Investigation of Protective Performance of a Mg-Rich Primer Containing Aluminum Tri-Polyphosphate on AZ91D Magnesium Alloy in Simulated Acid Rain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. SEM
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Electrochemical Measurements
3. Results
3.1. OCP Measurements
3.2. Polarization Measurement
3.3. EIS Measurement
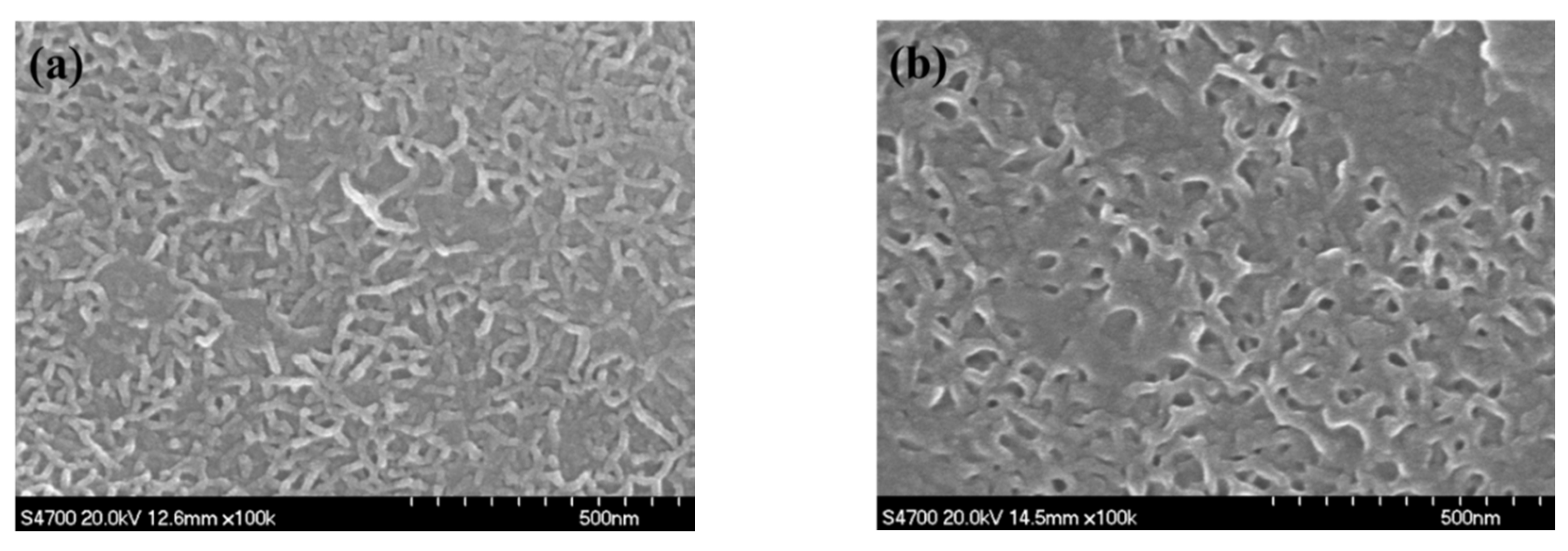
3.4. SEM Observation
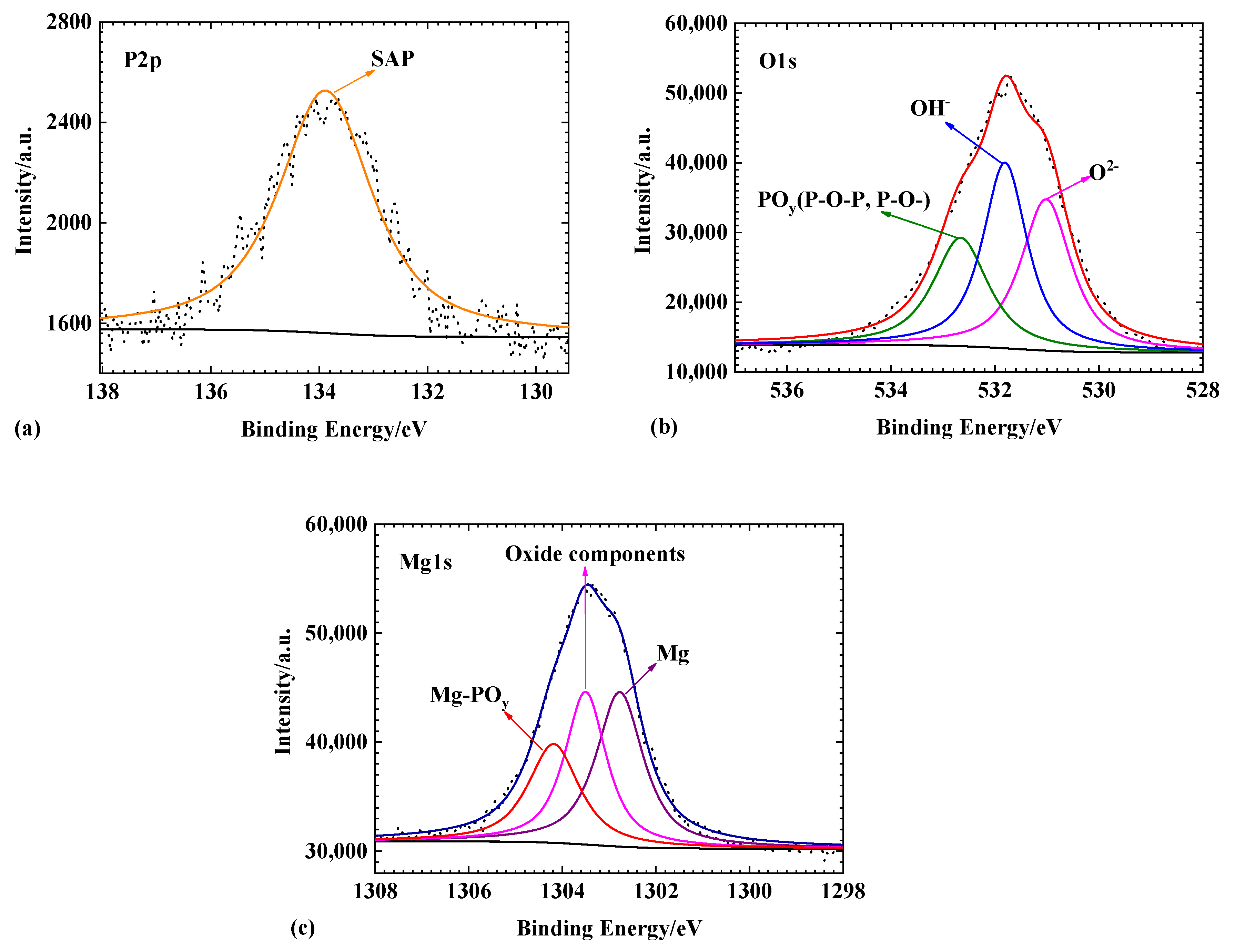
3.5. XPS Analysis
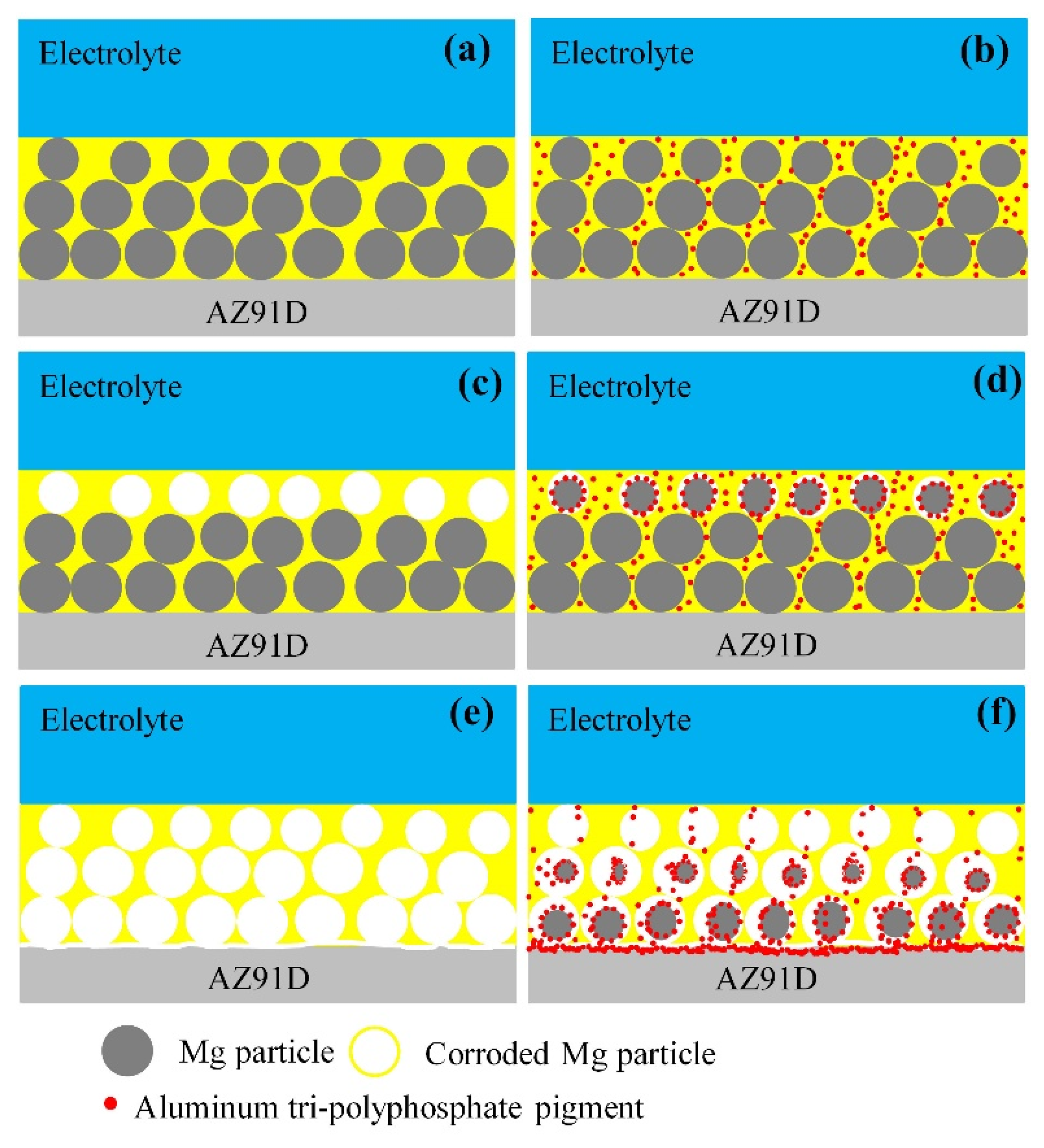
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, N.; Chen, Y.; Deng, B.; Yue, J.; Qu, W.; Yang, H.; He, Y.; Xia, W.; Li, L. Low temperature UV assisted sol-gel preparation of ZrO2 pore-sealing films on micro-arc oxidized magnesium alloy AZ91D and their electrochemical corrosion behaviors. J. Alloy. Compd. 2019, 792, 1036–1044. [Google Scholar] [CrossRef]
- Gan, R.; Wang, D.; Xie, Z.H.; He, L. Improving surface characteristic and corrosion inhibition of coating on Mg alloy by trace stannous (II) chloride. Corros. Sci. 2017, 123, 147–157. [Google Scholar] [CrossRef]
- Abatti, G.P.; Pires, A.T.N.; Spinelli, A.; Schrnagl, N.; da Conceição, T.F. Conversion coating on magnesium alloy sheet (AZ31) by vanillic acid treatment: Preparation, characterization and corrosion behavior. J. Alloy. Compd. 2018, 738, 224–232. [Google Scholar] [CrossRef]
- Xie, Z.H.; Shan, S. Nanocontainers-enhanced self-healing Ni coating for corrosion protection of Mg alloy. J. Mater. Sci. 2018, 53, 3744–3755. [Google Scholar] [CrossRef]
- Duan, G.; Yang, L.; Liao, S.; Zhang, C.; Lu, X.; Yang, Y.; Zhang, B.; Wei, Y.; Zhang, T.; Yu, B.; et al. Designing for the chemical conversion coating with high corrosion resistance and low electrical contact resistance on AZ91D magnesium alloy. Corros. Sci. 2018, 135, 197–206. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Xie, Z.H.; Yu, G. Duplex coating combining layered double hydroxide and 8-quinolinol layers on Mg alloy for corrosion protection. Electrochim. Acta 2018, 283, 1845–1857. [Google Scholar] [CrossRef]
