Dental Adhesion Enhancement on Zirconia Inspired by Mussel’s Priming Strategy Using Catechol
Abstract
1. Introduction
2. Materials and Methods
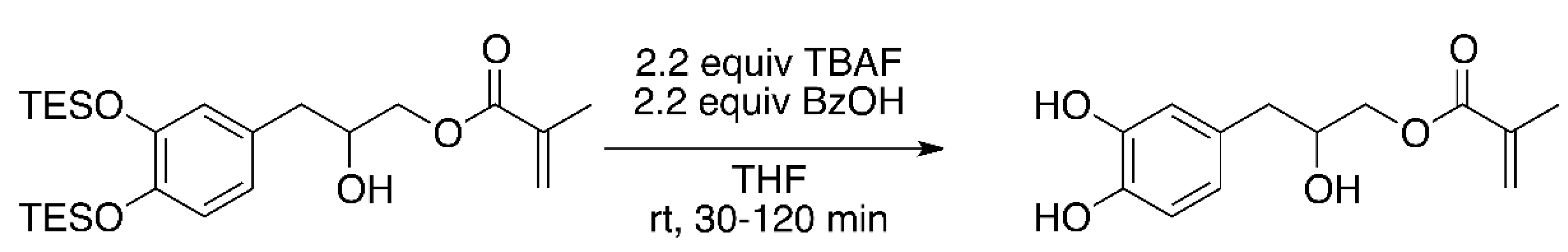
2.1. Synthesis of Catechol Methacrylate Primer
2.2. Zirconia Specimen
2.3. Surface Treatment
2.4. Surface Morphology
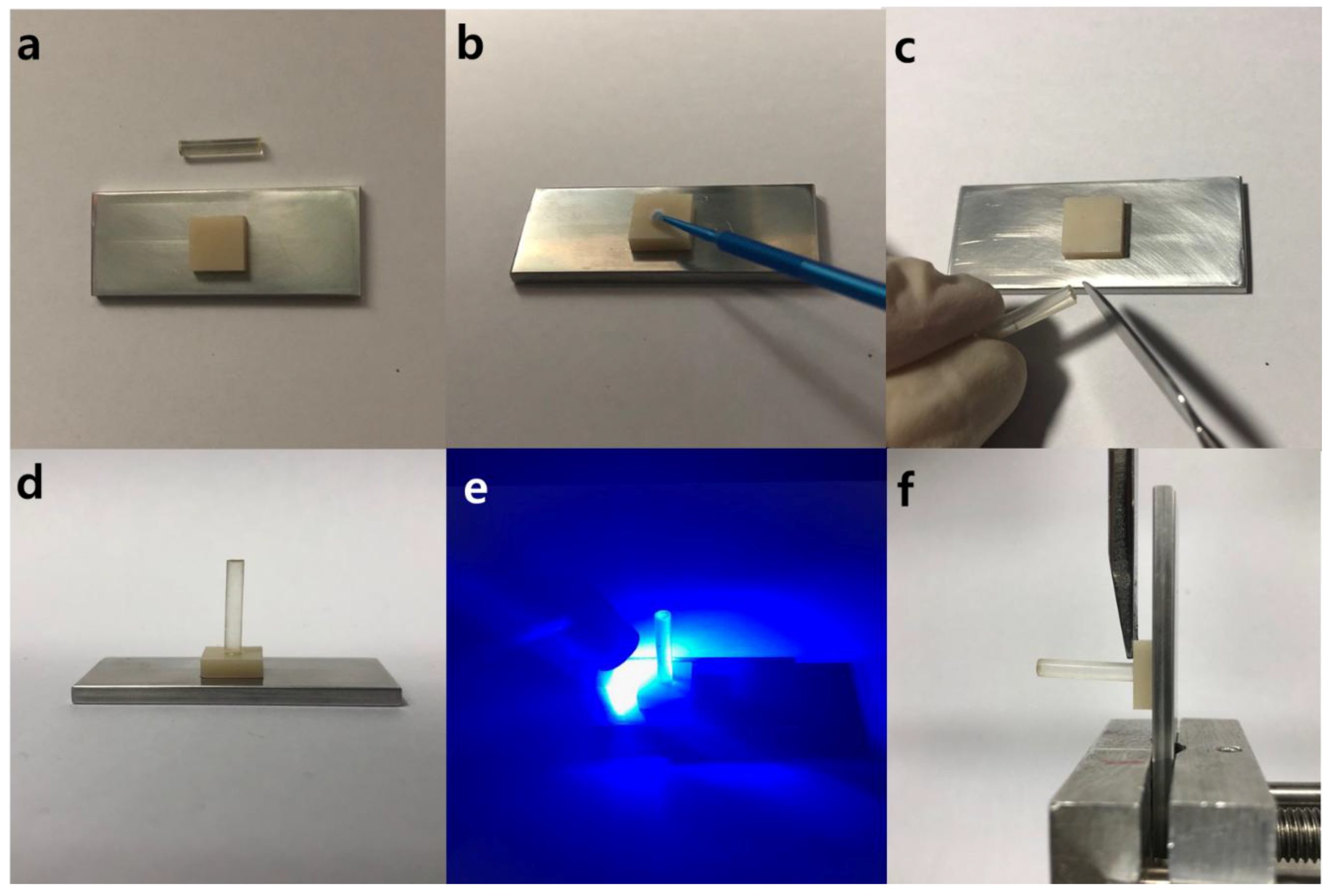
2.5. Knife-Edge Shear Bond Strength
2.6. Statistical Analysis
3. Results and Discussion
3.1. Improved Synthesis of Catechol Methacrylate Primer
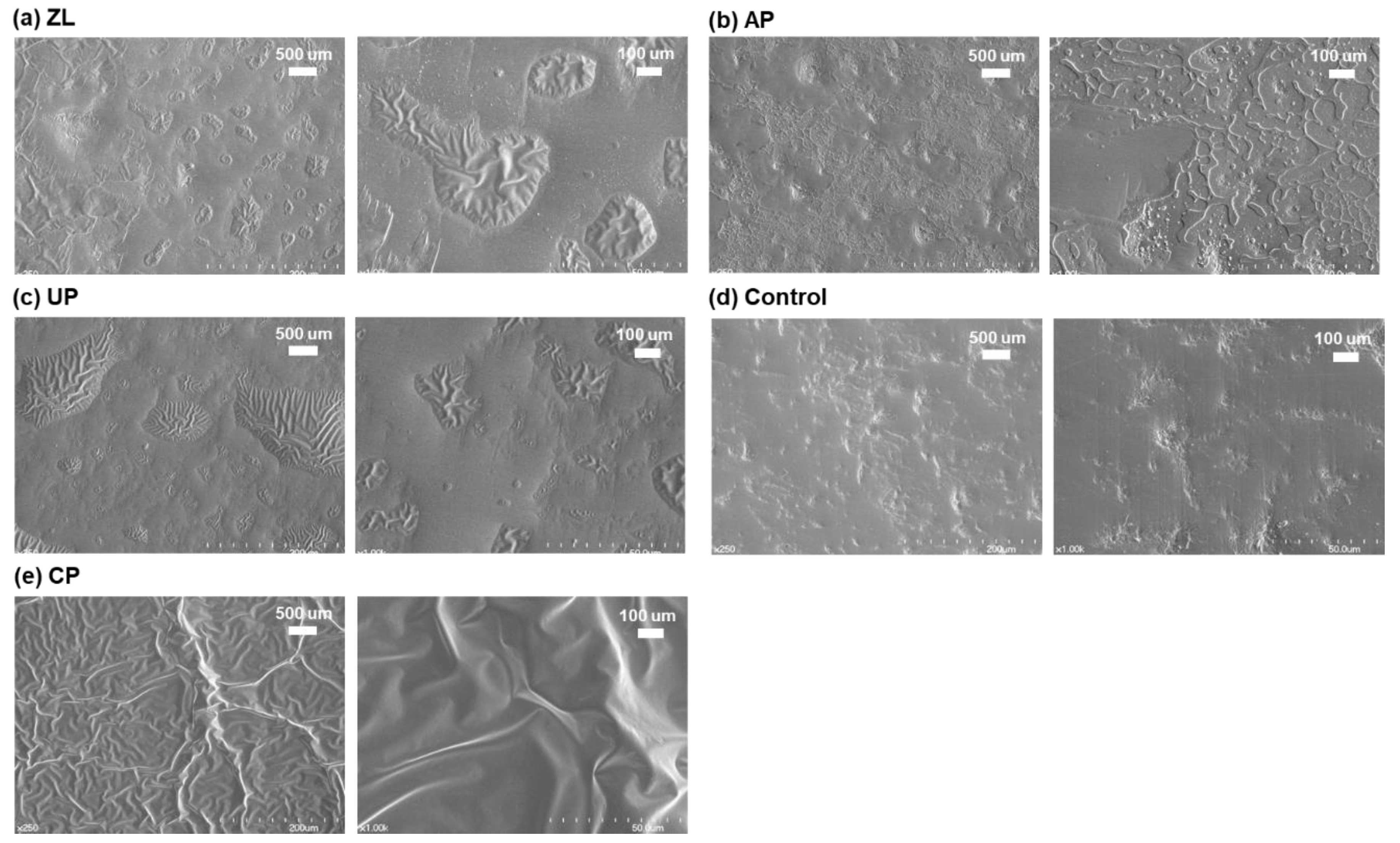
3.2. Scanning Electron Microscope (SEM) Imaging of Primed Zirconia Surface
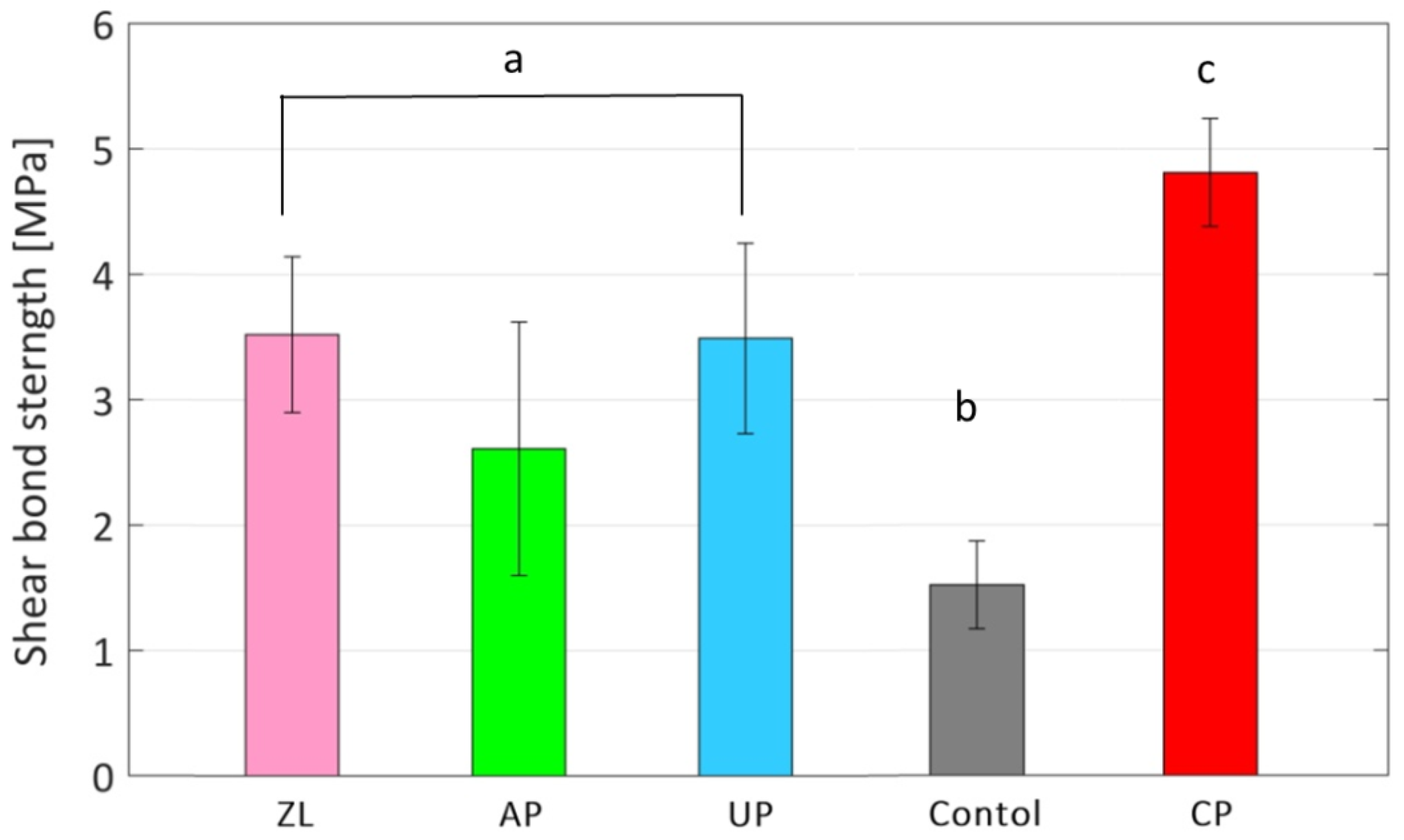
3.3. Shear Bond Strength of Dental Adhesives on Bioinspired Catechol Primed Zirconica Surface
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Paes, P.N.G.; Bastian, F.L.; Jardim, P.M. The influence of Y-TZP surface treatment on topography and ceramic/resin cement interfacial fracture toughness. Dent. Mater. 2017, 33, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-F.; Kang, L.-L.; Liu, Y.-C.; Lin, J.-C.; Wang, C.-C.; Chen, H.-M.; Tai, C.-K. Effects of silane- and MDP-based primers application orders on zirconia-resin adhesion—A ToF-SIMS study. Dent. Mater. 