Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Xerogels
2.2.1. Preformed TiO2/AuNRs
2.2.2. Mesoporous Coating
2.3. Sol-Gel Characterization
2.4. Application on Stone and Characterization
2.5. Photocatalysis Experiments
3. Results and Discussion
3.1. TiO2/AuNRs Characterization
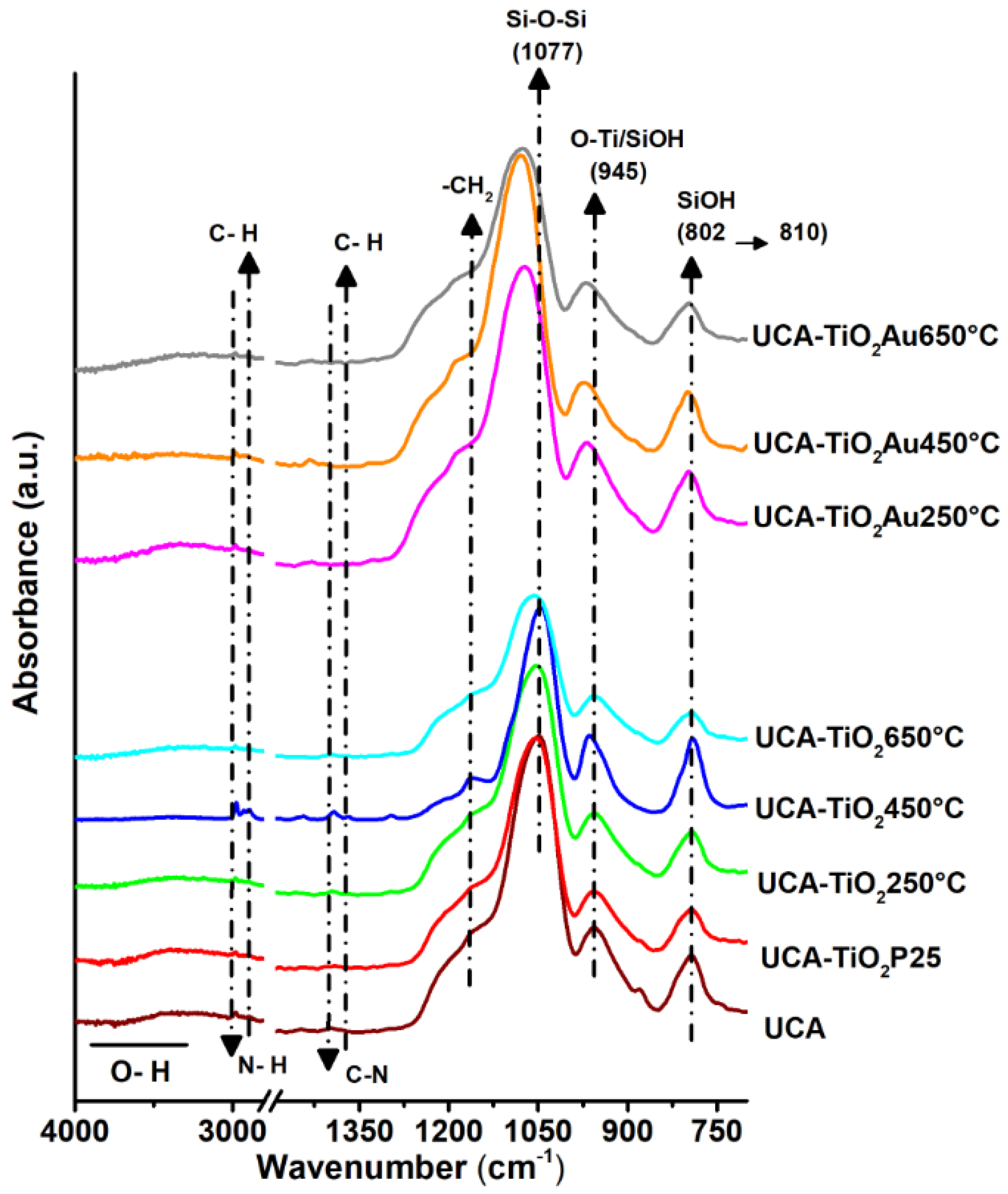
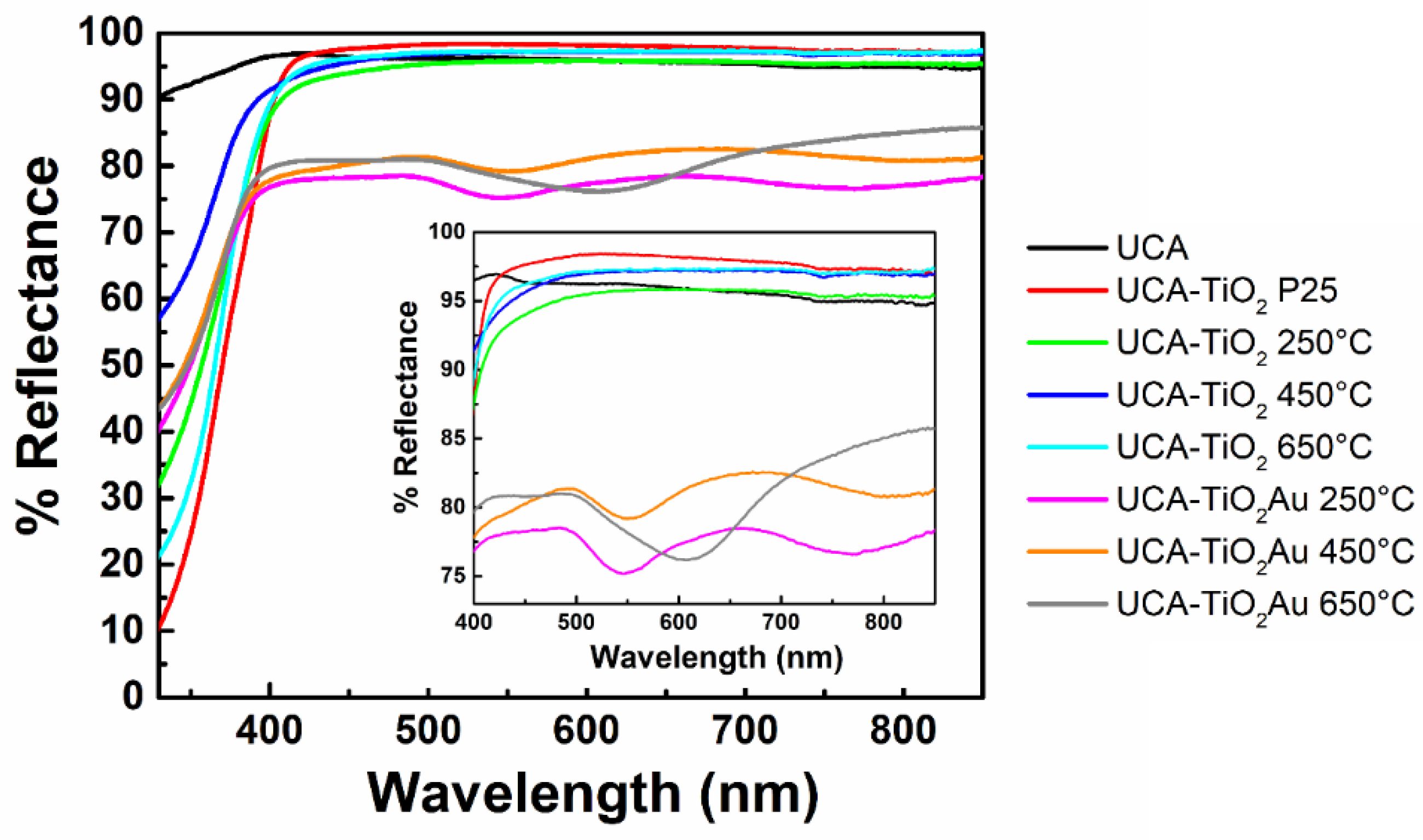
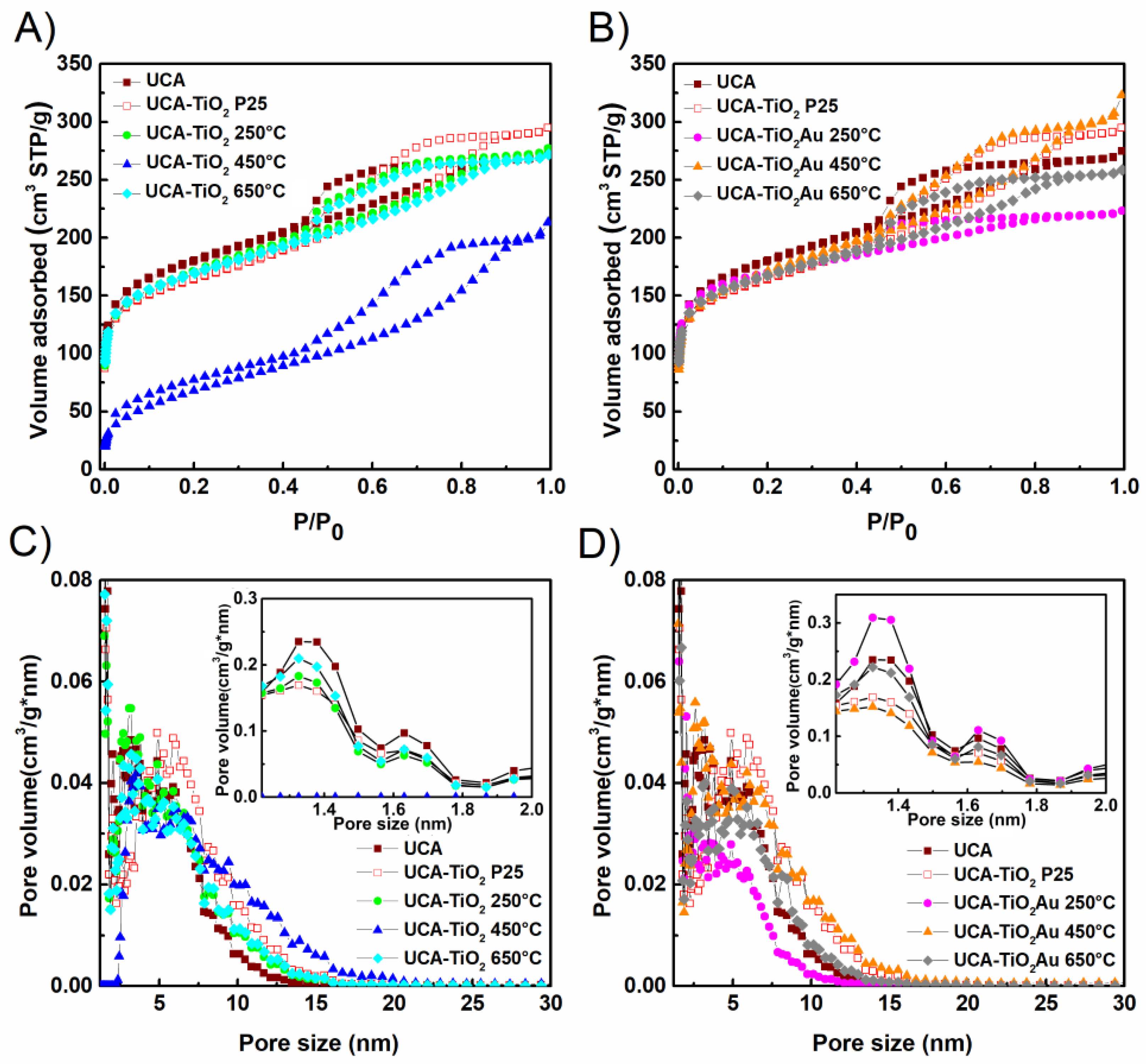
3.2. Sol-Gel Characterization
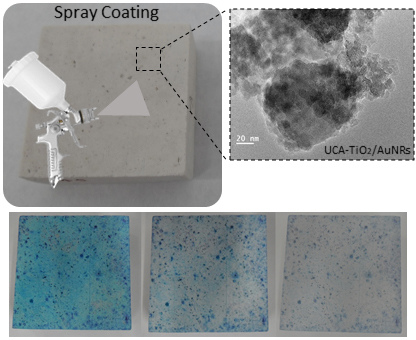
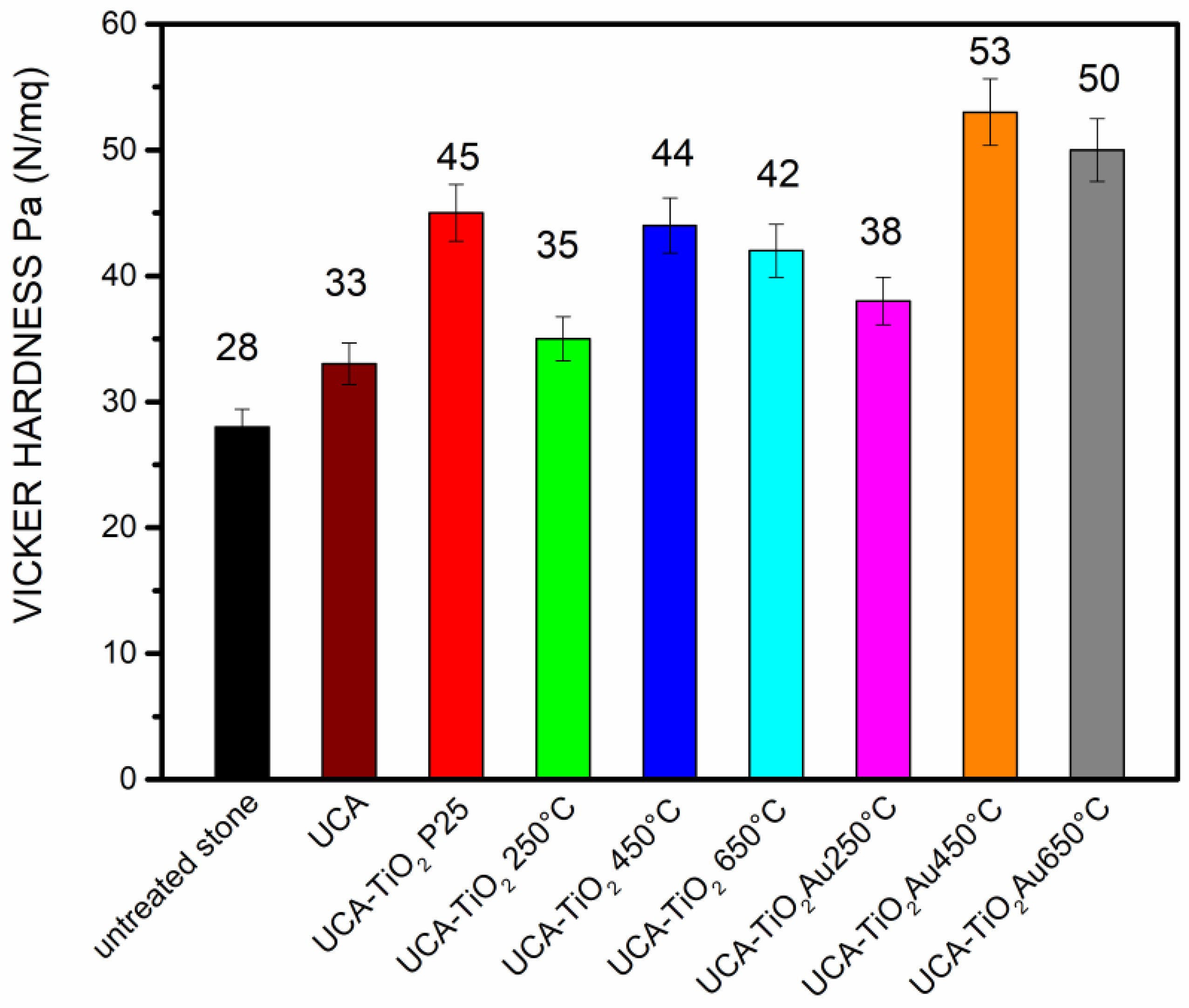
3.3. Application on Stone and Characterization
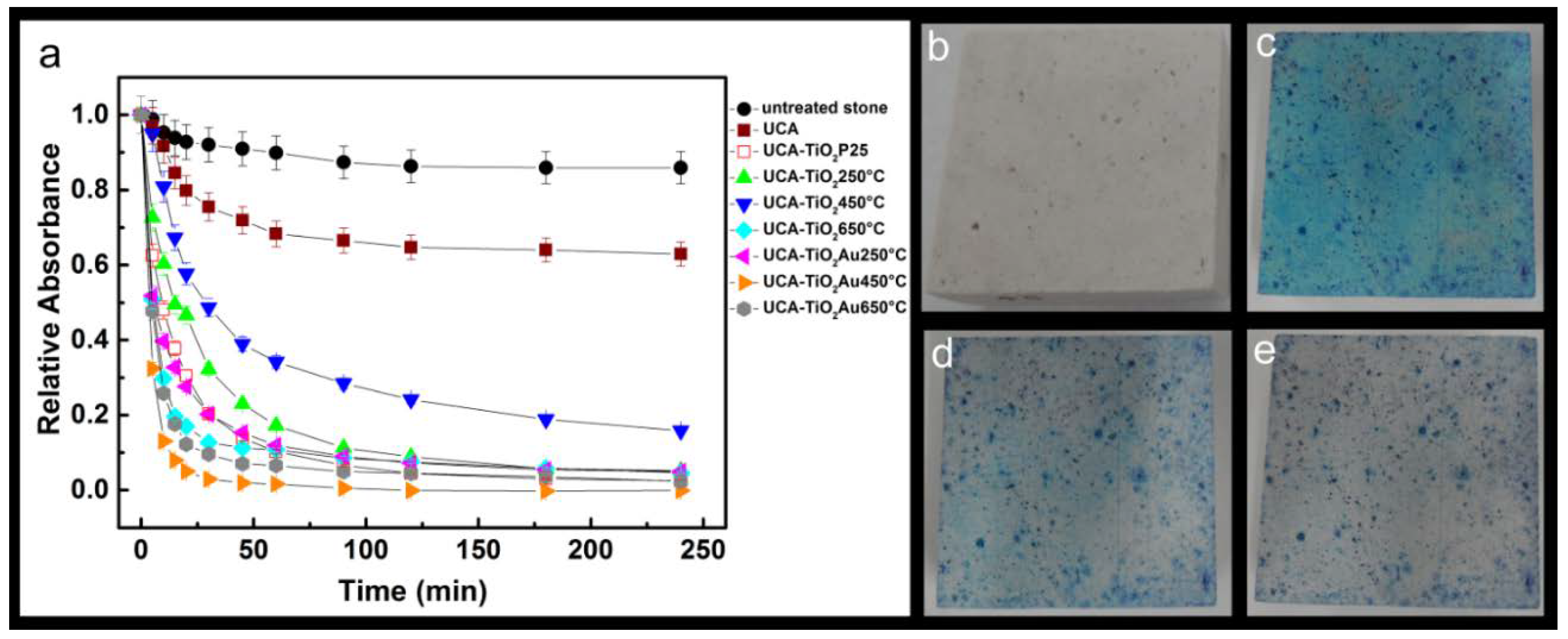
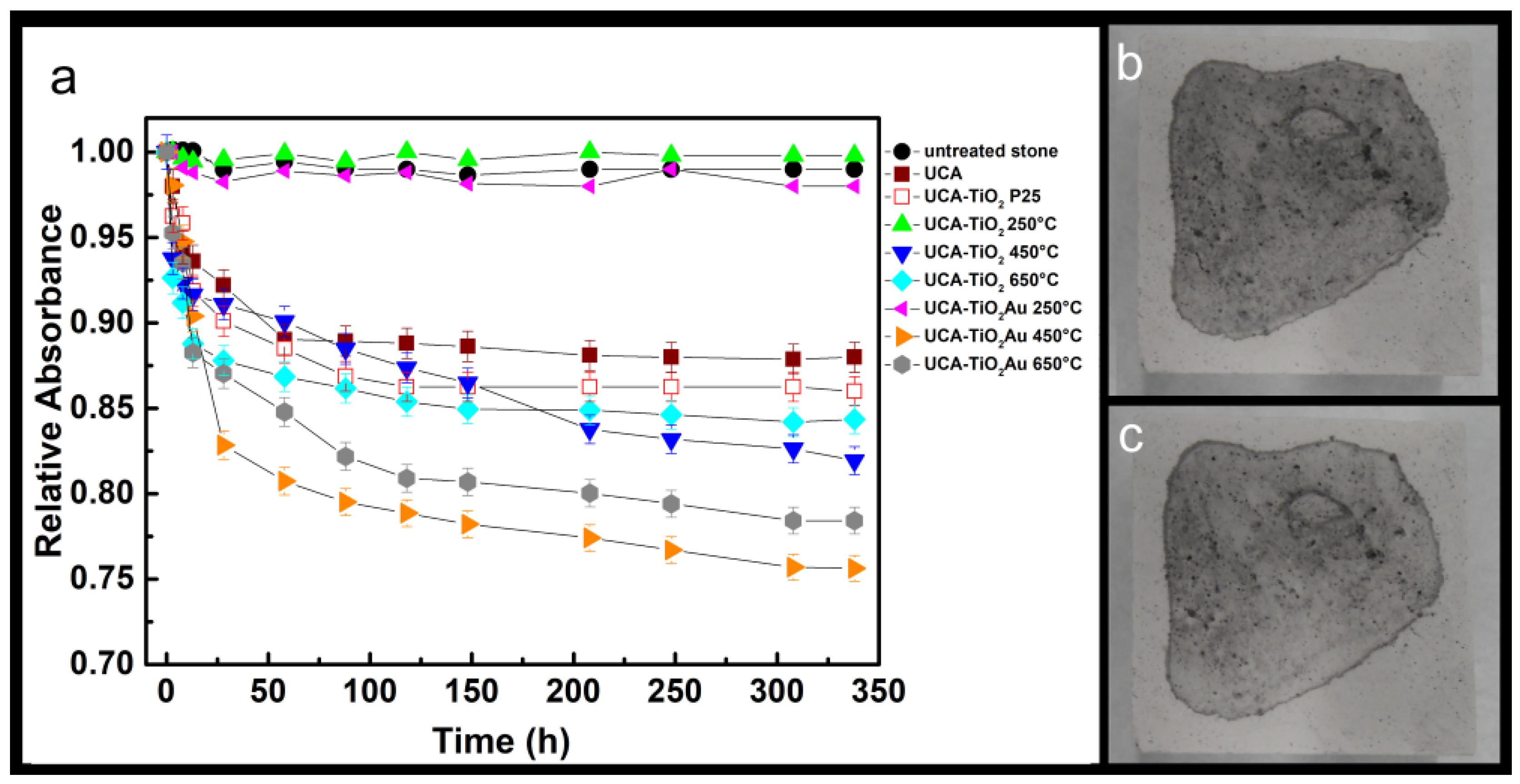
3.4. Photocatalysis Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinho, L.; Mosquera, M.J. Photocatalytic activity of TiO2-SiO2 nanocomposites applied to buildings: Influence of particle size and loading. Appl. Catal. B Environ. 2013, 134–135, 205–221. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- International Council on Monuments and Sites-International Scientific Committe for Stone (ICOMOS-ISCS). Illustrated Glossary on Stone Deterioration Patterns; ICOMOS: Charenton-le-Pont, France, 2010. [Google Scholar]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.; Comparelli, R. Visible-Light-Active TiO2-Based Hybrid Nanocatalysts for Environmental Applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Midtdal, K.; Jelle, B.P. Self-cleaning glazing products: A state-of-the-art review and future research pathways. Sol. Energy Mater. Sol. Cells 2013, 109, 126–141. [Google Scholar] [CrossRef]
- Smits, M.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic degradation of soot deposition: Self-cleaning effect on titanium dioxide coated cementitious materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J.; Crow, M. Photocatalytic oxidation of soot by P25 TiO2 films. Chemosphere 2006, 64, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Chin, P.; Roberts, G.W.; Ollis, D.F. Kinetic Modeling of Photocatalyzed Soot Oxidation on Titanium Dioxide Thin Films. Ind. Eng. Chem. Res. 2007, 46, 7598–7604. [Google Scholar] [CrossRef]
- Chin, P.; Grant, C.S.; Ollis, D.F. Quantitative photocatalyzed soot oxidation on titanium dioxide. Appl. Catal. B Environ. 2009, 87, 220–229. [Google Scholar] [CrossRef]
- Herrmann, J.M. Photocatalysis fundamentals revisited to avoid several misconceptions. Appl. Catal. B Environ. 2010, 99, 461–468. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Petronella, F.; Curri, M.L.; Striccoli, M.; Fanizza, E.; Mateo-Mateo, C.; Alvarez-Puebla, R.A.; Sibillano, T.; Giannini, C.; Correa-Duarte, M.A.; Comparelli, R. Direct growth of shape controlled TiO2 nanocrystals onto SWCNTs for highly active photocatalytic materials in the visible. Appl. Catal. B Environ. 