Preparation of Micro-Patterned CaMn7O12 Ceramic Films via a Photosensitive Sol-Gel Method
Abstract
:1. Introduction
2. Experimental
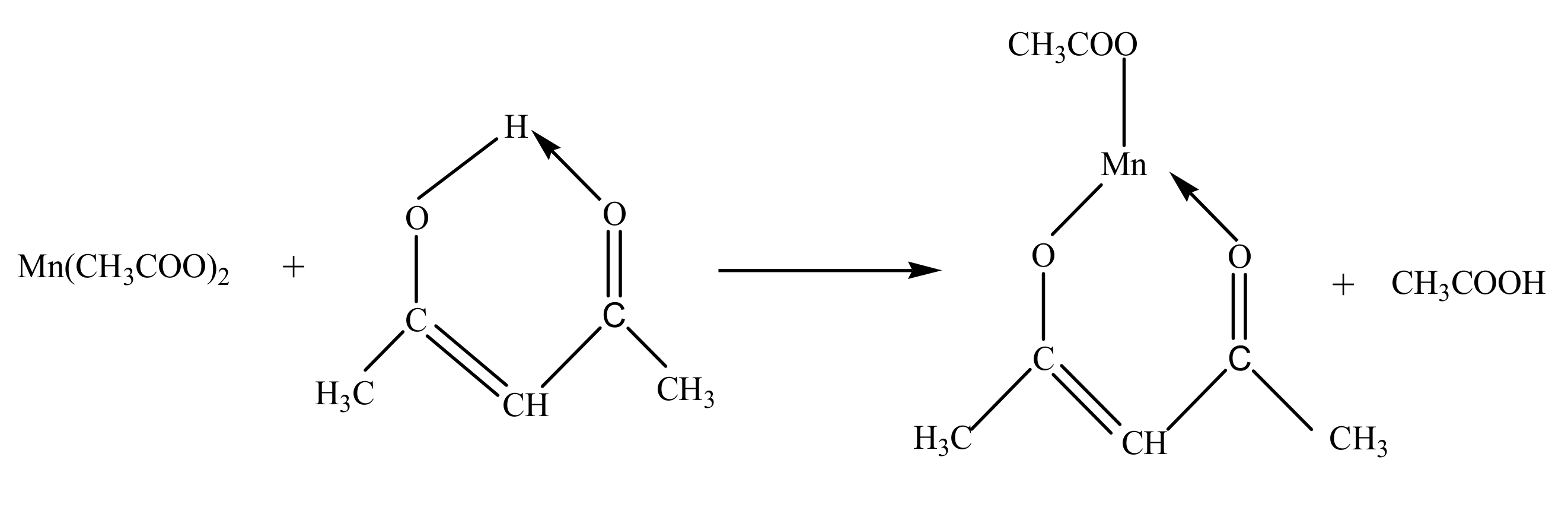
2.1. Preparation of Photosensitive CaMn7O12 Sol
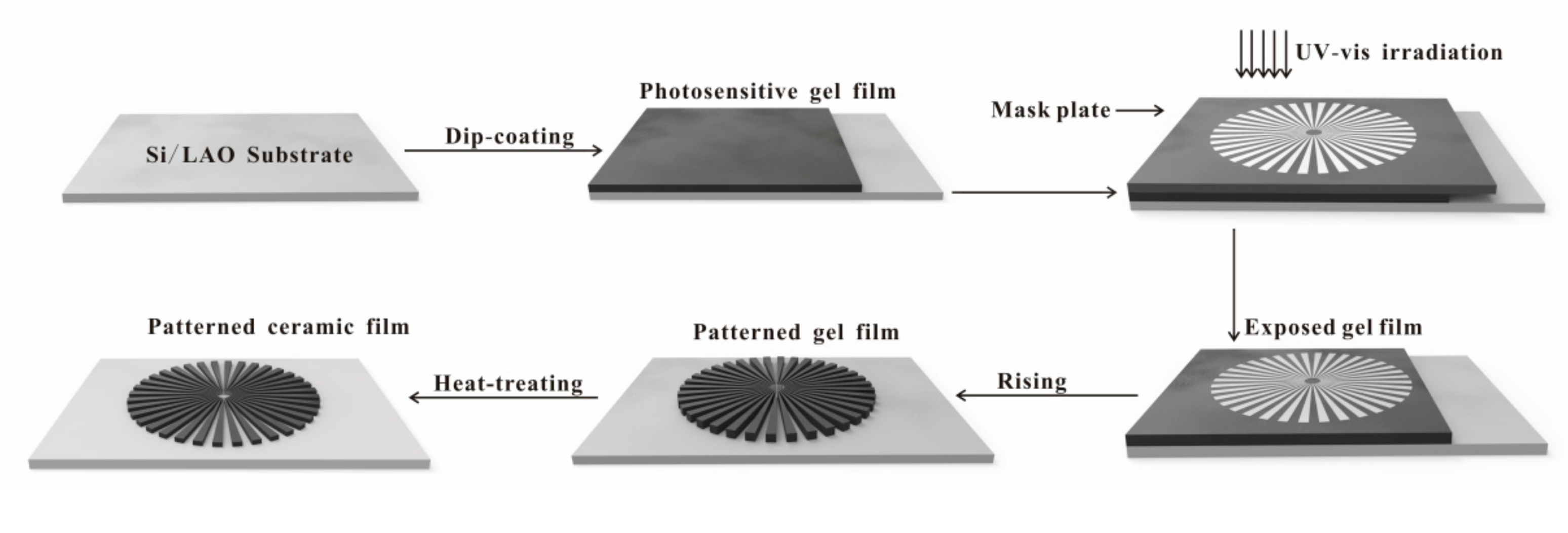
2.2. Preparation of Micro-Patterned CaMn7O12 Ceramic Film
2.3. Characterizations
3. Results and Discussion
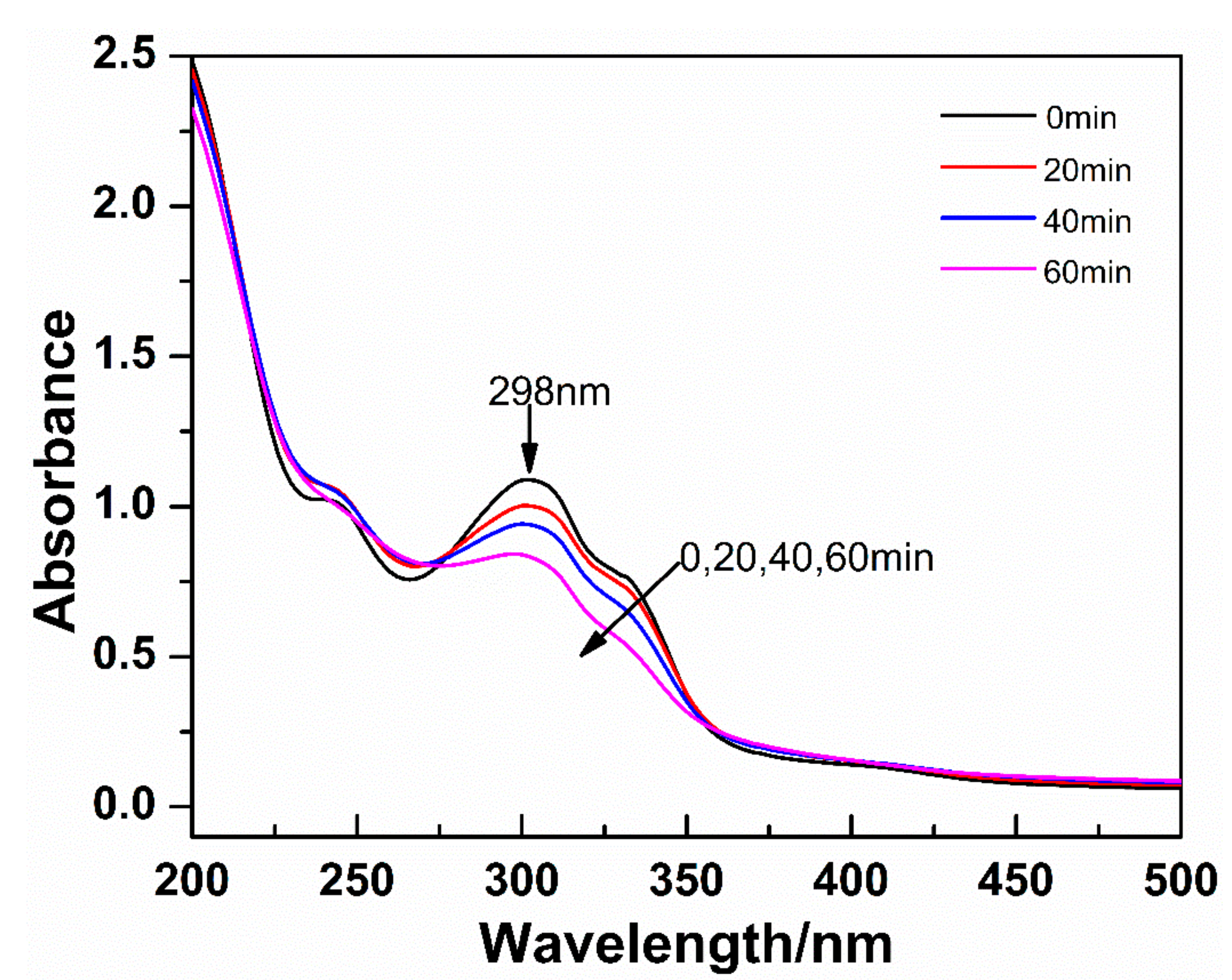
3.1. Photo-Sensitivity of the CaMn7O12 Sol and Its Gel Thin Film
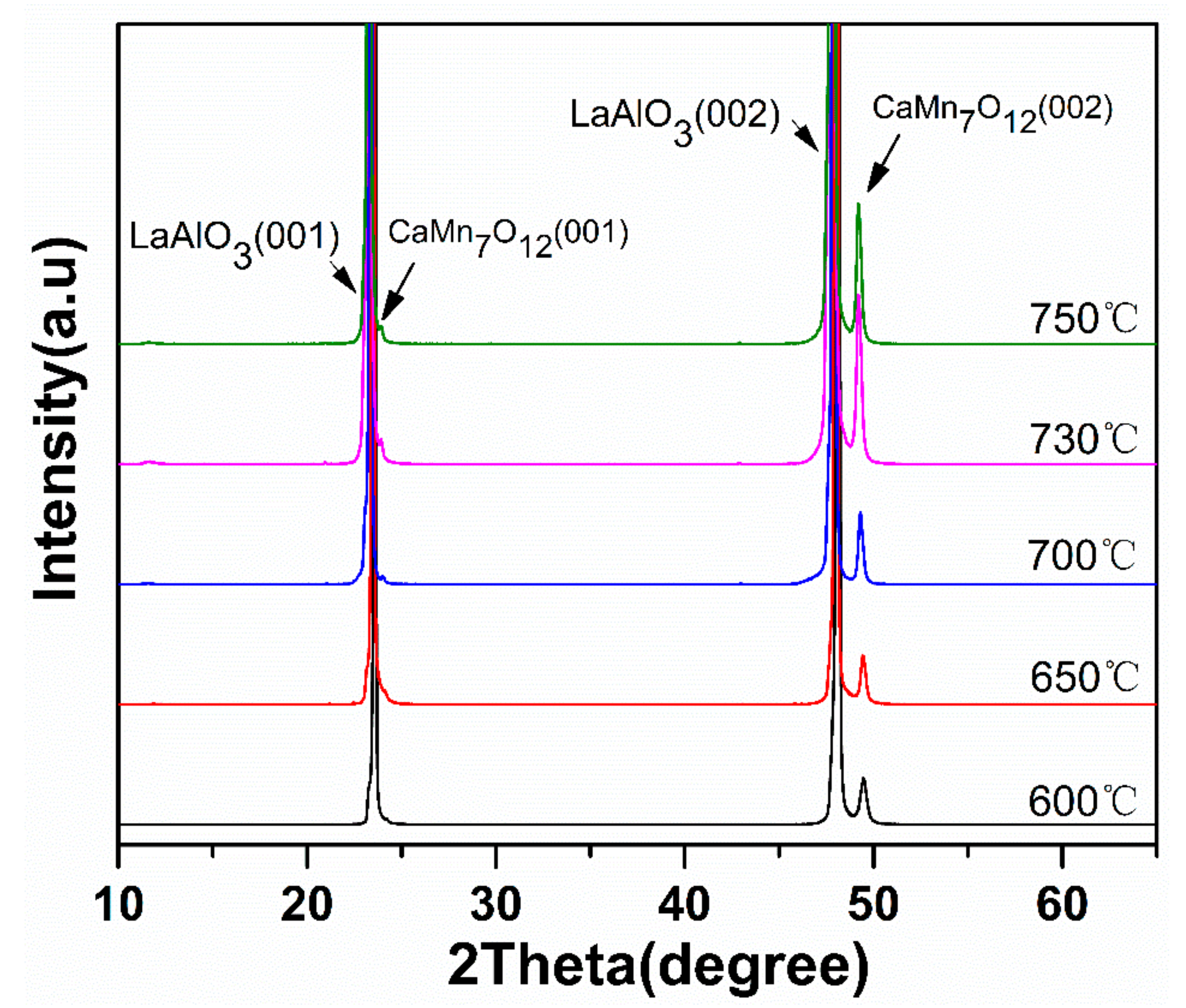
3.2. Phase Composition of Micro-Patterned CaMn7O12 Ceramic Film
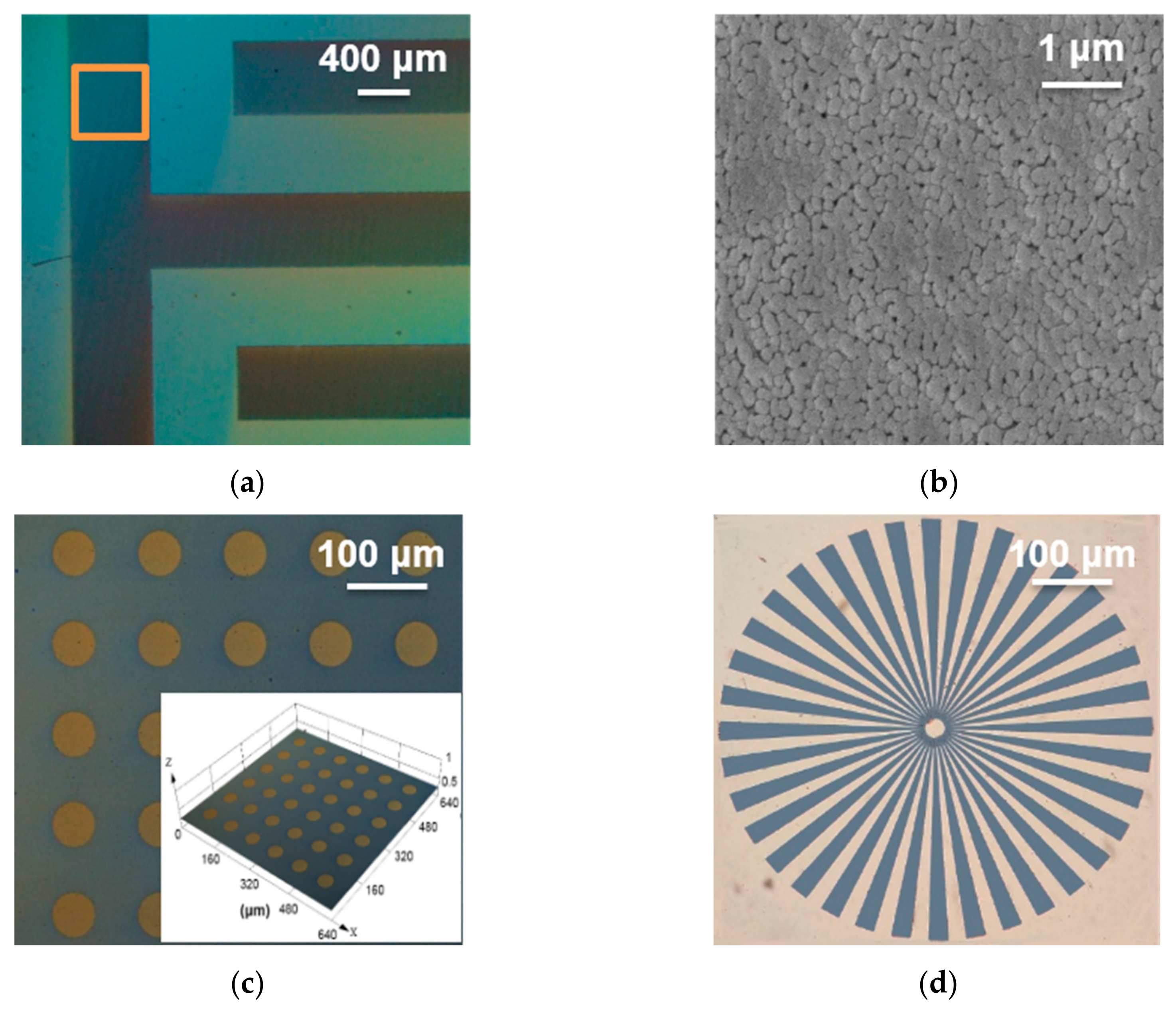
3.3. Micrograph of Micro-Patterned CaMn7O12 Ceramic Film
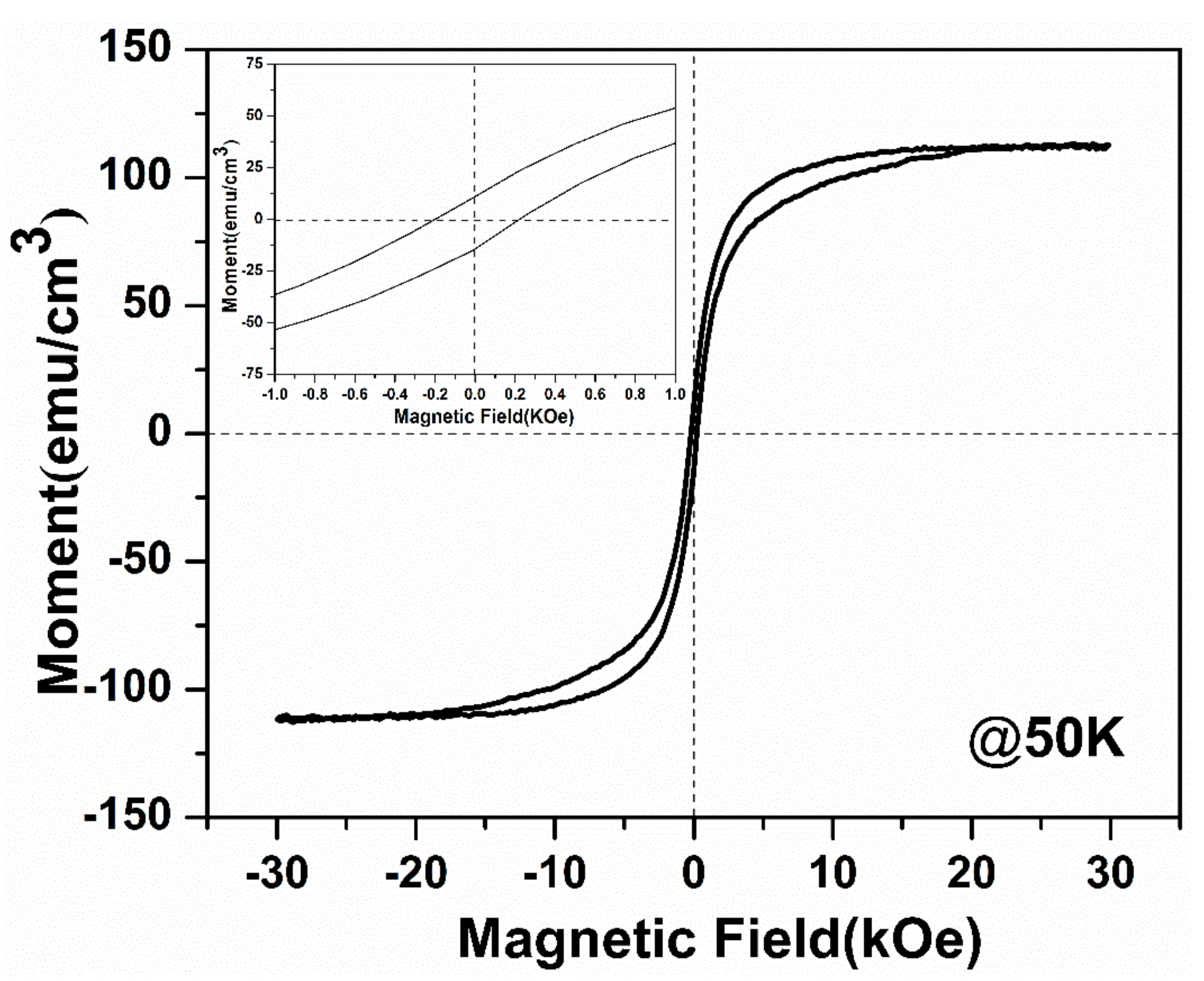
3.4. Magnetic Properties of the Micro-Patterned CaMn7O12 Ceramic Film
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, C.L.; Hu, W.J.; Tian, Y.F.; Wu, T. Multiferroic oxide thin films and heterostructures. Appl. Phys. Rev. 2015, 2, 021304. [Google Scholar] [CrossRef]
