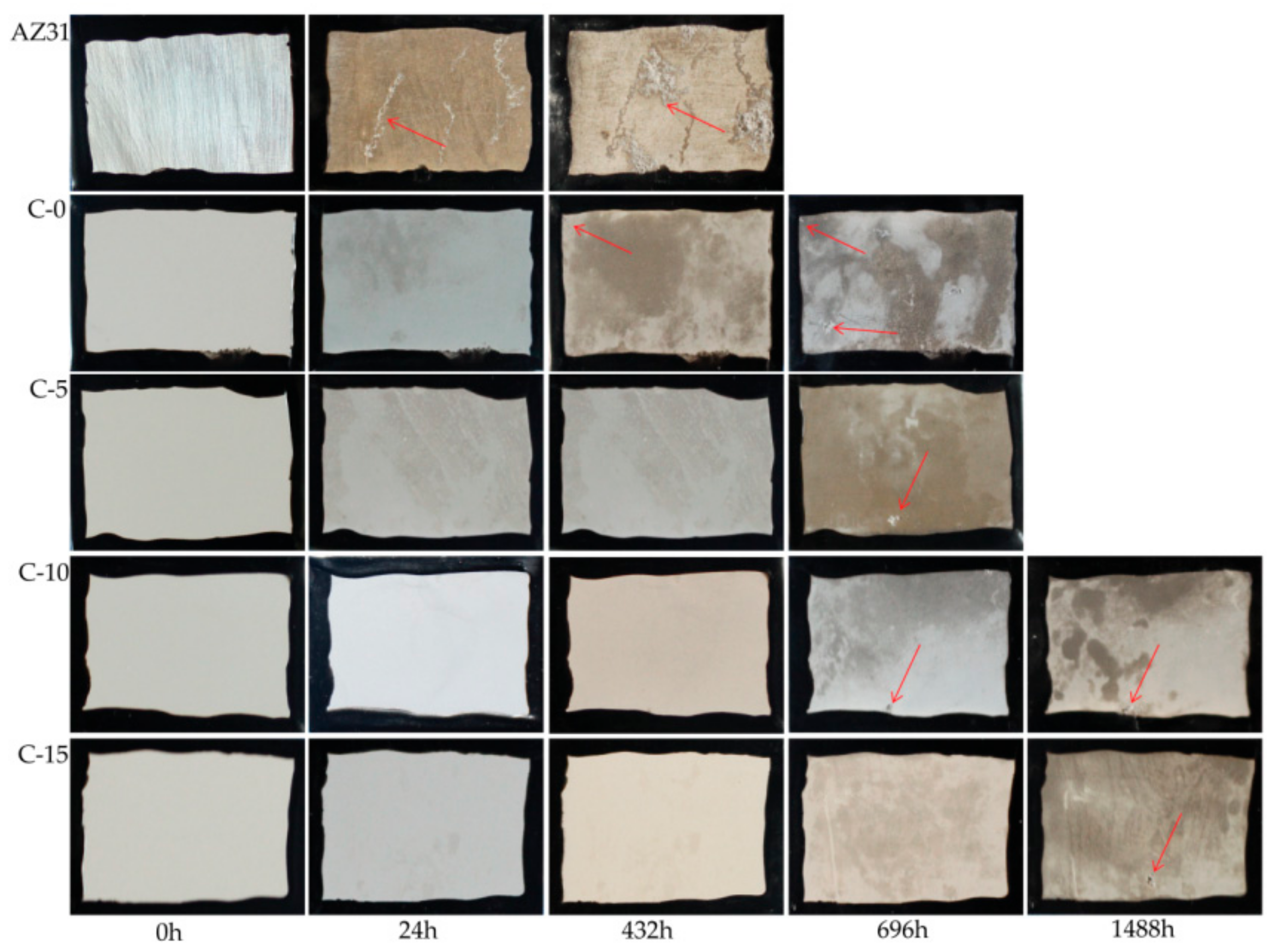
3.4.3. Long-Term Immersion Test
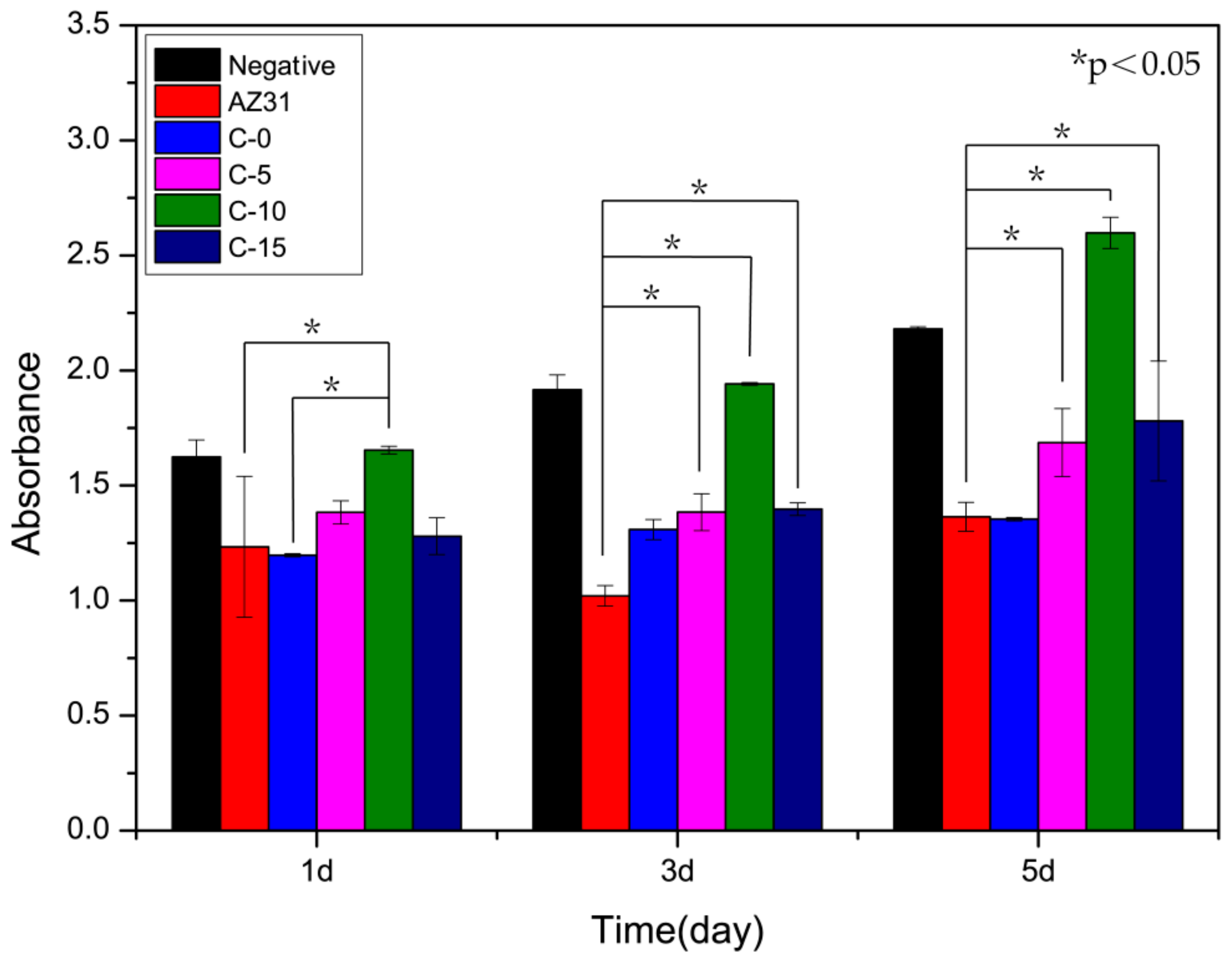
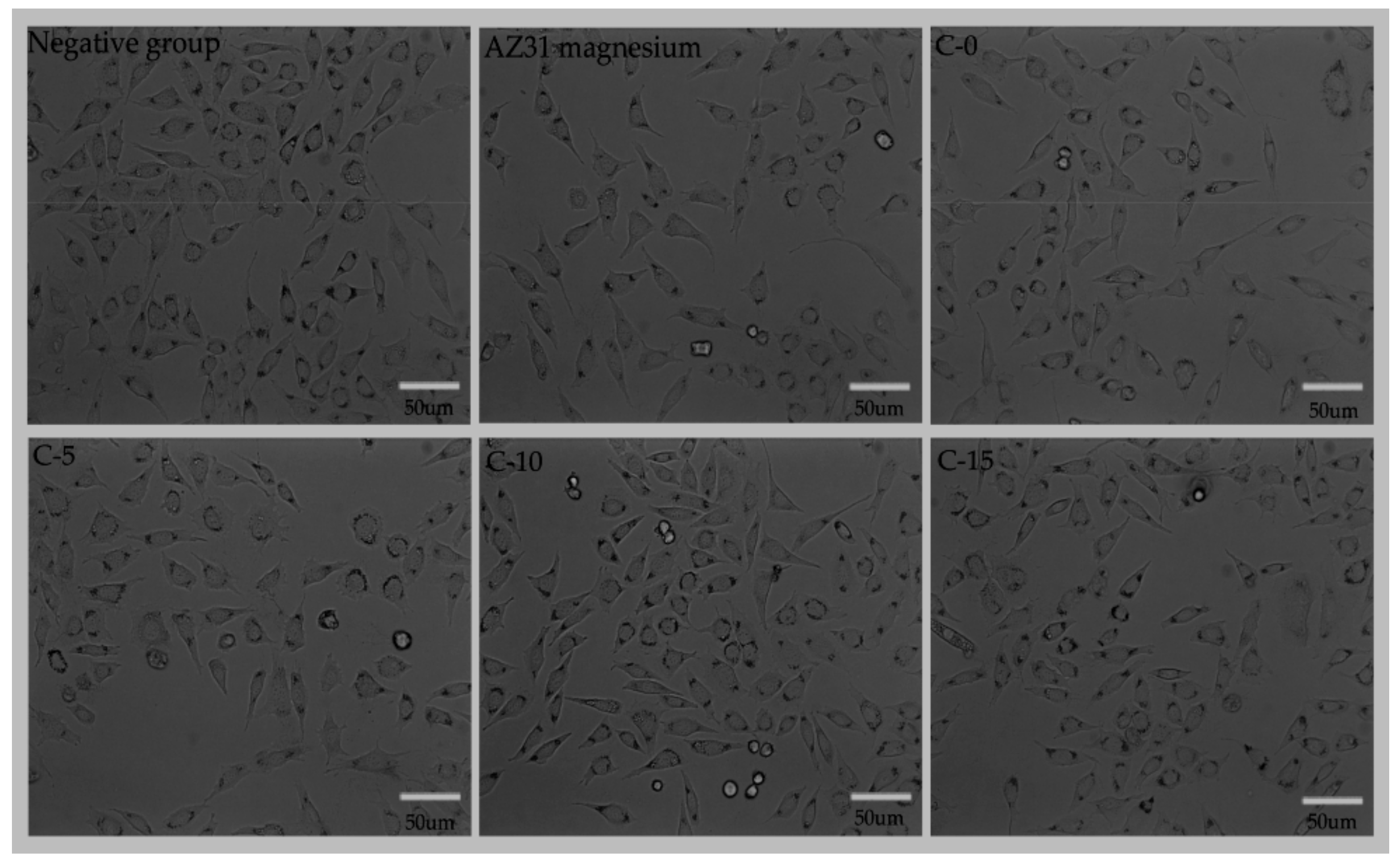
3.5. Cytotoxicity Test
4. Conclusions
- (1).
- The main component of the coating prepared in the fluorine-free electrolyte was MgO, and the coating components in the fluorine electrolyte were MgO and MgF2. Moreover, with increasing KF contents in the electrolyte, the porosity of the coating surface decreased and the coating thickness increased.
- (2).
- A corrosion resistance analysis showed that the corrosion resistance of the fluorine-containing plasma electrolytic oxidation film was superior to that without fluorine. Moreover, with increasing KF content in the electrolyte, corrosion resistance increased, which reduced the degradation rate of the AZ31 magnesium alloy.
- (3).