- López, A.D.F.; Lehr, I.L.; Saidman, S.B. Anodisation of AZ91D magnesium alloy in molybdate solution for corrosion protection. J. Alloy. Compd. 2017, 702, 338–345. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.; Yao, X.; Wang, J.; Chen, J. Stable preparation and characterization of yellow micro arc oxidation coating on magnesium alloy. J. Alloy. Compd. 2018, 745, 609–616. [Google Scholar] [CrossRef]
- Li, D.; Chen, F.; Xie, Z.H.; Shan, S.; Zhong, C.J. Enhancing structure integrity and corrosion resistance of Mg alloy by a two-step deposition to avoid F ions etching to nano-SiO2 reinforcement. J. Alloy. Compd. 2017, 705, 70–78. [Google Scholar] [CrossRef]
- Staišiūnas, L.; Miečinskas, P.; Leinartas, K.; Selskis, A.; Grigucevičiene, A.; Juzeliūnas, E. Sputter-deposited Mg–Al–Zn–Cr alloys–Electrochemical characterization of single films and multilayer protection of AZ31 magnesium alloy. Corros. Sci. 2014, 80, 487–493. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Xin, Y.; Yuan, N.; Ding, J. Facile formation of super-hydrophobic nickel coating on magnesium alloy with improved corrosion resistance. Colloid. Surface. A 2018, 538, 500–505. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.K.; Singh, R. Exploring environment friendly nickel electrodeposition on AZ91 magnesium alloy: Effect of prior surface treatments and temperature of the bath on corrosion behavior. Corros. Sci. 2019, 151, 1–19. [Google Scholar] [CrossRef]
- Hu, R.G.; Zhang, S.; Bu, J.F.; Lin, C.J.; Song, G.L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y.; Feng, X. The study of a Mg-rich epoxy primer for protection of AZ91D magnesium alloy. Corros. Sci. 2011, 53, 153–160. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Shen, S. The effects of magnesium particles in Mg-rich primers applied on AZ91D magnesium alloy. Int. J. Electrochem. Sci. 2015, 10, 9586–9604. [Google Scholar]
