Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica papaya L., and Its Physicochemical and Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Papaya Harvest
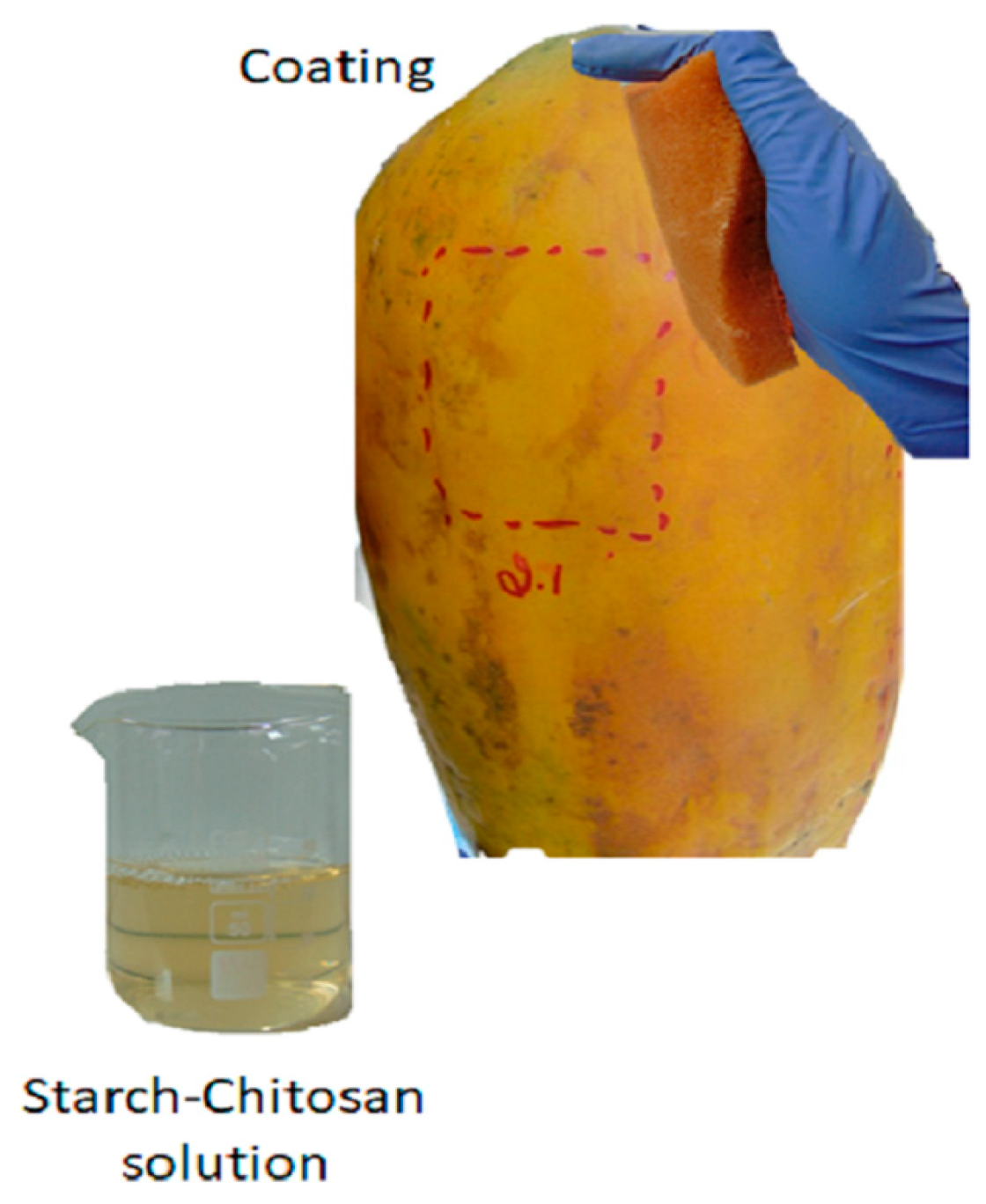
2.2.2. Coating Preparation
2.2.3. Coating Application
2.2.4. Papayas Characterization
Texture and Weight Loss
pH, Soluble Solids, Titratable Acidity, and Vitamin C
Color
Volatile Compounds
2.2.5. Image Analysis
2.2.6. Microbiological Analysis
Total Coliforms
Molds and Yeasts
Mesophilic Aerobic Bacteria
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Edible Coating Characterization
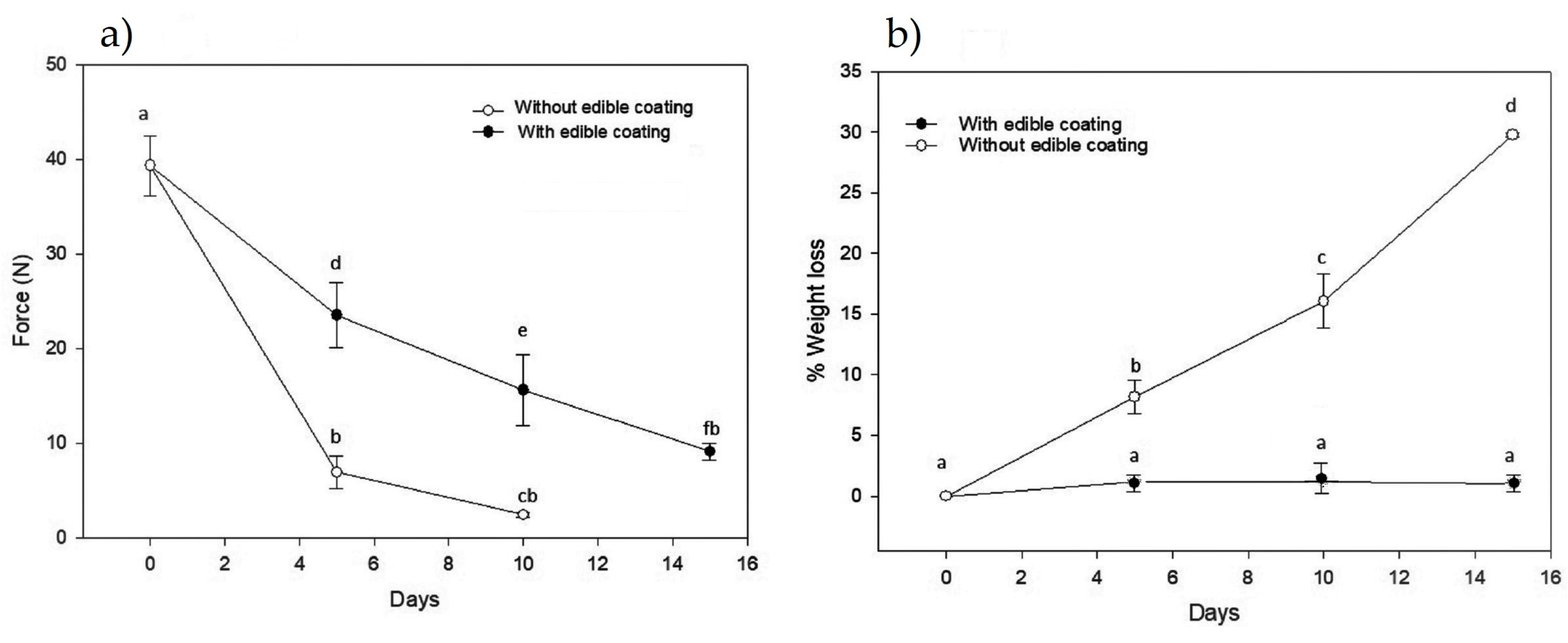
3.1.1. Texture and Weight Loss
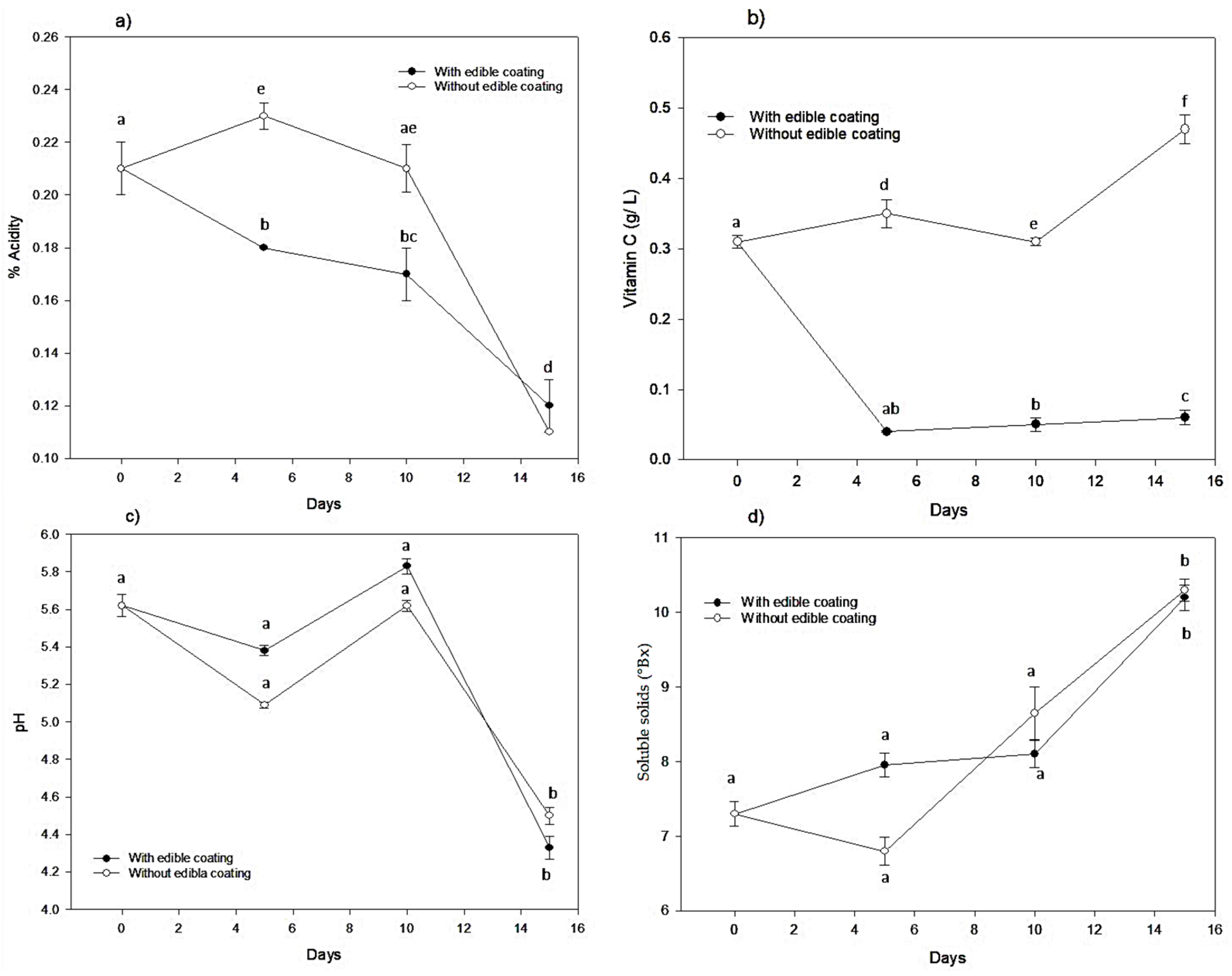
3.1.2. pH, Soluble Solids, Titratable Acidity, and Vitamin C
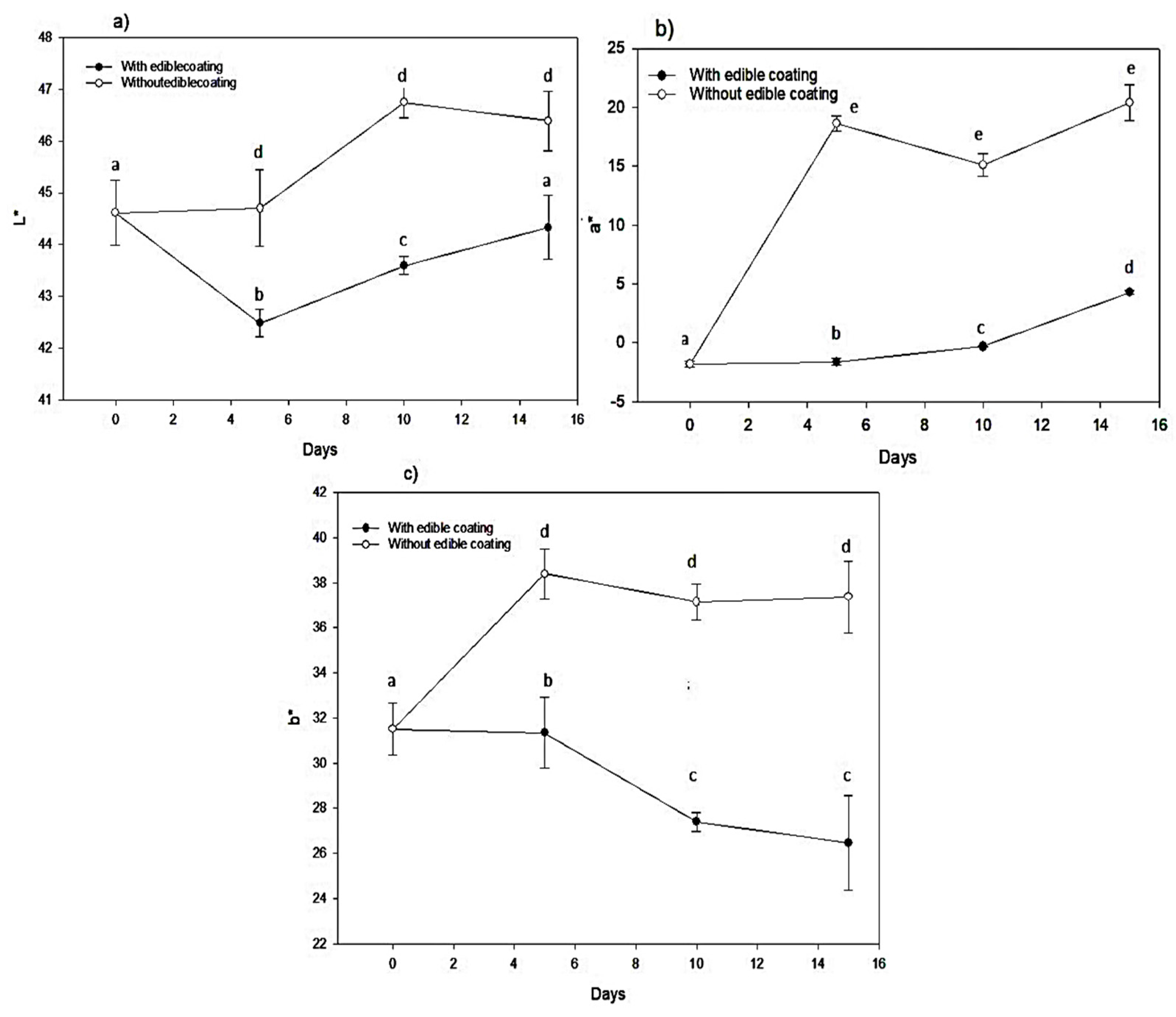
3.1.3. Color
3.1.4. Volatile Compounds
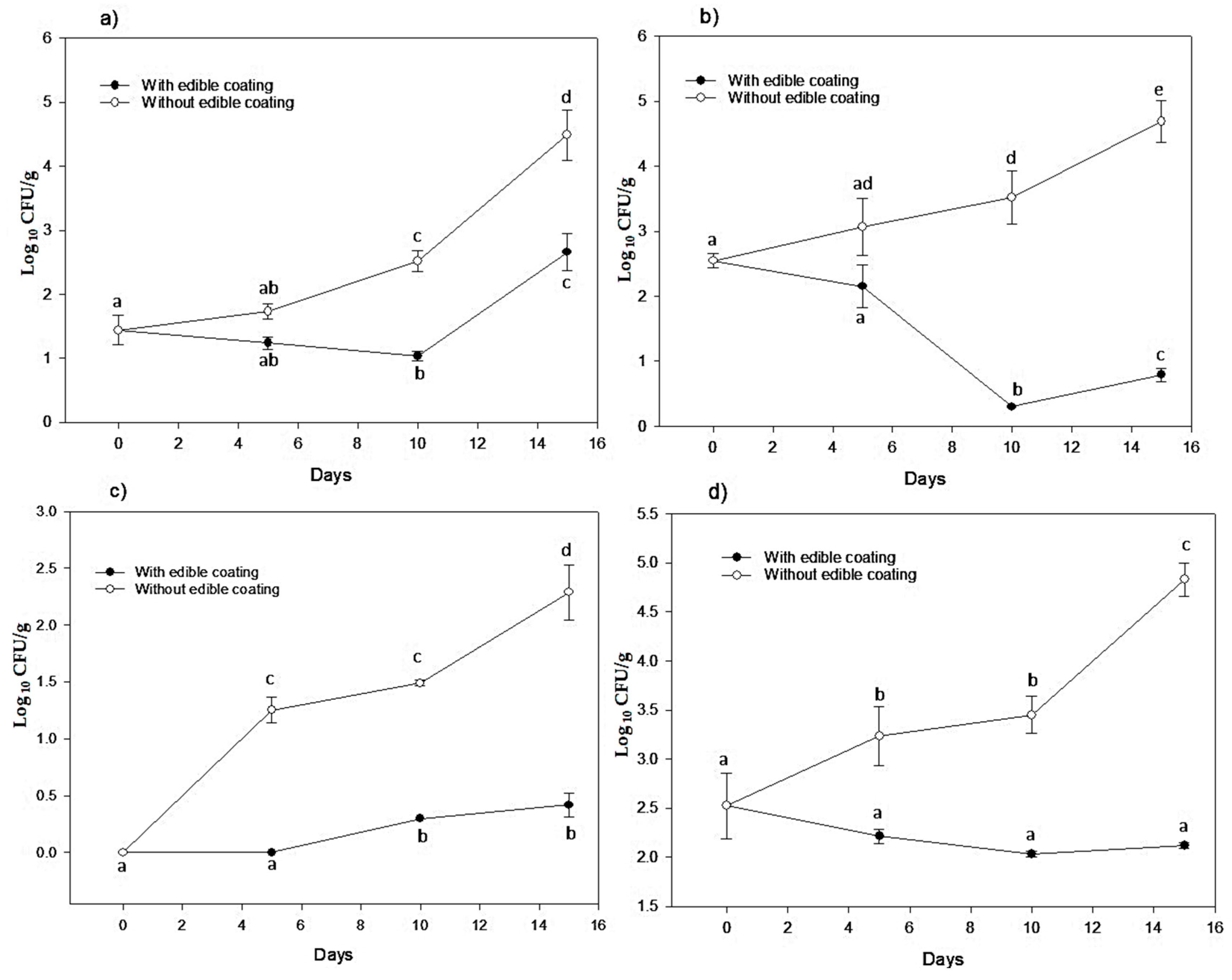
3.2. Microbiological Analysis
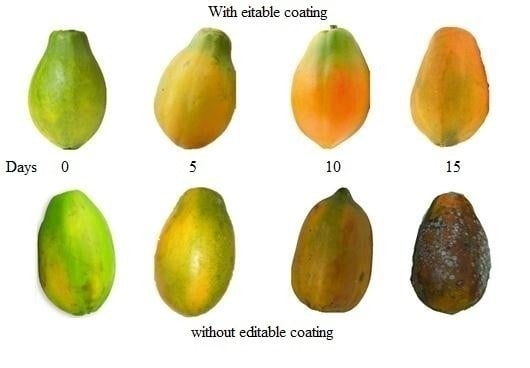
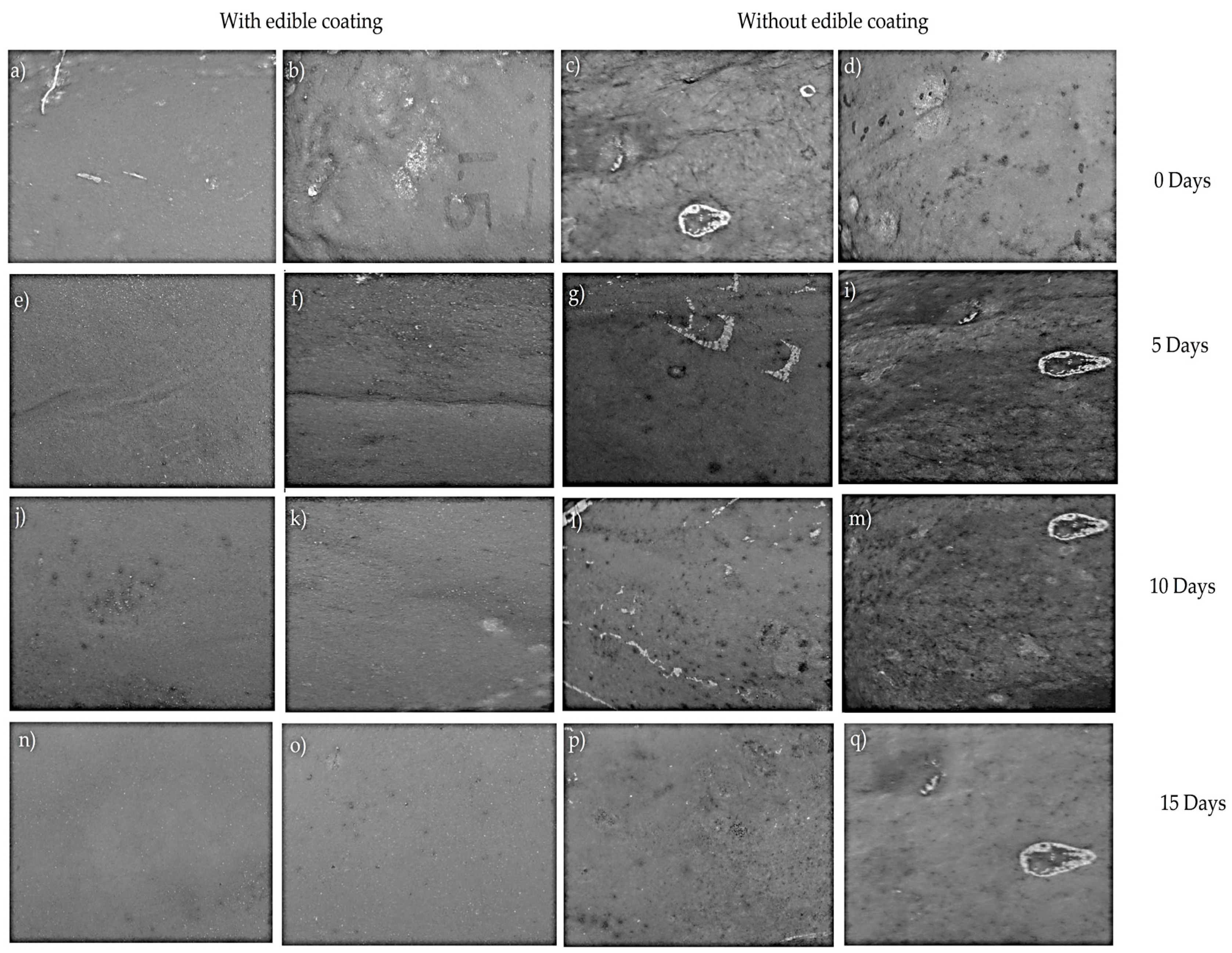
3.3. Image Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojo, O.A.; Ojo, A.B.; Awoyinka, O.; Ajiboye, B.O.; Oyinloye, B.E.; Osukoya, O.A.; Olayide, I.I.; Ibitayo, A. Aqueous extract of Carica papaya Linn. roots potentially attenuates arsenic induced biochemical and genotoxic effects in Wistar rats. J. Tradit. Complement. Med. 2018, 8, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, T.M.; Rodrigues, W.P.; Netto, A.T.; de Oliveira Reis, F.; Peçanha, A.L.; de Assis Figueiredo, F.A.M.M.; de Sousa, E.F.; Glenn, D.M.; Campostrini, E. Comparison between single-leaf and whole-canopy gas exchange measurements in papaya (Carica papaya L.) plants. Sci. Hortic. 2016, 209, 73–78. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://fenix.fao.org/faostat/internal/en/#data/QC (accessed on 3 August 2018).
- Zillo, R.R.; da Silva, P.P.M.; de Oliveira, J.; da Glória, E.M.; Spoto, M.H.F. Carboxymethylcellulose coating associated with essential oil can increase papaya shelf life. Sci. Hortic. 2018, 239, 70–77. [Google Scholar] [CrossRef]