- In vitro cytotoxicity tests indicated that the cytocompatibility of the fluorine-containing coating was better than that of the fluorine-free coating and that the MgF2/AZ31 coating had no cytotoxic effect on the L-929 cells. In particular, with the increasing fluorine content, the cell proliferation was accelerated. However, after a certain amount of fluorine was added, it inhibited the cell growth. Thus, the proper fluoride content in the coating should be carefully determined to meet a balance between good corrosion resistance and cytocompatibility.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.K.; Vince, K.; Hodgson, M.A. Biodegradable surgical implants based on magnesium alloys—A review of current research. IOP Conf. Ser. Mater. Sci. Eng. 2009, 4, 012011. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar]
- Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W.; et al. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2015, 11, 554–562. [Google Scholar]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar]
- Saris, N.E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Acad. Nutr. Diet. 2002, 102, 1621. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.-M.; Reifenrath, J.; Weizbauer, A.; Bach, F.-W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2012, 48, 39–50. [Google Scholar] [CrossRef]
- Tian, P.; Peng, F.; Wang, D.; Liu, X. Corrosion behavior and cytocompatibility of fluoride-incorporated plasma electrolytic oxidation coating on biodegradable AZ31 alloy. Regen. Biomater. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Gao, J.H.; Shi, X.Y.; Yang, B.; Hou, S.S.; Meng, E.C.; Guan, F.X.; Guan, S.K. Fabrication and characterization of bioactive composite coatings on Mg–Zn–Ca alloy by MAO/sol–gel. J. Mater. Sci. Mater. Med. 2011, 22, 1681–1687. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, Y.; Sun, J.; Yang, L.; Guo, C.; Liang, J.; Cao, B. Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy. Metals 2017, 7, 214. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, S.; Wang, L.; Yang, Y.; Liang, J.; Cao, B. Preparation of Hydroxyapatite/Tannic Acid Coating to Enhance the Corrosion Resistance and Cytocompatibility of AZ31 Magnesium Alloys. Coatings 2017, 7, 105. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Liang, J.; Chen, J. Microstructure and corrosion behavior of plasma electrolytic oxidation coated magnesium alloy pre-treated by laser surface melting. Surf. Coat. Technol. 2012, 206, 3109–3115. [Google Scholar] [CrossRef]
- Jiang, H.B.; Kim, Y.K.; Ji, J.H.; Park, I.S.; Bae, T.S.; Lee, M.H. Surface modification of anodized Mg in ammonium hydrogen fluoride by various voltages. Surf. Coat. Technol. 2014, 259, 310–317. [Google Scholar] [CrossRef]
- Wang, S.; Fu, L.; Nai, Z.; Liang, J.; Cao, B. Comparison of Corrosion Resistance and Cytocompatibility of MgO and ZrO2 Coatings on AZ31 Magnesium Alloy Formed via Plasma Electrolytic Oxidation. Coatings 2018, 8, 441. [Google Scholar] [CrossRef]
- Dou, J.; Chen, Y.; Chi, Y.; Li, H.; Gu, G.; Chen, C. Preparation and characterization of a calcium-phosphate-silicon coating on a Mg-Zn-Ca alloy via two-step micro-arc oxidation. Phys. Chem. Chem. Phys. 2017, 19, 15110–15119. [Google Scholar] [CrossRef]
- Jia, Z.J.; Li, M.; Liu, Q.; Xu, X.C.; Cheng, Y.; Zheng, Y.F.; Xi, T.F.; Wei, S.C. Micro-arc oxidization of a novel Mg–1Ca alloy in three alkaline KF electrolytes: Corrosion resistance and cytotoxicity. Appl. Surf. Sci. 2014, 292, 1030–1039. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Shin, S.H.; Hussain, I.; Koo, B.H. Investigation of hybrid PEO coatings on AZ31B magnesium alloy in alkaline K2ZrF6–Na2SiO3 electrolyte solution. Prot. Met. Phys. Chem. 2017, 53, 495–502. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Tailoring the composition of fluoride conversion coatings to achieve better corrosion protection of magnesium for biomedical applications. J. Mater. Chem. B 2014, 2, 3365–3382. [Google Scholar] [CrossRef]