2017, 33, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Waite, J.H. Mussel-designed protective coatings for compliant substrates. J. Dent. Res. 2008, 87, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.M.; Das, S.; Kaufman, Y.; Wei, W.; Israelachvili, J.N.; Waite, J.H. Mussel adhesive protein provides cohesive matrix for collagen type-1α. Biomaterials 2015, 51, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, D.W.; Ahn, J.S.; Cunha, K.; Filippidi, E.; Ju, S.W.; Shin, E.; Kim, B.S.; Levine, Z.A.; Lins, R.D.; et al. Significant performance enhancement of polymer resins by bioinspired dynamic bonding. Adv. Mater. 2017, 29, 703026. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, C.J.; Nahrendorf, M.; Figueiredo, J.L.; Lee, H.; Kambhampati, S.; Lee, T.; Cho, S.W.; Gorbatov, R.; Iwamoto, Y.; Dang, T.T.; et al. Painting blood vessels and atherosclerotic plaques with an adhesive drug depot. Proc. Nat. Acad. Sci. USA 2012, 109, 21444–21449. [Google Scholar] [CrossRef] [PubMed]
- Kivelio, A.; Dekoninck, P.; Perrini, M.; Brubaker, C.E.; Messersmith, P.B.; Mazza, E.; Deprest, J.; Zimmermann, R.; Ehrbar, M.; Ochsenbein-Koelble, N. Mussel mimetic tissue adhesive for fetal membrane repair: Initial in vivo investigation in rabbits. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.X.; Prajatelistia, E.; Ju, S.-W.; Jeong Kim, H.; Baek, S.-J.; Joon Cha, H.; Ho Jun, S.; Ahn, J.-S.; Soo Hwang, D.S. A rapid, efficient, and facile solution for dental hypersensitivity: The tannin–iron complex. Sci. Rep. 2015, 5, 10884. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; González-Cabezas, C.; Kim, K.-M.; Kim, K.-N.; Kuroda, K. Catechol-functionalized synthetic polymer as a dental adhesive to contaminated dentin surface for a composite restoration. Biomacromolecules 2015, 16, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.K. Perspectives on mussel-inspired wet adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef] [PubMed]
- Danner, E.W.; Kan, Y.J.; Hammer, M.U.; Israelachvili, J.N.; Waite, J.H. Adhesion of mussel foot protein Mefp-5 to mica: An underwater superglue. Biochemistry 2012, 51, 6511–6518. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.K.; Das, S.; Linstadt, R.; Kaufman, Y.; Martinez-Rodriguez, N.R.; Mirshafian, R.; Kesselman, E.; Talmon, Y.; Lipshutz, B.H.; Israelachvili, J.N.; et al. High-performance mussel-inspired adhesives of reduced complexity. Nat. Commun. 2015, 6, 8633. [Google Scholar] [CrossRef] [PubMed]