- Song, G.; Dudney, N.A.; Li, J.; Sacci, R.J.; Thomson, J.K. The possibility of forming a sacrificial anode coating for Mg. Corros. Sci. 2014, 87, 11–14. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of die cast AZ91D. Corros. Sci. 1999, 41, 249–273. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y. The improved performance of a Mg-rich epoxy coating on AZ91D magnesium alloy by silane pretreatment. Corros. Sci. 2012, 60, 165–172. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y. The influence of aluminum tri-polyphosphateon the protective behavior of Mg-rich epoxy coating on AZ91D magnesium alloy. Electrochim. Acta 2013, 93, 53–64. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Zuo, Y.; Zheng, C.; Lu, S.; Xu, L. Evaluation of the micro-arc oxidation treatment effect on the protective performance of a Mg-rich epoxy coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2015, 270, 227–235. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Zuo, Y.; Zhang, P.; Zheng, C. Improvement of protection performance of Mg-rich epoxy coating on AZ91D magnesium alloy by DC anodic oxidation. Prog. Org. Coat. 2017, 104, 188–198. [Google Scholar] [CrossRef]
- Battocchi, D.; Simões, A.M.; Tallman, D.E.; Bierwagen, G.P. Electrochemical behaviour of a Mg-rich primer in the protection of Al alloys. Corros. Sci. 2006, 48, 1292–1306. [Google Scholar] [CrossRef]
- Vilche, J.R.; Bucharsky, E.C.; Giúdice, C.A. Application of EIS and SEM to evaluate the influence of pigment shape and content in ZRP formulations on the corrosion prevention of naval steel. Corros. Sci. 2002, 44, 1287–1309. [Google Scholar] [CrossRef]
- Shen, S.; Zuo, Y.; Zhao, X. The effects of 8-hydroxyquinoline on corrosion performance of a Mg-rich coating on AZ91D magnesium alloy. Corros. Sci. 2013, 76, 275–283. [Google Scholar] [CrossRef]