- Ramesh, R.; Spaldin, N.A. Multiferroics: Progress and prospects in thin films. Nat. Mater. 2007, 6, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, M. Revival of the magnetoelectric effect. J. Phys. D Appl. Phys. 2005, 38, R1–R30. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J. Multiferroic and magnetoelectric materials. Nature 2006, 44, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Spaldin, N.A.; Fiebig, M. The renaissance of magnetoeletric multiferroics. Science 2005, 309, 391–392. [Google Scholar] [CrossRef]
- Lu, X.Z.; Whangbo, M.H.; Dong, S.; Gong, X.G.; Xiang, H.J. Giant ferroelectric polarization of CaMn7O12 induced by combined effect of Dzyaloshinskii-Moriya interaction and exchange striction. Phys. Rev. Lett. 2012, 108, 187204. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.D.; Chapon, L.C.; Khalyavin, D.D.; Manuel, P.; Radaelli, P.G.; Martin, C. Giant improper ferroelectricity in the ferroaxial magnet CaMn7O12. Phys. Rev. Lett. 2012, 108, 067201. [Google Scholar] [CrossRef] [PubMed]
- Perks, N.J.; Johnson, R.D.; Martin, C.; Chapon, L.C.; Radaelli, P.G. Magneto-orbital helices as a route to route to coupling magnetism and ferroelectricity in multiferroic CaMn7O12. Nat. Commun. 2012, 3, 1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Dong, S.; Yan, Z.B.; Guo, Y.Y.; Zhang, Q.F.; Yunoki, S.; Dagotto, E.; Liu, J.M. Multiferroic properties of CaMn7O12. Phys. Rev. B 2011, 84, 174413. [Google Scholar] [CrossRef]
- Zhang, H.G.; Ma, X.C.; Xie, L. The structural and magnetic properties of Sr-doped multiferroic CaMn7O12. Int. J. Mod. Phys. B 2015, 29, 1550221. [Google Scholar] [CrossRef]
- Zhang, J.T.; Lu, X.M.; Zhou, J.; Sun, H.; Huang, F.Z.; Zhu, J.S. Magnetic properties and origins of ferroelectric polarization in multiferrioic CaMn7O12. Phys. Rev. B 2013, 87, 075127. [Google Scholar] [CrossRef]
- Gautam, K.; Shukla, D.K.; Francoual, S.; Bednarcik, J.; Mardegan, J.R.L.; Liermann, H.-P.; Sankar, R.; Chou, F.C.; Phase, D.M.; Strempfer, J. Large negative thermal expansion in the cubic phase CaMn7O12. Phys. Rev. B 2017, 95, 144112. [Google Scholar] [CrossRef]
- Przenioslo, R.; Sosnowska, I.; Suard, E.; Hansen, T. Magnetic order parameter in the perovskite system CaMn7O12. Appl. Phys. A Mater. 2002, 74, S1731–S1733. [Google Scholar] [CrossRef]