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Ghazali, F.M. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika). Postharvest. Biol. Technol. 2013, 75, 142–146. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Poverenov, E. Improving food products’ quality and storability by using layer by layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81–92. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT-Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Lima Oliveira, P.D.; de Oliveira, K.Á.R.; dos Santos Vieira, W.A.; Câmara, M.P.S.; de Souza, E.L. Control of anthracnose caused by Colletotrichum species in guava, mango and papaya using synergistic combinations of chitosan and Cymbopogon citratus (D.C. ex Nees) Stapf. essential oil. Int. J. Food Microbiol. 2018, 266, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries postharvest life. Food Hydrocoll. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- Guimarães, I.C.; dos Reis, K.C.; Menezes, E.G.T.; Rodrigues, A.C.; da Silva, T.F.; de Oliveira, I.R.N.; de Barros Vilas Boas, E.V. Cellulose microfibrillated suspension of carrots obtained by mechanical defibrillation and their application in edible starch films. Ind. Crops Prod. 2016, 89, 285–294. [Google Scholar] [CrossRef]
- Zhu, F. Modifications of starch by electric field based techniques. Trends Food Sci. Technol. 2018, 75, 158–169. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Pezo, D.; Navascués, B.; Salafranca, J.; Nerín, C. Analytical procedure for the determination of Ethyl Lauroyl Arginate (LAE) to assess the kinetics and specific migration from a new antimicrobial active food packaging. Anal. Chim. Acta 2012, 745, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Oh, D.-H. Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. Technol. 2016, 34, 376–384. [Google Scholar] [CrossRef]
- Albertini, S.; Reyes, A.E.L.; Trigo, J.M.; Sarriés, G.A.; Spoto, M.H.F. Effects of chemical treatments on fresh-cut papaya. Food Chem. 2016, 190, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.E.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Márquez, G.; Regalado-González, C. Modified starch-chitosan edible films: Physicochemical and mechanical characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Reyes-González, L.R.; Regalado, C. Characterization and antimicrobial effect of starch-based edible coating suspensions. Food Hydrocoll. 2016, 52, 906–913. [Google Scholar] [CrossRef]