- Shen, S.; Zuo, Y. The improved performance of Mg-rich epoxy primer on AZ91D magnesium alloy by addition of ZnO. Corros. Sci. 2014, 87, 167–178. [Google Scholar] [CrossRef]
- Pathak, S.S.; Blanton, M.D.; Mendon, S.K.; Rawlins, J.W. Carbonation of Mg powder to enhance the corrosion resistance of Mg-rich primers. Corros. Sci. 2010, 52, 3782–3792. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, Y.; Tang, Y.; Lu, X. Phosphatizing of Mg particles to improve the protective performance of Mg-rich primer on A2024 Al alloy. Appl. Surf. Sci. 2014, 292, 93–99. [Google Scholar] [CrossRef]
- Ahnia, F.; Khelfaoui, Y.; Zaid, B.; Pérez, F.J.; Miroud, D.; Ahmed, A.S.; Alcalá, G. Thermally sprayed Al/Mo coatings on industrial steel E335 and effects on electrochemical parameters in simulated acid rain. J. Alloy. Compd. 2017, 696, 1282–1291. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, M.; Huang, H.; Xie, L.; Wang, C. Circular concrete filled steel tubular (CFST) columns under cyclic load and acid rain attack: Test simulation. Thin-Wall. Struct. 2018, 122, 90–101. [Google Scholar] [CrossRef]
- Xie, L.; Chen, M.; Sun, W.; Yuan, F.; Huang, H. Behaviour of concrete-filled steel tubular members under pure bending and acid rain attack: Test simulation. Adv. Struct. Eng. 2019, 22, 240–253. [Google Scholar] [CrossRef]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric corrosion, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 32–45. [Google Scholar]
- Liu, H.; Cao, F.; Song, G.L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Cui, Z.; Ge, F.; Lin, Y.; Wang, L.; Lei, L.; Tian, H.; Yu, M.; Wang, X. Corrosion behavior of AZ31 magnesium alloy in the chloride solution containing ammonium nitrate. Electrochim. Act 2018, 278, 421–437. [Google Scholar] [CrossRef]
- Hu, T.; Xiang, B.; Liao, S.G.; Huang, W.Z. Corrosion of AM60B magnesium alloy in simulated acid rain. Anti-Corros. Method. Mat. 2010, 57, 244–248. [Google Scholar] [CrossRef]