- Bochu, B.; Buevoz, J.L.; Chenavas, J.; Collomb, A.; Joubert, J.C.; Marezio, M. Bond lengths in [CaMn3](Mn4)O12: A new Jahn-Teller distortion of Mn3+ octahedra. Solid State Commun. 1980, 36, 133–138. [Google Scholar] [CrossRef]
- Bochu, B.; Chenavas, J.; Joubert, J.C.; Marezio, M. High Pressure Synthesis and crystal structure of a new series of perovskite-like compounds CMn7O12(C=Na,Ca,Cd,Sr,La,Nd). J. Solid State Chem. 1974, 11, 88–93. [Google Scholar] [CrossRef]
- Przeniosło, R.; Sosnowska, I.; Zoltek, M.; Hohlwein, D.; Troyanchuk, I.O. A modulated magnetic structure in CaMn7O12. Physica B 1997, 241–243, 730–732. [Google Scholar] [CrossRef]
- Przeniosło, R.; Sosnowska, I.; Hohlwein, D.; Hauß, T.; Troyanchuk, I.O. Magnetic ordering in the manganese perovskite CaMn7O12. Solid State Commun. 1999, 111, 687–692. [Google Scholar] [CrossRef]
- Przeniosło, R.; Sosnowska, I.; Strunz, P.; Hohlwein, D.; Hauß, T.; Troyanchuk, I.O. SANS study of magnetic phase transitions in CaMn7O12. Physica B 2000, 276–278, 547–548. [Google Scholar] [CrossRef]