- Arevalos-Sánchez, M.; Regalado, C.; Martin, S.E.; Domínguez-Domínguez, J.; García-Almendárez, B.E. Effect of neutral electrolyzed water and nisin on Listeria monocytogenes biofilms, and on listeriolysin O activity. Food Control 2012, 24, 116–122. [Google Scholar] [CrossRef]
- Arevalos-Sánchez, M.; Regalado, C.; Martin, S.E.; Meas-Vong, Y.; Cadena-Moreno, E.; García-Almendárez, B.E. Effect of neutral electrolyzed water on lux-tagged Listeria monocytogenes EGDe biofilms adhered to stainless steel and visualization with destructive and non-destructive microscopy techniques. Food Control 2013, 34, 472–477. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Khan, I.; Oh, D.H. Electrolyzed water as a novel sanitizer in the food industry: Current trends and future perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef]
- Waghmare, R.B.; Annapure, U.S. Combined effect of chemical treatment and/or modified atmosphere packaging (MAP) on quality of fresh-cut papaya. Postharvest. Biol. Technol. 2013, 85, 147–153. [Google Scholar] [CrossRef]
- Dhital, R.; Mora, N.B.; Watson, D.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. LWT-Food Sci. Technol. 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Chander, R.; Sharma, A. Characterization of nutritional, organoleptic and functional properties of intermediate moisture shelf stable ready-to-eat Carica papaya cubes. Food Biosci. 2015, 10, 69–79. [Google Scholar] [CrossRef]
- García-Aguilar, L.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Rojas-Molina, J.I.; Vázquez-Landaverde, P.A.; Luna-Vázquez, F.J.; Zavala-Sánchez, M.A. Nutritional value and volatile compounds of black cherry (Prunus serotina) seeds. Molecules 2015, 20, 3479–3495. [Google Scholar] [CrossRef] [PubMed]