- Golabadi, M.; Aliofkhazraei, M.; Toorani, M.; Rouhaghdam, A.S. Evaluation of La containing PEO pretreatment on protective performance of epoxy coating on magnesium. Prog. Org. Coat. 2017, 105, 258–266. [Google Scholar] [CrossRef]
- Tsai, D.S.; Tsai, Y.C.; Chou, C.C. Corrosion passivation of magnesium alloy with the duplex coatings of plasma electrolytic oxidation and tetrafluoroethylene-based polymers. Surf. Coat. Tech. 2019, 366, 15–23. [Google Scholar] [CrossRef]
- Hu, C.; Xu, M.; Zhang, J.; Hu, B.; Yu, G. High corrosion resistance of electroless Ni/Ni–B coating from fluoride-free baths on AZ31 magnesium alloy. J. Alloy. Compd. 2019, 770, 48–57. [Google Scholar] [CrossRef]
- Wu, H.; Shi, Z.; Zhang, X.; Qasim, A.M.; Xiao, S.; Zhang, F.; Wu, Z.; Wu, G.; Ding, K.; et al. Achieving an acid resistant surface on magnesium alloy via bio-inspired design. Appl. Surf. Sci. 2019, 478, 150–161. [Google Scholar] [CrossRef]
- Battocchi, D.; Simões, A.M.; Tallman, D.E.; Bierwagen, G.P. Comparison of testing solutions on the protection of Al-alloys using a Mg-rich primer. Corros. Sci. 2006, 48, 2226–2240. [Google Scholar] [CrossRef]
- Pathak, S.S.; Blanton, M.D.; Mendon, S.K.; Rawlins, J.W. Investigation on dual corrosion performance of magnesium-rich primer for aluminum alloys under salt spray test (ASTM B117) and natural exposure. Corros. Sci. 2010, 48, 1453–1463. [Google Scholar] [CrossRef]
- Yuwono, J.A.; Birbilis, N.; Taylor, C.D.; Williams, K.S.; Samin, A.J.; Medhekar, N.V. Aqueous electrochemistry of the magnesium surface: Thermodynamic and kinetic profiles. Corros. Sci. 2019, 147, 53–68. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Q. Characteristics of the acid rain variation in China during 1993–2006 and associated causes. J. Meteorol. Res. 2010, 24, 239–250. [Google Scholar]
- Chen, M.C.; Wang, K.; Xie, L. Deterioration mechanism of cementitious materials under acid attack. Eng. Fail. Anal. 2013, 27, 272–285. [Google Scholar] [CrossRef]