- Huon, A.; Lang, A.C.; Saldana-Greco, D.; Lim, J.S.; Moon, E.J.; Rappe, A.M.; Taheri, M.L.; May, S.J. Electronic transition above room temperature in CaMn7O12 films. Appl. Phys. Lett. 2015, 107, 142901. [Google Scholar] [CrossRef]
- Huon, A.; Lee, D.; Herklotz, A.; Fitzsimmons, M.R.; Lee, H.N.; May, S.J. Effect of chemical pressure on the electronic phase transition in Ca1-xSrxMn7O12 films. Appl. Mater. 2017, 5, 096105. [Google Scholar] [CrossRef]
- Huon, A.; Vibhakar, A.M.; Grutter, A.J.; Borchers, J.A.; Disseler, S.; Liu, Y.H.; Tian, W.; Orlandi, F.; Manuel, P.; Khalyavin, D.D.; et al. Helical magnetism in sr-doped CaMn7O12 films. Phys. Rev. B 2018, 98, 224419. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zhao, G.Y.; Wu, C.B.; Duan, Z.F. Preparation of epitaxial CaMn7O12 film via sol-gel method and its ferromagnetic properties. J. Sol Gel Sci. Technol. 2018, 88, 639–645. [Google Scholar] [CrossRef]
- Shiinmou, K.; Tohge, N.; Minami, T. Fine-patterning of ZrO2 thin films by the photolysis of chemically Modified gel films. Jpn. J. Appl. Phys. 1994, 33, 1181–1184. [Google Scholar] [CrossRef]