- Arzate-Vázquez, I.; Chanona-Pérez, J.J.; de Jesus Perea-Flores, M.; Calderón-Domínguez, G.; Moreno-Armendáriz, M.A.; Calvo, H.; Godoy-Calderón, S.; Quevedo, R.; Gutiérrez-López, G. Image processing applied to classification of avocado variety Hass (Persea americana Mill.) during the ripening process. Food Bioprocess Technol. 2011, 4, 1307–1313. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- NORMA Official Mexicana NOM-110-SSA1-1994, Bienes y Servicios. Available online: http://www.salud.gob.mx/unidades/cdi/nom/110ssa14.html (accessed on 28 June 2018).
- Alsharjabi, F.A.; Al-Qadasi, A.M.; Al-Shorgani, N.K. Bacteriological evaluation of weaning dried foods consumed in Taiz City, Republic of Yemen. J. Saudi Soc. Agric. Sci. 2017, in press. [Google Scholar] [CrossRef]
- Pérez-Grijalva, B.; Herrera-Sotero, M.; Mora-Escobedo, R.; Zebadúa-García, J.C.; Silva-Hernández, E.; Oliart-Ros, R.; Pérez-Cruz, C.; Guzmán-Gerónimo, R. Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT-Food Sci. Technol. 2018, 87, 47–53. [Google Scholar] [CrossRef]
- Cortez-Vega, W.R.; Pizato, S.; de Souza, J.T.A.; Prentice, C. Using edible coatings from Whitemouth croaker (Micropogonias furnieri) protein isolate and organo-clay nanocomposite for improve the conservation properties of fresh-cut ‘Formosa’ papaya. Innov. Food Sci. Emerg. Technol. 2014, 22, 197–202. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Ogundare, M.O.; Ogunkunle, A.T.J.; Kolawole, O.M.; Adetunji, J.B. Effects of edible coatings from xanthum gum produced from xanthomonas campestris pammel on the shelf life of Carica papaya Linn fruits. Asian J. Agric. Biol. 2014, 2, 8–13. [Google Scholar]
- Hazarika, T.; Lalthanpuii, M.D. Influence of edible coatings on physico-chemical characteristics and shelf-life of papaya (Carica papaya) fruits during ambient storage. Indian J. Agric. Sci. 2017, 87, 1077–1083. [Google Scholar]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Kaewseejan, N. Quality, bioactive compounds and antioxidant capacity of selected climacteric fruits with relation to their maturity. Sci. Hortic. 2017, 221, 33–42. [Google Scholar] [CrossRef]
- Hernández, Y.; Lobo, M.G.; González, M. Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chem. 2006, 96, 654–664. [Google Scholar] [CrossRef]