2015, 178, 91–99. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Madkour, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Yang, X.; Wang, S.; Shen, J.; Lin, B.; Li, C. Enhanced visible light photocatalytic activity of interlayer-isolated triplex Ag@SiO2@TiO2 core–shell nanoparticles. Nanoscale 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A.; Afrin, T.; Sun, L.; Wang, X. Self-cleaning wool: Effect of noble metals and silica on visible-light-induced functionalities of nano TiO2 colloid. J. Text. Inst. 2015, 106, 1348–1361. [Google Scholar] [CrossRef]
- Luna, M.; Delgado, J.; Gil, M.L.A.; Mosquera, M. TiO2-SiO2 Coatings with a Low Content of AuNPs for Producing Self-Cleaning Building Materials. Nanomaterials 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Mosquera, M.J. Titania-Silica Nanocomposite Photocatalysts with Application in Stone Self-Cleaning. J. Phys. Chem. C 2011, 115, 22851–22862. [Google Scholar] [CrossRef]
- Facio, D.S.; Luna, M.; Mosquera, M.J. Facile preparation of mesoporous silica monoliths by an inverse micelle mechanism. Microporous Mesoporous Mater. 2017, 247, 166–176. [Google Scholar] [CrossRef]
- Ancora, R.; Borsa, M.; Cassar, L. WO/2009/080647 Titanium Dioxide Based Photocatalytic Composites and Derived Products on a Metakaolin Support; Italcementi: Bergamo, Italy, 2009. [Google Scholar]
- De Sio, L.; Caracciolo, G.; Annesi, F.; Placido, T.; Pozzi, D.; Comparelli, R.; Pane, A.; Curri, M.L.; Agostiano, A.; Bartolino, R. Photo-thermal effects in gold nanorods/DNA complexes. Micro Nano Syst. Lett. 2015, 3. [Google Scholar] [CrossRef]
- Thommes, M.; Smarsly, B.; Groenewolt, M.; Ravikovitch, P.I.; Neimark, A.V. Adsorption Hysteresis of Nitrogen and Argon in Pore Networks and Characterization of Novel Micro and Mesoporous Silicas. Langmuir 2006, 22, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Tandon, S.P.; Gupta, J.P. Measurement of Forbidden Energy Gap of Semiconductors by Diffuse Reflectance Spectra. Phys. Status Solidi B 1970, 38, 363–367. [Google Scholar] [CrossRef]
- Drdácký, M.; Lesák, J.; Rescic, S.; Slížková, Z.; Tiano, P.; Valach, J. Standardization of peeling tests for assessing the cohesion and consolidation characteristics of historic stone surfaces. Mater. Struct. 2012, 45, 505–520. [Google Scholar] [CrossRef] [PubMed]
- ASTM C1327-15-Standard Test Method for Vickers Indentation Hardness of Advanced Ceramics; ASTM: West Conshohocken, PA, USA, 2015.
- UNI-EN 15801: Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity; Italian Standards: Milan, Italy, 2010.
- UNI-EN 15886: Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces; Italian Standards: Milan, Italy, 2010.