- Marchebois, H.; Keddam, M.; Savall, C.; Bernard, J.; Touzain, S. Zinc-rich powder coatings characterisation in artificial sea water EIS analysis of the galvanic action. Electrochim. Acta 2004, 49, 1719–1729. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of water transport behavior and impedance models of epoxy-coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]
- Gabrielli, C.; Keddam, M.; Mattos, O.R.; Takenouti, H. Charge transfer resistance measurements by galvanostatic double pulse and impedance methods. J. Electroanal. Chem. Interfacial Electrochem. 1981, 117, 147–153. [Google Scholar] [CrossRef]
- Elsner, C.I.; Cavalcanti, E.; Ferraz, O.; Di Sarli, A.R. Evaluation of the surface treatment effect on the anticorrosive performance of paint systems on steel. Prog. Org. Coat. 2003, 48, 50–62. [Google Scholar] [CrossRef]
- Del Amo, B.; Véleva, L.; Di Sarli, A.R.; Elsner, C.I. Performance of coated steel systems exposed to different media Part, I. Painted galvanized steel. Prog. Org. Coat. 2004, 50, 179–192. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279. [Google Scholar] [CrossRef]
- Kendig, M.; Mansfeld, F.; Tsai, S. Determination of the long term corrosion behavior of coated steel with AC impedance measurements. Corros. Sci. 1983, 23, 317–329. [Google Scholar] [CrossRef]
- Mcintyre, N.S.; Chen, C. Role of impurities on Mg surfaces under ambient exposure conditions. Corros. Sci. 1998, 40, 1697–1709. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film onmagnesium metal: A characterization by X-ray photoelectron spectroscopy(XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef]
- Ardelean, H.; Frateur, I.; Zanna, S.; Atrens, A.; Marcus, P. Corrosion protection of AZ91 magnesium alloy by anodizing in niobium and zirconium-containing electrolytes. Corros. Sci. 2009, 51, 3030–3038. [Google Scholar] [CrossRef]