- Rickman, R.D.; Verkhoturov, S.V.; Balderas, S.; Bestaoui, N.; Clearfield, A.; Schweikert, E.A. Characterization of surface structure by cluster coincidental ion mass spectrometry. Appl. Surf. Sci. 2004, 231, 106–112. [Google Scholar] [CrossRef]
- Shin, E.; Ju, S.W.; An, L.; Ahn, E.; Ahn, J.-S.; Kim, B.-S.; Ahn, B.K. Bioinspired catecholic primers for rigid and ductile dental resin composites. ACS Appl. Mater. Interfaces 2018, 10, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.K.; Lee, D.W.; Israelachvili, J.N.; Waite, J.H. Surface-initiated self-healing of polymers in aqueous media. Nat. Mater. 2014, 13, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Das, S.; Zalicki, P.J.; Mirshafian, R.; Eisenbach, C.D.; Israelachvili, J.N.; Waite, J.H.; Ahn, B.K. Microphase behavior and enhanced wet-cohesion of synthetic copolyampholytes inspired by a mussel foot protein. J. Am. Chem. Soc. 2015, 137, 9214–9217. [Google Scholar] [CrossRef] [PubMed]
- Gelbke, H.P.; Knuppen, R. A new method for preventing oxidative decomposition of catechol estrogens during chromatography. J. Chromatogr. A 1972, 72, 465–471. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, M.; Kim, K.-N.; Hwang, C.-J. Resin bonding of metal brackets to glazed zirconia with a porcelain primer. Korean J. Orthod. 2015, 45, 299–307. [Google Scholar] [CrossRef] [PubMed]