- Wall, M.M. Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J. Food Compost. Anal. 2006, 19, 434–445. [Google Scholar] [CrossRef]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Jayathunge, K.G.L.R.; Gunawardhana, D.K.S.N.; Illeperuma, D.C.K.; Chandrajith, U.G.; Thilakarathne, B.M.K.S.; Fernando, M.D.; Palipane, K.B. Physico-chemical and sensory quality of fresh cut papaya (Carica papaya) packaged in micro-perforated polyvinyl chloride containers. J. Sci. Technol. 2014, 51, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Iglesias, J.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Ripeness indexes and physicochemical changes of papaya (Carica papaya L. cv. Maradol) during ripening on-tree. Sci. Hortic. 2018, 236, 272–278. [Google Scholar] [CrossRef]
- Fuggate, P.; Wongs-Aree, C.; Noichinda, S.; Kanlayanarat, S. Quality and volatile attributes of attached and detached ‘Pluk Mai Lie’ papaya during fruit ripening. Sci. Hortic. 2010, 126, 120–129. [Google Scholar] [CrossRef]
- Lieb, V.M.; Esquivel, P.; Castillo, E.C.; Carle, R.; Steingass, C.B. GC–MS profiling, descriptive sensory analysis, and consumer acceptance of Costa Rican papaya (Carica papaya L.) fruit purees. Food Chem. 2018, 248, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.P.M.; Fabi, J.P.; De Rosso, V.V.; Cordenunsi, B.R.; Lajolo, F.M.; do Nascimento, J.R.O.; Mercadante, A.Z. Influence of ethylene on carotenoid biosynthesis during papaya postharvesting ripening. J. Food Compost. Anal. 2011, 24, 620–624. [Google Scholar] [CrossRef]
- Almora, K.; Pino, J.A.; Hernández, M.; Duarte, C.; González, J.; Roncal, E. Evaluation of volatiles from ripening papaya (Carica papaya L., var. Maradol roja). Food Chem. 2004, 86, 127–130. [Google Scholar] [CrossRef]
- Lee, P.-R.; Ong, Y.-L.; Yu, B.; Curran, P.; Liu, S.-Q. Profile of volatile compounds during papaya juice fermentation by a mixed culture of Saccharomyces cerevisiae and Williopsis saturnus. Food Microbiol. 2010, 27, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; He, S.; Liu, Q.; Xiang, J.; Huang, D. Quantifying muskmelon fruit attributes with A-TEP-based model and machine vision measurement. J. Integr. Agric. 2018, 17, 1369–1379. [Google Scholar] [CrossRef]