- He, W.; Zhang, E.; Yang, K. Effect of Y on the bio-corrosion behavior of extruded Mg–Zn–Mn alloy in Hank’s solution. Mat. Sci. Eng. C 2010, 30, 167–174. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, S.; Jiang, F.; Wang, F. Cerium conversion coatings for AZ91D magnesium alloy in ethanol solution and its corrosion resistance. Corros. Sci. 2009, 51, 2916–2923. [Google Scholar] [CrossRef]
- Holt, L.E., Jr.; Pierce, J.A.; Kajdi, C.N. The solubility of the phosphates of strontium, barium, and magnesium and their relation to the problem of calcification. J. Colloid Sci. 1954, 9, 409–426. [Google Scholar] [CrossRef]
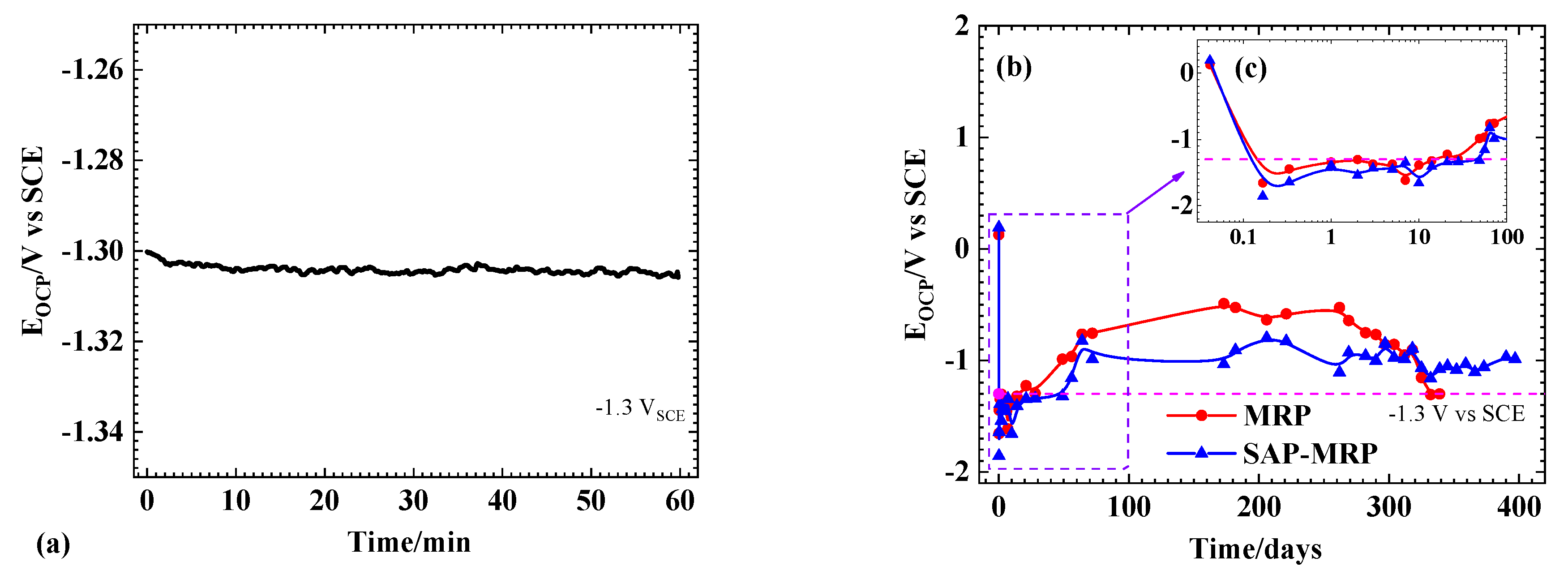
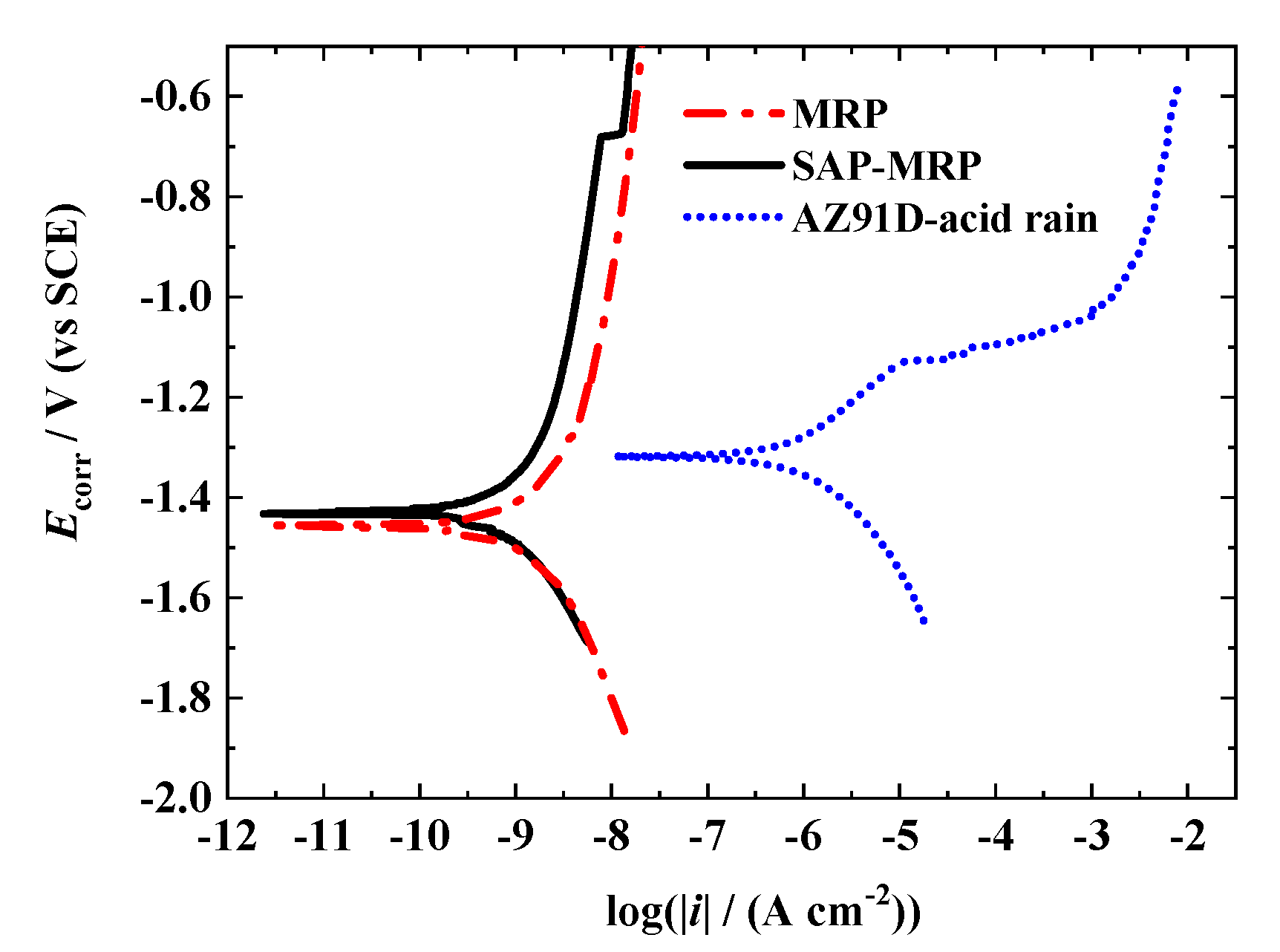
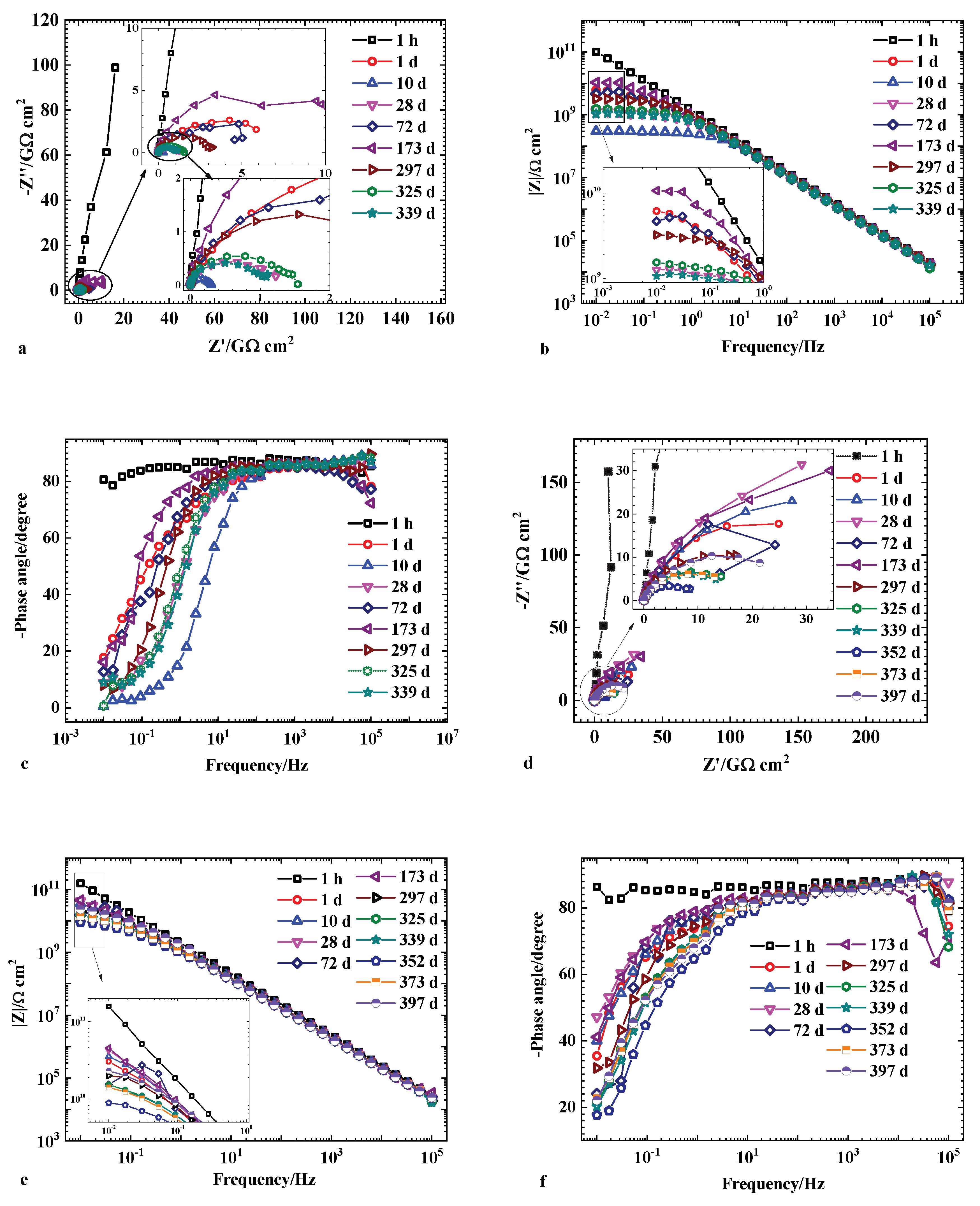
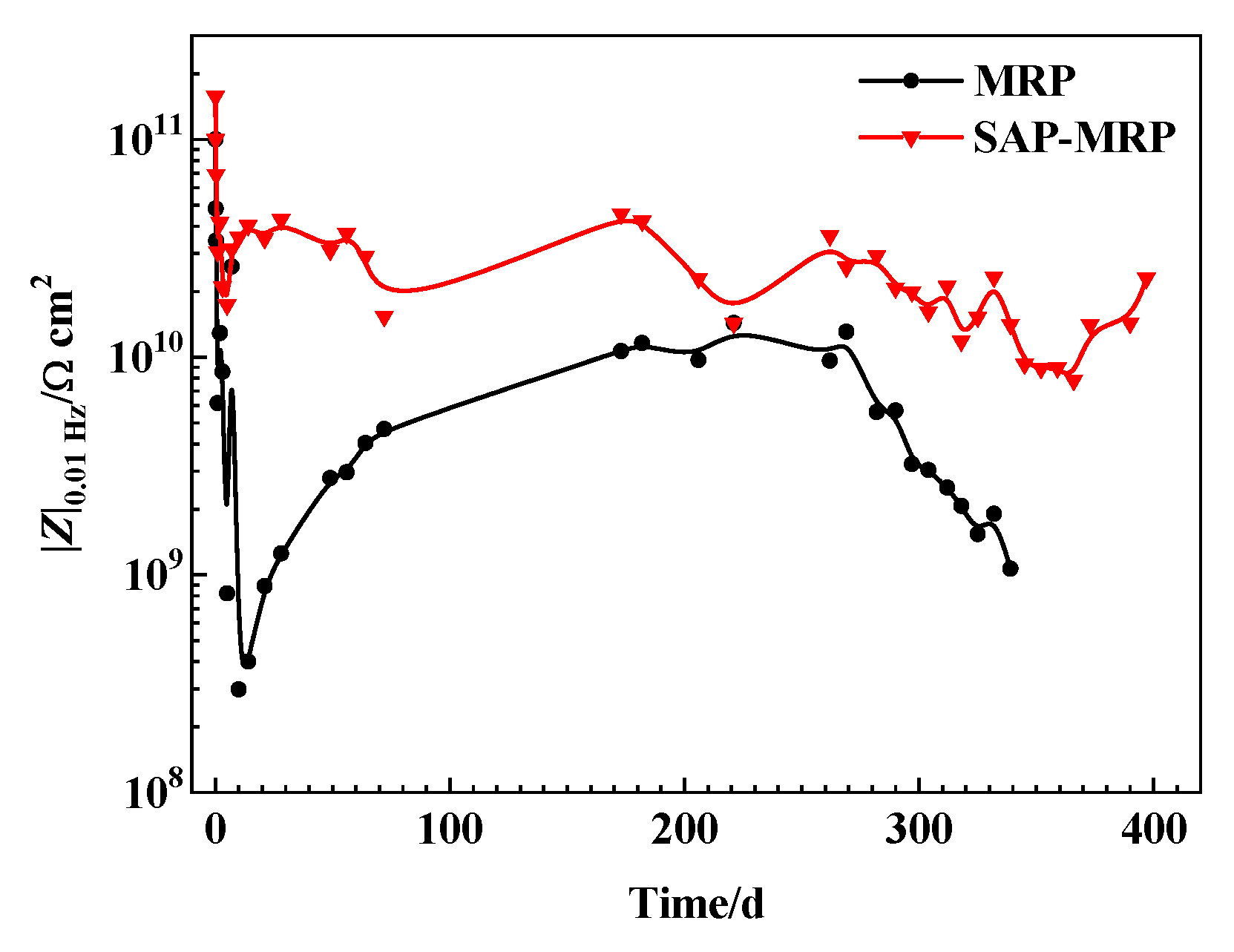
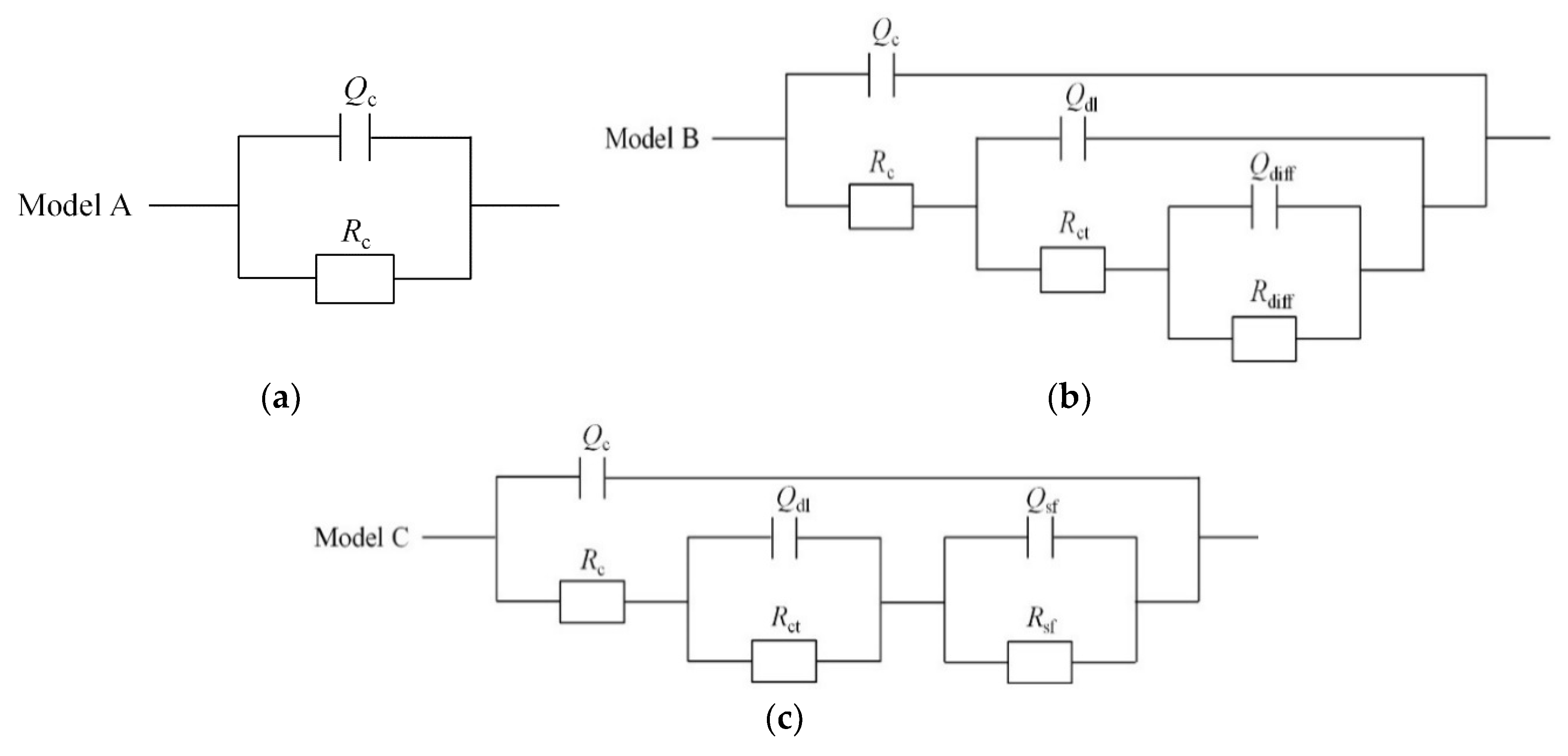
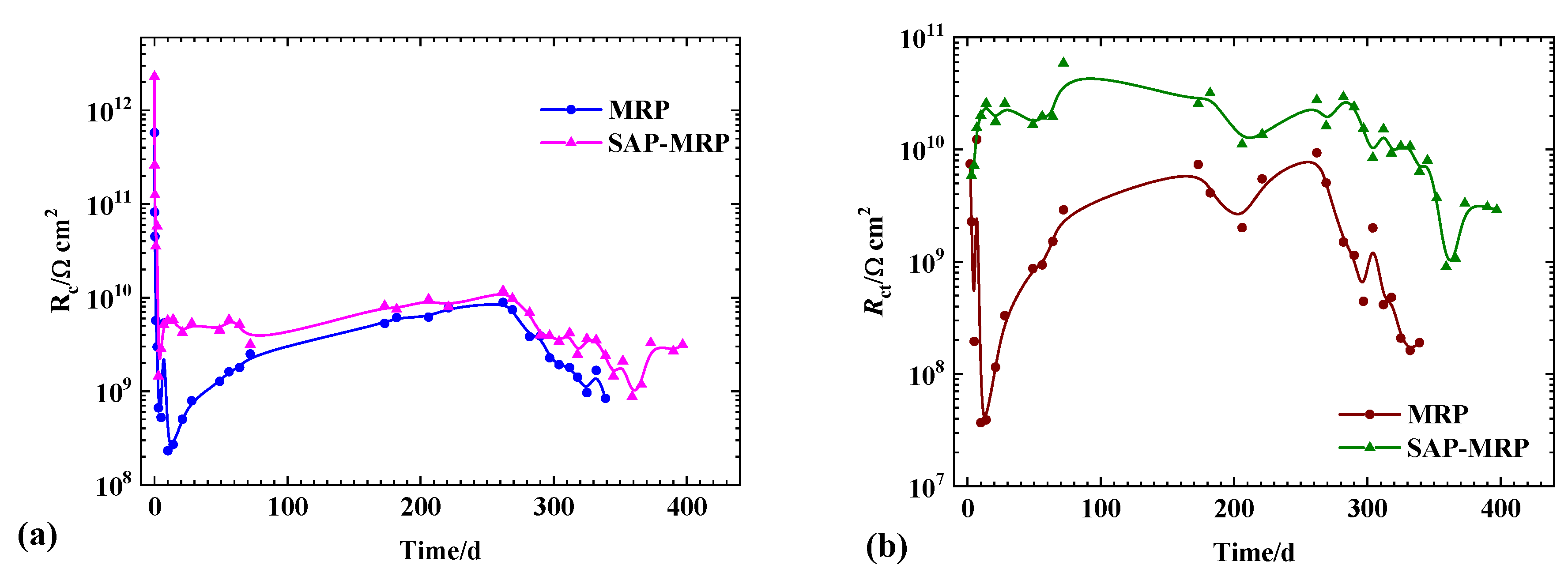
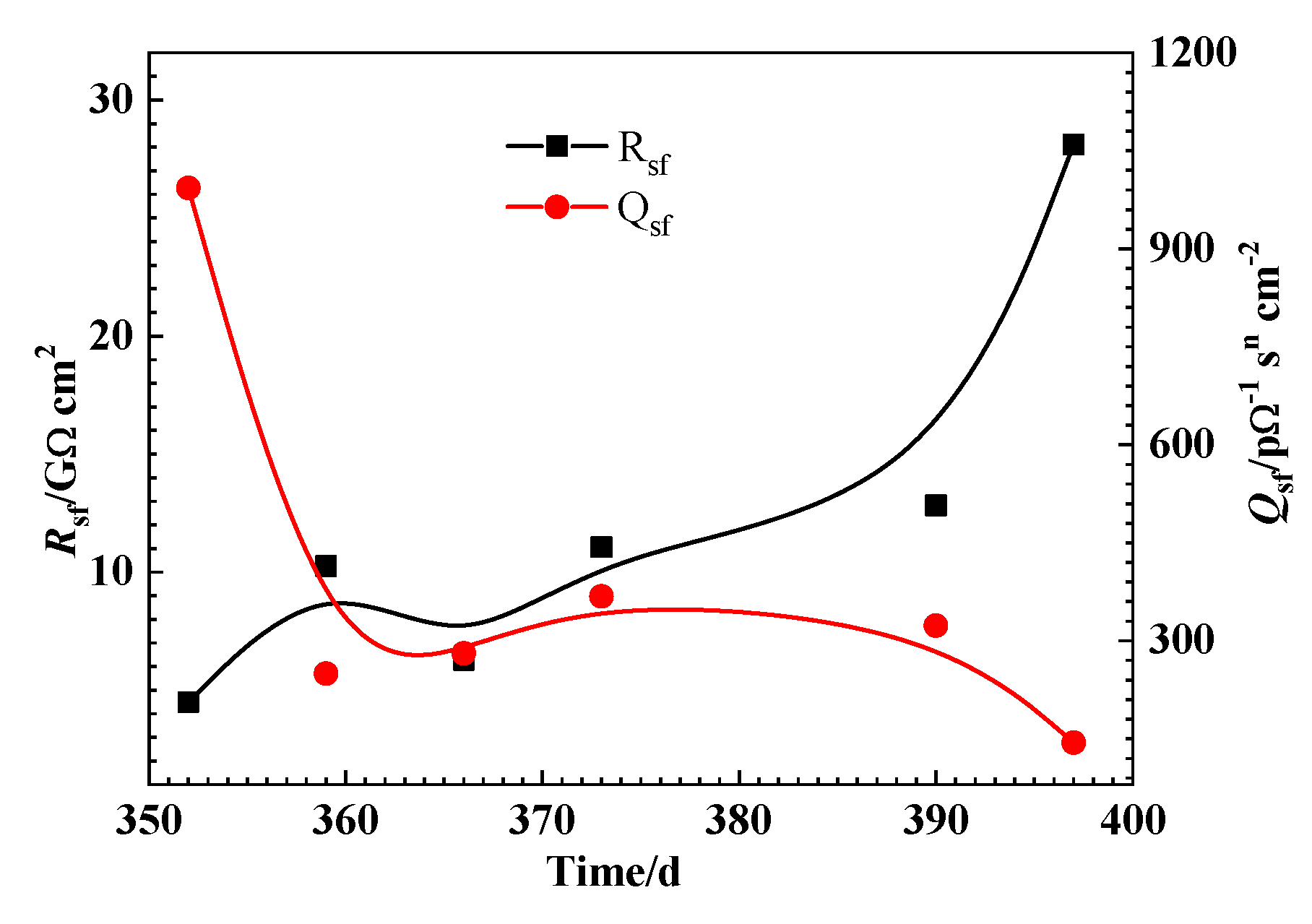
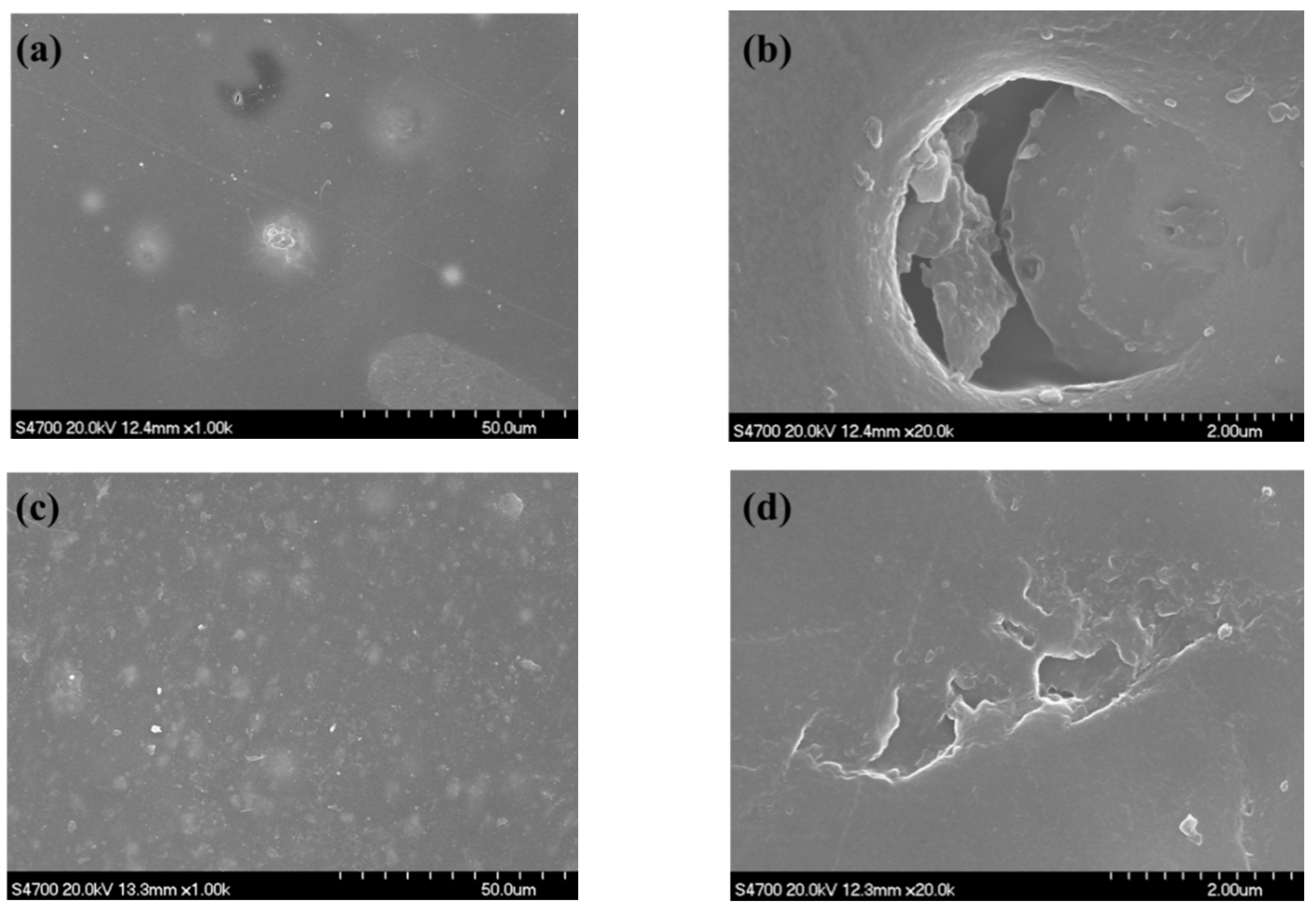
| Al | Mn | Zn | Fe | Si | Ni | Cu | Mg |
|---|---|---|---|---|---|---|---|
| 9.4 | 0.23 | 0.82 | 0.005 | 0.01 | 0.002 | 0.02 | remainder |
| SO42− | NO3− | Mg2+ | NH4+ | Ca2+ |
|---|---|---|---|---|
| 0.1 | 0.015 | 0.002 | 0.002 | 0.001 |
| Sample | Ecorr (V vs. SCE) | icorr (A cm−2) | βa (mV/decade) | βc (mV/decade) |
|---|---|---|---|---|
| AZ91D | −1.3185 | 1.3793 × 10−6 | 256.30 | 238.45 |
| MRP | −1.4589 | 1.1817 × 10−9 | 456.32 | 366.95 |
| SAP-MRP | −1.4301 | 1.3192 × 10−9 | 566.02 | 360.21 |
| Samples | MRP | SAP-MRP | ||||
|---|---|---|---|---|---|---|
| Immersion Time | 1 h | 10 Days | 339 Days | 1 h | 10 Days | 373 Days |
| Qc (Ω−1 sn cm−2) | 1.27 × 10−10 | 1.73 × 10−10 | 1.84 × 10−10 | 9.01 × 10−11 | 1.27 × 10−10 | 1.50 × 10−10 |
| error (%) | 1.684 | 0.7086 | 0.6032 | 1.172 | 0.9542 | 0.9875 |
| nc | 0.96743 | 0.95634 | 0.9565 | 0.96844 | 0.95032 | 0.9409 |
| error (%) | 0.2293 | 0.1261 | 0.6987 | 0.1677 | 0.1513 | 0.2241 |
| Rc (Ω·cm2) | 5.79 × 1011 | 2.32 × 108 | 8.40 × 108 | 2.29 × 1012 | 5.68 × 109 | 3.38 × 109 |
| error | 3.384 | 3.631 | 2.414 | 3.572 | 2.134 | 1.397 |
| Qdl (Ω−1.sn.cm−2) | − | 1.63 × 10−9 | 3.08 × 10−9 | − | 4.69 × 10−11 | 2.33 × 10−10 |