| Primers | Composition 1 | Manufacturer |
|---|---|---|
| Zirconia liner (ZL) | 4-META | SunMedical Co. Ltd (Shiga, Japan) |
| Alloy primer (AP) | 10-MDP, VBATDT | Kuraray Dental, Inc. (Osaka, Japan) |
| Universal primer (UP) | MAC-10, MTU-6 | Tokuyama Dental, Corp. (Tokyo, Japan) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Ju, S.; Linstadt, R.; Ahn, J.; Ahn, K. Dental Adhesion Enhancement on Zirconia Inspired by Mussel’s Priming Strategy Using Catechol. Coatings 2018, 8, 298. https://doi.org/10.3390/coatings8090298
Park M, Ju S, Linstadt R, Ahn J, Ahn K. Dental Adhesion Enhancement on Zirconia Inspired by Mussel’s Priming Strategy Using Catechol. Coatings. 2018; 8(9):298. https://doi.org/10.3390/coatings8090298
Chicago/Turabian StylePark, Minsu, Sungwon Ju, Roscoe Linstadt, Jinsoo Ahn, and Kollbe Ahn. 2018. "Dental Adhesion Enhancement on Zirconia Inspired by Mussel’s Priming Strategy Using Catechol" Coatings 8, no. 9: 298. https://doi.org/10.3390/coatings8090298
APA StylePark, M., Ju, S., Linstadt, R., Ahn, J., & Ahn, K. (2018). Dental Adhesion Enhancement on Zirconia Inspired by Mussel’s Priming Strategy Using Catechol. Coatings, 8(9), 298. https://doi.org/10.3390/coatings8090298