- Chen, A.; Yang, Z.; Zhang, N.; Zhao, S.; Chen, M. Quantitative evaluation and prediction for preservation quality of cold shocked cucumber based on entropy. Innov. Food Sci. Emerg. Technol. 2016, 35, 58–66. [Google Scholar] [CrossRef]
- Calvo, H.; Moreno-Armendáriz, M.A.; Godoy-Calderón, S. A practical framework for automatic food products classification using computer vision and inductive characterization. Neurocomputing 2016, 175, 911–923. [Google Scholar] [CrossRef]

| No. | Compound | 0 Day | 5 Days | 10 Days | 15 Days | |||
|---|---|---|---|---|---|---|---|---|
| Control | With Edible Coating | Without Edible Coating | With Edible Coating | Without Edible Coating | With Edible Coating | Without Edible Coating | ||
| Peak area ×10−7 | ||||||||
| 1 | 4-Methyloctane | 9.36 | 11.70 | 6.98 | 12.05 | 6.54 | 12.29 | 6.06 |
| 2 | 1-Heptene, 2-methyl- | 0.58 | 0.59 | 0.57 | 0.35 | 0.33 | 0.17 | 0.15 |
| 3 | 1-Hexene, 2,5-dimethyl- | 0.33 | 0.34 | 033 | 0.33 | 0.29 | 0.21 | 0.13 |
| 4 | 1-Butanol | – | 2.38 | – | 2.30 | – | 2.27 | 1.30 |
| 5 | 2-Butenoic acid, ethyl ester | 0.37 | 0.35 | 0.38 | 0.35 | 0.38 | 0.35 | 0.38 |
| 6 | 2-Ethylhexanol | – | – | – | – | – | 1.89 | 1.87 |
| 7 | 2-Ethylpentane | – | – | – | 6.15 | 6.08 | 2.28 | 1.07 |
| 8 | α-Methylfuran | – | – | 1.24 | – | 1.33 | – | 1.68 |
| 9 | Acetoin | – | – | – | – | – | 5.79 | 6.11 |
| 10 | Acetone | – | – | 1.64 | 1.88 | 1.56 | 1.97 | 1.90 |
| 11 | Acetic acid | – | – | – | – | – | 1.40 | 5.19 |
| 12 | Benzene | 0.49 | 0.58 | 0.39 | 0.43 | 0.27 | 0.38 | 0.17 |
| 13 | Benzothiazole | 0.36 | 0.31 | 0.41 | 0.35 | – | 0.20 | 0.39 |
| 14 | Benzyl Alcohol | – | 0.64 | 0.63 | 0.77 | 0.75 | 0.72 | 0.67 |
| 15 | Benzyl cyanide | 0.15 | 0.15 | 0.14 | 0.15 | 0.14 | 0.15 | 0.14 |
| 16 | Benzyl nitrile | – | – | – | 0.18 | – | 0.24 | – |
| 17 | Butanoic acid | – | – | – | 1.07 | – | 1.25 | – |
| 18 | Butyric acid | 12.20 | 21.90 | 222.00 | 22.80 | 254.00 | 30.10 | 271.00 |
| 19 | Caproic acid | – | – | 3.18 | 2.27 | 2.40 | 1.87 | 1.23 |
| 20 | Cetyl chloride | 0.80 | 0.96 | 0.65 | 1.02 | 0.72 | 1.11 | 0.82 |
| 21 | cis-Linalool oxide | – | – | 4.95 | – | 5.21 | – | 5.84 |