- Khiabani, A.B.; Ghanbari, A.; Yarmand, B.; Zamanian, A.; Mozafari, M. Improving corrosion behavior and in vitro bioactivity of plasma electrolytic oxidized AZ91 magnesium alloy using calcium fluoride containing electrolyte. Mater. Lett. 2018, 212, 98–102. [Google Scholar] [CrossRef]
- Němcová, A.; Skeldon, P.; Thompson, G.E.; Pacal, B. Effect of fluoride on plasma electrolytic oxidation of AZ61 magnesium alloy. Surf. Coat. Technol. 2013, 232, 827–838. [Google Scholar] [CrossRef]
- Mousny, M.; Omelon, S.; Wise, L.; Everett, E.T.; Dumitriu, M.; Holmyard, D.P.; Banse, X.; Devogelaer, J.P.; Grynpas, M.D. Fluoride effects on bone formation and mineralization are influenced by genetics. Bone 2008, 43, 1067–1074. [Google Scholar] [CrossRef]
- Yan, T.; Tan, L.; Xiong, D.; Liu, X.; Zhang, B.; Yang, K. Fluoride treatment and in vitro corrosion behavior of an AZ31B magnesium alloy. Mater. Sci. Eng. C 2010, 30, 740–748. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.J. Surface modification of Li[Li0.2Ni0.2Mn0.6]O2 nanoparticles with polydopamine-assisted MgF2 coating. Mater. Res. Bull. 2014, 58, 169–173. [Google Scholar] [CrossRef]
- Liu, X.; Zhen, Z.; Liu, J.; Xi, T.; Zheng, Y.; Guan, S.; Zheng, Y.; Cheng, Y. Multifunctional MgF2/Polydopamine Coating on Mg Alloy for Vascular Stent Application. J. Mater. Sci. Technol. 2015, 31, 733–743. [Google Scholar] [CrossRef]
- Riaz, U.; Rahman, Z.U.; Asgar, H.; Shah, U.; Shabib, I.; Haider, W. An insight into the effect of buffer layer on the electrochemical performance of MgF2 coated magnesium alloy ZK60. Surf. Coat. Technol. 2018, 344, 514–521. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, H.; Ding, Z.; Zhang, Z.; Sun, B.; Shen, J.; Chen, S.; Zhang, B.; Yang, K.; Liu, M.; et al. In vitro and in vivo evaluation of MgF2 coated AZ31 magnesium alloy porous scaffolds for bone regeneration. Colloids Surf. B. Biointerfaces 2017, 149, 330–340. [Google Scholar] [CrossRef]
- Marc, B.; Jacques, L. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Jabbarzare, S.; Iqbal, N.; Kadir, M.R.A. Deposition of nanostructured fluorine-doped hydroxyapatite–polycaprolactone duplex coating to enhance the mechanical properties and corrosion resistance of Mg alloy for biomedical applications. Mater. Sci. Eng. C 2016, 60, 526–537. [Google Scholar] [CrossRef]
- ISO 10993-12:2007 Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2007.
- Li, Z.; Shizhao, S.; Chen, M.; Fahlman, B.D.; Debao, L.; Bi, H. In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF2-coated Mg-Zn-Zr alloy as cancellous screws. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1268–1280. [Google Scholar] [CrossRef]
- Tang, M.; Liu, H.; Li, W.; Zhu, L. Effect of zirconia sol in electrolyte on the characteristics of microarc oxidation coating on AZ91D magnesium. Mater. Lett. 2011, 65, 413–415. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Wang, H.; Peng, J. Effect of potassium fluoride on structure and corrosion resistance of plasma electrolytic oxidation films formed on AZ31 magnesium alloy. J. Alloys Compd. 2009, 480, 469–474. [Google Scholar] [CrossRef]
- Nashrah, N.; Kamil, M.P.; Yoon, D.K.; Kim, Y.G.; Ko, Y.G. Formation mechanism of oxide layer on AZ31 Mg alloy subjected to micro-arc oxidation considering surface roughness. Appl. Surf. Sci. 2019, 143772. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Wang, Q.; Bu, S. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros. Sci. 2010, 52, 2228–2234. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate–fluoride solutions and evaluation of corrosion resistance. Appl. Surf. Sci. 2005, 246, 229–238. [Google Scholar]