- Berns, R.S. Billmeyer and Saltzman’s Principles of Color Technology; Wiley-Interscience: New York, NY, USA, 2000. [Google Scholar]
- Smits, M.; Huygh, D.; Craeye, B.; Lenaerts, S. Effect of process parameters on the photocatalytic soot degradation on self-cleaning cementitious materials. Catal. Today 2014, 230, 250–255. [Google Scholar] [CrossRef]
- Vasiliev, P.O.; Faure, B.; Ng, J.B.S.; Bergström, L. Colloidal aspects relating to direct incorporation of TiO2 nanoparticles into mesoporous spheres by an aerosol-assisted process. J. Colloid Interface Sci. 2008, 319, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Elhaddad, F.; Facio, D.S.; Mosquera, M.J. A novel TiO2-SiO2 nanocomposite converts a very friable stone into a self-cleaning building material. Appl. Surf. Sci. 2013, 275, 389–396. [Google Scholar] [CrossRef]
- Sekhar, Y.R.; Sharma, K.V. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2015, 10, 86–102. [Google Scholar] [CrossRef]
- Giannini, C.; Ladisa, M.; Altamura, D.; Siliqi, D.; Sibillano, T.; De Caro, L. X-ray Diffraction: A Powerful Technique for the Multiple-Length-Scale Structural Analysis of Nanomaterials. Crystals 2016, 6, 87. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Zhang, Q.; Zhao, Q.; Peng, F.; Wang, H.; Yu, H.; Yang, J. Thermal stability of gold nanorods in an aqueous solution. Colloids Surf. A 2010, 372, 177–181. [Google Scholar] [CrossRef]
- Liu, L.; Ouyang, S.; Ye, J. Gold-Nanorod-Photosensitized Titanium Dioxide with Wide-Range Visible-Light Harvesting Based on Localized Surface Plasmon Resonance. Angew. Chem. Int. Ed. 2013, 52, 6689–6693. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Rojas, M.; Mosquera, M.J. Ag-SiO2-TiO2 nanocomposite coatings with enhanced photoactivity for self-cleaning application on building materials. Appl. Catal. B Environ. 2015, 178, 144–154. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Takeuchi, F.; Todoroki, S.; Sakka, Y.; Inoue, S. Spherical Mesoporous Silica Particles with Titanium Dioxide Nanoparticles by an Aerosol-assisted Coassembly. Chem. Lett. 2008, 37, 72–73. [Google Scholar] [CrossRef]
- Suzuki, N.; Jiang, X.; Radhakrishnan, L.; Takai, K.; Shimasaki, K.; Huang, Y.T.; Miyamoto, N.; Yamauchi, Y. Hybridization of Photoactive Titania Nanoparticles with Mesoporous Silica Nanoparticles and Investigation of Their Photocatalytic Activity. Bull. Chem. Soc. Jpn. 2011, 84, 812–817. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, J.; Xu, R.; Ma, G. Hydrophilicity, photocatalytic activity and stability of tetraethyl orthosilicate modified TiO2 film on glazed ceramic surface. Appl. Surf. Sci. 2013, 266, 141–147. [Google Scholar] [CrossRef]
- Ting, H.F.; Chen, C.M.; Lu, F.H.; Suen, S.Y. Adsorption and photodegradation of methylene blue using a bulk Ti material with porous titania layer prepared by chemical oxidation. J. Taiwan Inst. Chem. Eng. 2014, 45, 617–624. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Ishak, M.A.M.; Ismail, K.; Nawawi, W.I. Kinetics of photocatalytic decolourization of cationic dye using porous TiO2 film. J. Taibah Univ. Sci. 2016, 10, 352–362. [Google Scholar] [CrossRef]
- Zhang, J.; Grabstanowicz, L.R.; Gao, S.; Hosmane, N.S.; Huang, B.; Dai, Y.; Liu, D.; Xu, T. Visible-light photocatalytic SiO2/TiO2−x Cx/C nanoporous composites using TiCl4 as the precursor for TiO2 and polyhydroxyl tannin as the carbon source. Catal. Sci. Technol. 2012, 2, 390–399. [Google Scholar] [CrossRef]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K.; Fujishima, A. A film-type photocatalyst incorporating highly active TiO2 powder and fluororesin binder: Photocatalytic activity and long-term stability. J. Electroanal. Chem. 1996, 415, 183–186. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Szeifert, J.M.; Yu, Q.; Kalousek, V.; Rathouský, J.; Bein, T. Low-Temperature Synthesis of Mesoporous Titania−Silica Films with Pre-Formed Anatase Nanocrystals. Chem. Mater. 2009, 21, 2410–2417. [Google Scholar] [CrossRef]