| 22 | Cyclohexane, methyl- | – | – | – | 1.11 | – | 1.48 | – |
| 23 | Limonene | – | 1.13 | – | 1.69 | – | 1.85 | – |
| 24 | Decane | – | 1.12 | 0.98 | 4.16 | 2.57 | 6.13 | 5.71 |
| 25 | Dibutyl formamide | – | – | 115.00 | 0.01 | 200.00 | 0.009 | 243.00 |
| 26 | Diethyl Phthalate | 0.62 | 0.63 | 0.04 | 0.84 | 0.24 | 1.34 | 0.40 |
| 27 | Dimethyl sulfide | – | 0.30 | 0.25 | 0.25 | 0.24 | 0.17 | 0.13 |
| 28 | Dodecane | – | – | 0.69 | – | 0.76 | – | 0.83 |
| 29 | Dodecane, 4,6-dimethyl- | – | – | – | 0.69 | – | 0.81 | – |
| 30 | Ethanol | 18.00 | 34.10 | 18.69 | 11.80 | 22.40 | 8.31 | 31.50 |
| 31 | Ethyl Acetate | – | 29.10 | – | 18.70 | – | 12.90 | 6.29 |
| 32 | Ethyl butanoate | – | – | 23.00 | 41.40 | 41.50 | 34.70 | 161.00 |
| 33 | Ethyl hexanoate | – | – | – | – | – | 1.72 | 5.07 |
| 34 | Ethyl propanoate | – | – | – | – | – | 0.37 | 0.56 |
| 35 | Formic acid, pentyl ester | – | 0.39 | – | 0.40 | – | 0.42 | – |
| 36 | Heptane | 14.00 | 17.60 | 10.50 | 4.35 | 5.32 | 6.35 | 2.52 |
| 37 | Hexadecane | – | – | – | 0.11 | – | 0.34 | – |
| 38 | Isoheptane | – | – | 0.17 | 0.06 | 0.05 | 0.09 | 0.07 |
| 39 | Isobutenylcarbinol | – | – | – | 0.06 | 2.97 | 0.09 | 0.32 |
| 40 | Isopentyl alcohol, acetate | 0.83 | 0.24 | 1.42 | 0.11 | 1.84 | 0.08 | 1.95 |
| 41 | Isopreno | – | – | 0.15 | – | 0.21 | – | 0.71 |
| 42 | Isothymol methyl ether | – | – | – | – | – | 1.83 | 1.53 |
| 43 | Linalool | 1.79 | 0.33 | 3.24 | 0.08 | 3.41 | 0.08 | 3.63 |
| 44 | Methyl acetate | – | – | 4.96 | – | 5.02 | – | 5.21 |
| 45 | Methyl benzoate | 0.78 | 0.70 | 0.85 | 0.42 | 1.23 | 0.22 | 1.74 |
| 46 | Methylene Chloride | – | – | – | 1.76 | – | 1.94 | – |
| 47 | Methyl crotonate | – | – | 4.97 | – | 5.03 | – | 5.71 |
| 48 | Methyl nerolate | – | – | 0.26 | – | 0.31 | – | 0.37 |
| 49 | Methyl propanoate | 0.74 | 1.14 | 0.96 | 1.44 | 1.04 | 1.66 | 1.12 |
| 50 | Methyl salicylate | – | – | – | – | 0.13 | 0.17 | |
| 51 | Morpholine, 4-octadecyl- | – | – | 0.46 | – | 0.52 | – | 0.63 |
| 52 | N,N-Dibutylacetamide | – | – | 0.14 | – | 0.22 | – | 0.27 |
| 53 | Nonane | – | – | 0.48 | – | 0.53 | – | 0.61 |
| 54 | Nonane, 2,5-dimethyl- | – | – | 4.38 | – | 4.51 | – | 4.63 |