| error (%) | − | 3.161 | 2.834 | − | 2.271 | 3.946 |
| ndl | − | 0.93551 | 0.95069 | − | 0.94445 | 0.93198 |
| error (%) | − | 2.926 | 1.021 | − | 0.3079 | 1.239 |
| Rct (Ω·cm2) | − | 3.68 × 107 | 1.90 × 108 | − | 2.01 × 1010 | 3.31 × 109 |
| error (%) | − | 0.2583 | 1.184 | − | 1.831 | 4.641 |
| Qdiff/Qsf (Ω−1·sn·cm−2) | − | 2.28 × 10−8 | 3.74 × 10−8 | − | 2.23 × 10−10 | 3.68 × 10−10 |
| error (%) | − | 2.285 | 1.544 | − | 2.888 | 3.504 |
| ndiff/nsf | − | 0.82824 | 0.74574 | − | 0.83679 | 0.76716 |
| error (%) | − | 2.075 | 1.527 | − | 2.186 | 4.347 |
| Rdiff/Rsf (Ω·cm2) | − | 3.08 × 107 | 1.49 × 109 | − | 3.39 × 1010 | 1.11 × 1010 |
| error (%) | − | 2.502 | 3.898 | − | 1.463 | 1.741 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Sun, S.; Fan, Q.; Pei, X.; Dun, Y.; Feng, X.; Zou, C.; Lu, W. Investigation of Protective Performance of a Mg-Rich Primer Containing Aluminum Tri-Polyphosphate on AZ91D Magnesium Alloy in Simulated Acid Rain. Coatings 2019, 9, 649. https://doi.org/10.3390/coatings9100649
Lu X, Sun S, Fan Q, Pei X, Dun Y, Feng X, Zou C, Lu W. Investigation of Protective Performance of a Mg-Rich Primer Containing Aluminum Tri-Polyphosphate on AZ91D Magnesium Alloy in Simulated Acid Rain. Coatings. 2019; 9(10):649. https://doi.org/10.3390/coatings9100649
Chicago/Turabian StyleLu, Xiangyu, Sichen Sun, Qiqi Fan, Xiangjun Pei, Yuchao Dun, Xingguo Feng, Chen Zou, and Wang Lu. 2019. "Investigation of Protective Performance of a Mg-Rich Primer Containing Aluminum Tri-Polyphosphate on AZ91D Magnesium Alloy in Simulated Acid Rain" Coatings 9, no. 10: 649. https://doi.org/10.3390/coatings9100649
APA StyleLu, X., Sun, S., Fan, Q., Pei, X., Dun, Y., Feng, X., Zou, C., & Lu, W. (2019). Investigation of Protective Performance of a Mg-Rich Primer Containing Aluminum Tri-Polyphosphate on AZ91D Magnesium Alloy in Simulated Acid Rain. Coatings, 9(10), 649. https://doi.org/10.3390/coatings9100649