- Soyer, C.; Catttan, E.; Remiens, D.; Guilloux-viry, M. Ion beam etching of lead-zirconate-titanate thin films: Correlation between etching parameters and electrical properties evolution. J. Appl. Phys. 2002, 92, 1048. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, T.Y.; Chung, K.I.; Desu, S.B. Characterization and elimination of dry etching damaged layer in Pt/Pb(Zr0.53Ti0.47)O3/PtPt/Pb(Zr0.53Ti0.47)O3/Pt ferroelectric capacitor. Appl. Phys. Lett. 1999, 75, 334. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Yan, F.X.; Zhao, G.Y.; Wang, Z.Z. Photosensitive sol-gel preparation and direct micro-patterning of c-oriented ZnO film. Appl. Surf. Sci. 2011, 257, 6817–6820. [Google Scholar]
- Yan, F.X.; Zhao, G.Y.; Song, N.; Zhao, N.N.; Chen, Y.Q. In situ synthesis and characterization of fine-patterned La and Mn co-doped BiFeO3 film. J. Alloy. Compd. 2013, 507, 19–22. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Zhang, H.L.; Chen, Y.Q.; Peng, H.J. Fabrication of YBCO film patterns and their properties. Supercond. Sci. Technol. 2008, 21, 125016–125019. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, Y.; Zhao, G.Y. Facile single-step fabrications of electrochromic WO3 micro-patterned films using the novel photosensitive sol-gel method. Ceram. Int. 2015, 41, 403–408. [Google Scholar] [CrossRef]
- Zhao, G.R.; Zhao, G.Y. Preparation of amorphous ZrO-SiO2 thin films and their fine patterning. Chin. J. Mater. Res. 2002, 16, 168–171. [Google Scholar]
- Duan, Z.F.; Cui, Y.; Shi, X.J.; Wei, J.N.; Ren, P.R.; Zhao, G.Y. Facile fabrication of micro-patterned LSMO films with unchanged magnetic properties by photosensitive sol-gel method on LaAlO3 substrates. Ceram. Int. 2016, 42, 14100–14106. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhao, G.Y.; Chen, Z.M. Photosensitive PZT gel films and their preparation for fine patterning. Mater. Sci. Eng. B 2003, 99, 168–172. [Google Scholar]
- Duan, Z.F.; Zhao, Y.Z.; Ren, Y.; Jia, J.Q.; Ma, L.; Cui, J.; Wang, Y.W.; Zhao, G.Y. Facile micro-patterning of ferromagnetic CoFe2O4 films using a combined approach of sol–gel method and UV irradiation. Cream. Int. 2019, 45, 369–377. [Google Scholar] [CrossRef]