- Beyers, E.; Biermans, E.; Ribbens, S.; De Witte, K.; Mertens, M.; Meynen, V.; Bals, S.; Van Tendeloo, G.; Vansant, E.F.; Cool, P. Combined TiO2/SiO2 mesoporous photocatalysts with location and phase controllable TiO2 nanoparticles. Appl. Catal. B Environ. 2009, 88, 515–524. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol-gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photobleaching of methylene blue sensitised by TiO2: An ambiguous system? J. Photochem. Photobiol. A Chem. 1999, 127, 123–134. [Google Scholar] [CrossRef]
- Wang, G.; Xu, L.; Zhang, J.; Yin, T.; Han, D. Enhanced photocatalytic activity of TiO2 powders (P25) via calcination treatment. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]

| Product | Viscosity (mPa·s) | Band Gap ~ +10−2 | Surface Area (m2/g) ~ +1 | Pore Volume (cm3/g) ~ +5·10−2 | Pore Size (nm) ~ +10−1 |
|---|---|---|---|---|---|
| UCA | 4.8 ± 0.1 | – | 654 | 0.42 | 1.3 |
| UCA–TiO2 P25 | 6.1 ± 0.1 | 3.52 | 540 | 0.46 | 1.3 |
| UCA–TiO2 250 °C | 4.3 ± 0.1 | 3.35 | 614 | 0.43 | 1.3 |
| UCA–TiO2 450 °C | 4.4 ± 0.1 | 3.36 | 257 | 0.33 | 3.5 |
| UCA–TiO2 650 °C | 4.7 ± 0.1 | 3.34 | 615 | 0.42 | 1.3 |
| UCA–TiO2 Au 250 °C | 4.9 ± 0.1 | 3.29 | 635 | 0.34 | 1.3 |
| UCA–TiO2 Au 450 °C | 5.0 ± 0.1 | 3.24 | 610 | 0.50 | 1.3 |
| UCA–TiO2 Au 650 °C | 4.8 ± 0.1 | 3.22 | 613 | 0.40 | 1.3 |
| Sample | Dry Matter (% w/w) | ∆E* Color | Material Removed by Peeling (mg) | Contact Angles (CA) | Contact Angles after Irradiation |
|---|---|---|---|---|---|
| untreated | – | – | 1.7 ± 0.3 | – | – |
| UCA | 0.45 ± 0.05 | 3.0 ± 0.1 | 0.2 ± 0.1 | 77 ± 5 | 50 ± 2 |
| UCA–TiO2 P25 | 0.53 ± 0.02 | 4.0 ± 0.6 | 0.2 ± 0.1 | 82 ± 4 | 57 ± 3 |
| UCA–TiO2 250 °C | 0.62 ± 0.01 | 4.5 ± 0.2 | 0.3 ± 0.1 | 94 ± 8 | 65 ± 5 |
| UCA–TiO2 450 °C | 0.57 ± 0.04 | 4.6 ± 0.4 | 0.3 ± 0.1 | 101 ± 3 | 70 ± 5 |
| UCA–TiO2 650 °C | 0.57 ± 0.03 | 3.3 ± 0.8 | 0.3 ± 0.1 | 78 ± 3 | 55 ± 2 |
| UCA–TiO2 Au 250 °C | 0.49 ± 0.02 | 4.9 ± 0.1 | 0.2 ± 0.1 | 100 ± 5 | 68 ± 1 |
| UCA–TiO2 Au 450 °C | 0.48 ± 0.08 | 4.7 ± 0.3 | 0.5 ± 0.1 | 83 ± 5 | 54 ± 2 |
| UCA–TiO2 Au 650 °C | 0.51 ± 0.06 | 5.0 ± 0.1 | 0.2 ± 0.1 | 85 ± 7 | 55 ± 5 |
| Sample | WAC 0–60 min ± 0.01 | RCI 0–144 h ± 0.05 | TWU (% w/w) |
|---|---|---|---|
| Untreated stone | 2.83 | 1 | 3.50 ± 0.55 |
| UCA | 0.65 | 0.85 | 1.03 ± 0.40 |
| UCA–TiO2 P25 | 0.11 | 0.21 | 0.17 ± 0.08 |
| UCA–TiO2 250 °C | 0.10 | 0.10 | 0.11 ± 0.03 |
| UCA–TiO2 450 °C | 0.23 | 0.70 | 0.74 ± 0.02 |
| UCA–TiO2 650 °C | 0.20 | 0.40 | 0.55 ± 0.01 |
| UCA–TiO2Au 250 °C | 0.14 | 0.30 | 0.36 ± 0.03 |
| UCA–TiO2Au 450 °C | 0.24 | 0.34 | 0.52 ± 0.01 |
| UCA–TiO2Au 650 °C | 0.11 | 0.12 | 0.16 ± 0.05 |
| Samples | Kkinetic (min−1) |
|---|---|
| untreated stone | 0.004 ± 1·10−3 |
| UCA | 0.010 ± 1·10−3 |
| UCA–TiO2 P25 | 0.069 ± 4·10−3 |
| UCA–TiO2 250 °C | 0.049 ± 2·10−3 |
| UCA–TiO2 450 °C | 0.024 ± 2·10−3 |
| UCA–TiO2 650 °C | 0.114 ± 4·10−3 |
| UCA–TiO2Au 250 °C | 0.084 ± 8·10−3 |
| UCA–TiO2Au 450 °C | 0.183 ± 8·10−3 |
| UCA–TiO2Au 650 °C | 0.124 ± 6·10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truppi, A.; Luna, M.; Petronella, F.; Falcicchio, A.; Giannini, C.; Comparelli, R.; Mosquera, M.J. Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials. Coatings 2018, 8, 296. https://doi.org/10.3390/coatings8090296
Truppi A, Luna M, Petronella F, Falcicchio A, Giannini C, Comparelli R, Mosquera MJ. Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials. Coatings. 2018; 8(9):296. https://doi.org/10.3390/coatings8090296
Chicago/Turabian StyleTruppi, Alessandra, Manuel Luna, Francesca Petronella, Aurelia Falcicchio, Cinzia Giannini, Roberto Comparelli, and Maria J. Mosquera. 2018. "Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials" Coatings 8, no. 9: 296. https://doi.org/10.3390/coatings8090296
APA StyleTruppi, A., Luna, M., Petronella, F., Falcicchio, A., Giannini, C., Comparelli, R., & Mosquera, M. J. (2018). Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials. Coatings, 8(9), 296. https://doi.org/10.3390/coatings8090296