| 55 | Nonane, 2,2,4,4,6,8,8-heptamethyl- | 0.24 | 2.41 | 2.27 | 3.31 | 3.12 | 4.12 | 4.26 |
| 56 | Nonane, 3-methyl-5-propyl- | – | 0.22 | – | 0.28 | – | 0.33 | – |
| 57 | Octane | 1.05 | 0.25 | 0.66 | 0.30 | 0.72 | 0.39 | 0.89 |
| 58 | Oxime-, methoxy-phenyl | 2.34 | 3.54 | 2.48 | 4.50 | 3.71 | 0.63 | 0.64 |
| 59 | Pentadecane | – | 1.67 | – | 1.74 | – | 1.95 | – |
| 60 | Penta ethylene glycol monododecyl ether | – | – | 0.42 | – | 0.55 | – | 0.76 |
| 61 | Phenol | 0.46 | – | – | 0.25 | – | 0.42 | – |
| 62 | Propanoic acid, ethyl ester | – | 1.32 | – | 1.62 | – | 1.74 | – |
| 63 | Propanoic acid, 2-methyl-, ethyl ester | 0.25 | 0.26 | 0.24 | 0.76 | 0.39 | 1.11 | 0.44 |
| 64 | Toluene | – | 0.62 | 0.57 | 0.63 | 1.14 | 0.44 | 0.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-García, M.; Rodríguez-Hernández, M.J.; Hernández-Hernández, H.M.; Delgado-Sánchez, L.F.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica papaya L., and Its Physicochemical and Antimicrobial Properties. Coatings 2018, 8, 318. https://doi.org/10.3390/coatings8090318
Escamilla-García M, Rodríguez-Hernández MJ, Hernández-Hernández HM, Delgado-Sánchez LF, García-Almendárez BE, Amaro-Reyes A, Regalado-González C. Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica papaya L., and Its Physicochemical and Antimicrobial Properties. Coatings. 2018; 8(9):318. https://doi.org/10.3390/coatings8090318
Chicago/Turabian StyleEscamilla-García, Monserrat, María J. Rodríguez-Hernández, Hilda M. Hernández-Hernández, Luis F. Delgado-Sánchez, Blanca E. García-Almendárez, Aldo Amaro-Reyes, and Carlos Regalado-González. 2018. "Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica papaya L., and Its Physicochemical and Antimicrobial Properties" Coatings 8, no. 9: 318. https://doi.org/10.3390/coatings8090318
APA StyleEscamilla-García, M., Rodríguez-Hernández, M. J., Hernández-Hernández, H. M., Delgado-Sánchez, L. F., García-Almendárez, B. E., Amaro-Reyes, A., & Regalado-González, C. (2018). Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica papaya L., and Its Physicochemical and Antimicrobial Properties. Coatings, 8(9), 318. https://doi.org/10.3390/coatings8090318