- Duan, Z.F.; Hu, D.W.; Ohuchia, H.; Zhao, M.Q.; Zhao, G.Y.; Nishioka, Y. Organic field-effect transistors based on two phenylene–thiophene oligomer derivatives with a biphenyl or fluorene core. Synth. Met. 2012, 162, 1292–1298. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, G.; Wu, C.; Duan, Z. Preparation of Micro-Patterned CaMn7O12 Ceramic Films via a Photosensitive Sol-Gel Method. Coatings 2019, 9, 650. https://doi.org/10.3390/coatings9100650
Wang Y, Zhao G, Wu C, Duan Z. Preparation of Micro-Patterned CaMn7O12 Ceramic Films via a Photosensitive Sol-Gel Method. Coatings. 2019; 9(10):650. https://doi.org/10.3390/coatings9100650
Chicago/Turabian StyleWang, Yunwei, Gaoyang Zhao, Chuanbao Wu, and Zongfan Duan. 2019. "Preparation of Micro-Patterned CaMn7O12 Ceramic Films via a Photosensitive Sol-Gel Method" Coatings 9, no. 10: 650. https://doi.org/10.3390/coatings9100650
APA StyleWang, Y., Zhao, G., Wu, C., & Duan, Z. (2019). Preparation of Micro-Patterned CaMn7O12 Ceramic Films via a Photosensitive Sol-Gel Method. Coatings, 9(10), 650. https://doi.org/10.3390/coatings9100650