- Jiang, H.B.; Wu, G.; Lee, S.-B.; Kim, K.-M. Achieving controllable degradation of a biomedical magnesium alloy by anodizing in molten ammonium bifluoride. Surf. Coat. Technol. 2017, 313, 282–287. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Liang, J.; Chen, J. Corrosion Mechanism of Plasma Electrolytic Oxidation Coated Magnesium Alloy with Laser Surface Melting Pretreatment. J. Electrochem. Soc. 2014, 161, C20–C24. [Google Scholar]
- Shi, P.; Niu, B.; Shanshan, E.; Chen, Y.; Li, Q. Preparation and characterization of PLA coating and PLA/MAO composite coatings on AZ31 magnesium alloy for improvement of corrosion resistance. Surf. Coat. Technol. 2015, 262, 26–32. [Google Scholar]
- Zuo, Y.; Li, T.; Yu, P.; Zhao, Z.; Chen, X.; Zhang, Y.; Chen, F. Effect of graphene oxide additive on tribocorrosion behavior of MAO coatings prepared on Ti6Al4V alloy. Appl. Surf. Sci. 2019, 480, 26–34. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Controlling the degradation rate of AZ91 magnesium alloy via sol-gel derived nanostructured hydroxyapatite coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3817–3825. [Google Scholar]
- Tian, P.; Xu, D.; Liu, X. Mussel-inspired functionalization of PEO/PCL composite coating on a biodegradable AZ31 magnesium alloy. Colloids Surf. B. Biointerfaces 2016, 141, 327–337. [Google Scholar] [CrossRef]
- Iglesias, C.; Bodelon, O.G.; Montoya, R.; Clemente, C.; Garcia-Alonso, M.C.; Rubio, J.C.; Escudero, M.L. Fracture bone healing and biodegradation of AZ31 implant in rats. Biomed. Mater. 2015, 10, 025008. [Google Scholar]

| Group\Electrolytes | (NaPO3)6 (g/L) | NaOH (g/L) | KF (g/L) |
|---|---|---|---|
| C-0 | 3 | 2 | 0 |
| C-5 | 3 | 2 | 5 |
| C-10 | 3 | 2 | 10 |
| C-15 | 3 | 2 | 15 |
| Group | C-0 | C-5 | C-10 | C-15 |
|---|---|---|---|---|
| Thickness (µm) | 5.84 ± 2.37 | 9.49 ± 1.96 | 25.61 ± 3.26 | 27.83 ± 3.51 |
| Elements (wt %) | Mg | O | F | P | Na |
|---|---|---|---|---|---|
| C-0 | 33.00 ± 0.12 | 46.66 ± 0.32 | 0 | 18.38 ± 0.04 | 1.18 ± 0.10 |
| C-5 | 37.80 ± 0.02 | 38.70 ± 0.17 | 7.67 ± 0.09 | 12.66 ± 0.35 | 2.66 ± 0.13 |
| C-10 | 35.86 ± 0.02 | 32.96 ± 0.28 | 15.48 ± 0.04 | 10.20 ± 0.02 | 2.69 ± 0.04 |
| C-15 | 35.20 ± 0.04 | 25.61 ± 0.14 | 25.74 ± 0.07 | 7.90 ± 0.11 | 2.05 ± 0.04 |
| Samples | Ecorr (V vs. SCE) | Icorr (A/cm2) |
|---|---|---|
| AZ31 | −1.465 ± 0.003 | (4.804 ± 0.080) × 10-6 |
| C-0 | −1.448 ± 0.014 | (1.397 ± 0.004) × 10−7 * |
| C-5 | −1.457 ± 0.002 * | (2.419 ± 0.317) × 10−8 * |
| C-10 | −1.393 ± 0.006 * | (3.151 ± 0.071) × 10−8 * |
| C-15 | −1.375 ± 0.005 * | (8.677 ± 0.023) × 10−9 * |
| Samples | Rs (Ω·cm2) | CPE1 | R1 (Ω·cm2) | CPE2 | R2 (Ω·cm2) |
|---|---|---|---|---|---|
| C-0 | 0.01 | 3.52 × 10−8 | 2693 | 5.90 × 10−6 | 1.98 × 104 |
| C-5 | 0.01 | 1.94 × 10−8 | 2721 | 4.05 × 10−6 | 2.81 × 104 |
| C-10 | 0.01 | 1.68 × 10−8 | 4238 | 2.81 × 10−6 | 3.02 × 104 |
| C-15 | 0.01 | 2.08 × 10−8 | 7012 | 2.83 × 10−7 | 3.80 × 104 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Yang, Y.; Zhang, L.; Wu, Y.; Liang, J.; Cao, B. Preparation and Characterization of Fluoride-Incorporated Plasma Electrolytic Oxidation Coatings on the AZ31 Magnesium Alloy. Coatings 2019, 9, 826. https://doi.org/10.3390/coatings9120826
Fu L, Yang Y, Zhang L, Wu Y, Liang J, Cao B. Preparation and Characterization of Fluoride-Incorporated Plasma Electrolytic Oxidation Coatings on the AZ31 Magnesium Alloy. Coatings. 2019; 9(12):826. https://doi.org/10.3390/coatings9120826
Chicago/Turabian StyleFu, Lingxia, Yanxia Yang, Longlong Zhang, Yuanzhi Wu, Jun Liang, and Baocheng Cao. 2019. "Preparation and Characterization of Fluoride-Incorporated Plasma Electrolytic Oxidation Coatings on the AZ31 Magnesium Alloy" Coatings 9, no. 12: 826. https://doi.org/10.3390/coatings9120826
APA StyleFu, L., Yang, Y., Zhang, L., Wu, Y., Liang, J., & Cao, B. (2019). Preparation and Characterization of Fluoride-Incorporated Plasma Electrolytic Oxidation Coatings on the AZ31 Magnesium Alloy. Coatings, 9(12), 826. https://doi.org/10.3390/coatings9120826




