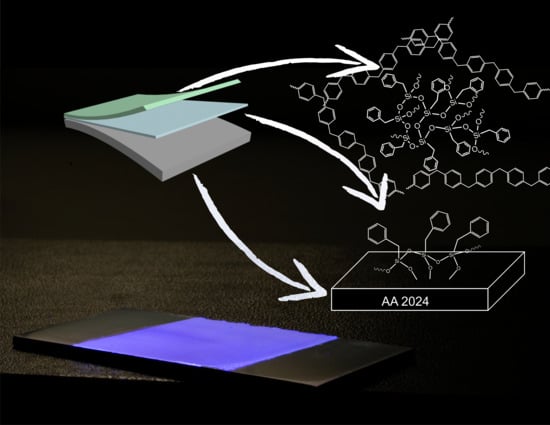
Poly(Phenylene Methylene): A Multifunctional Material for Thermally Stable, Hydrophobic, Fluorescent, Corrosion-Protective Coatings
Abstract
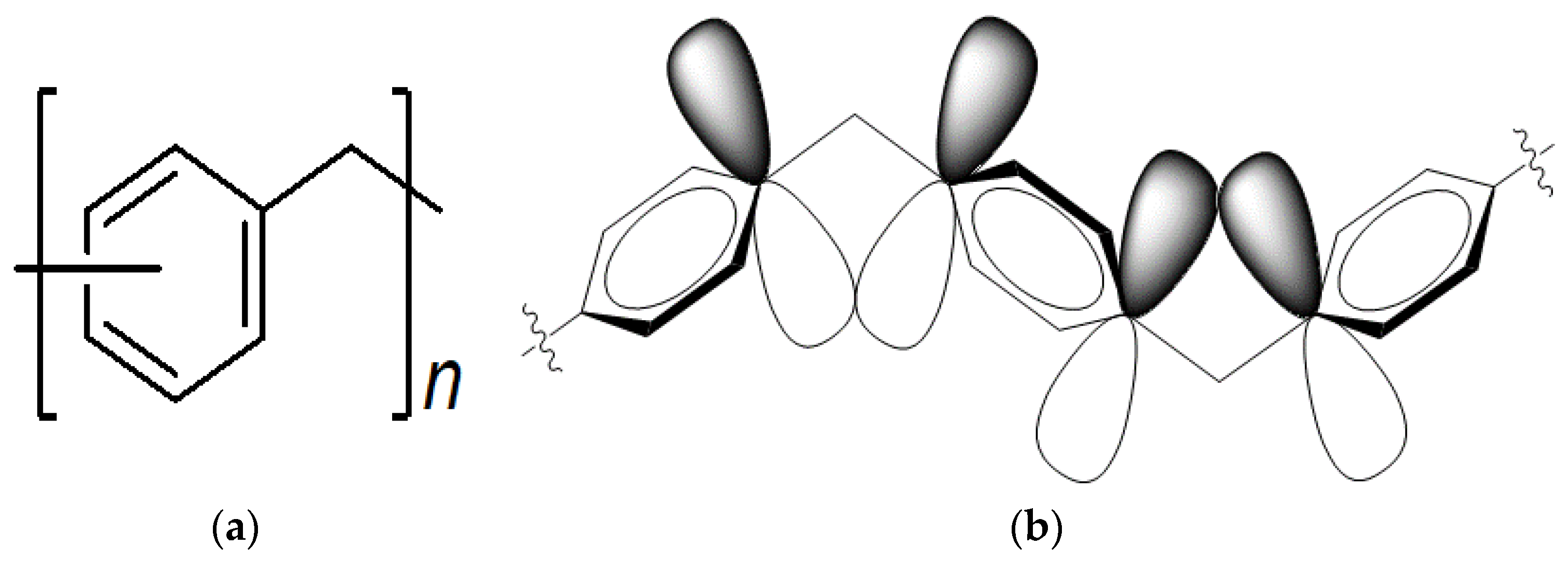
:1. Introduction
2. Materials and Methods
3. Results
3.1. Surface-Modified Metal Substrates
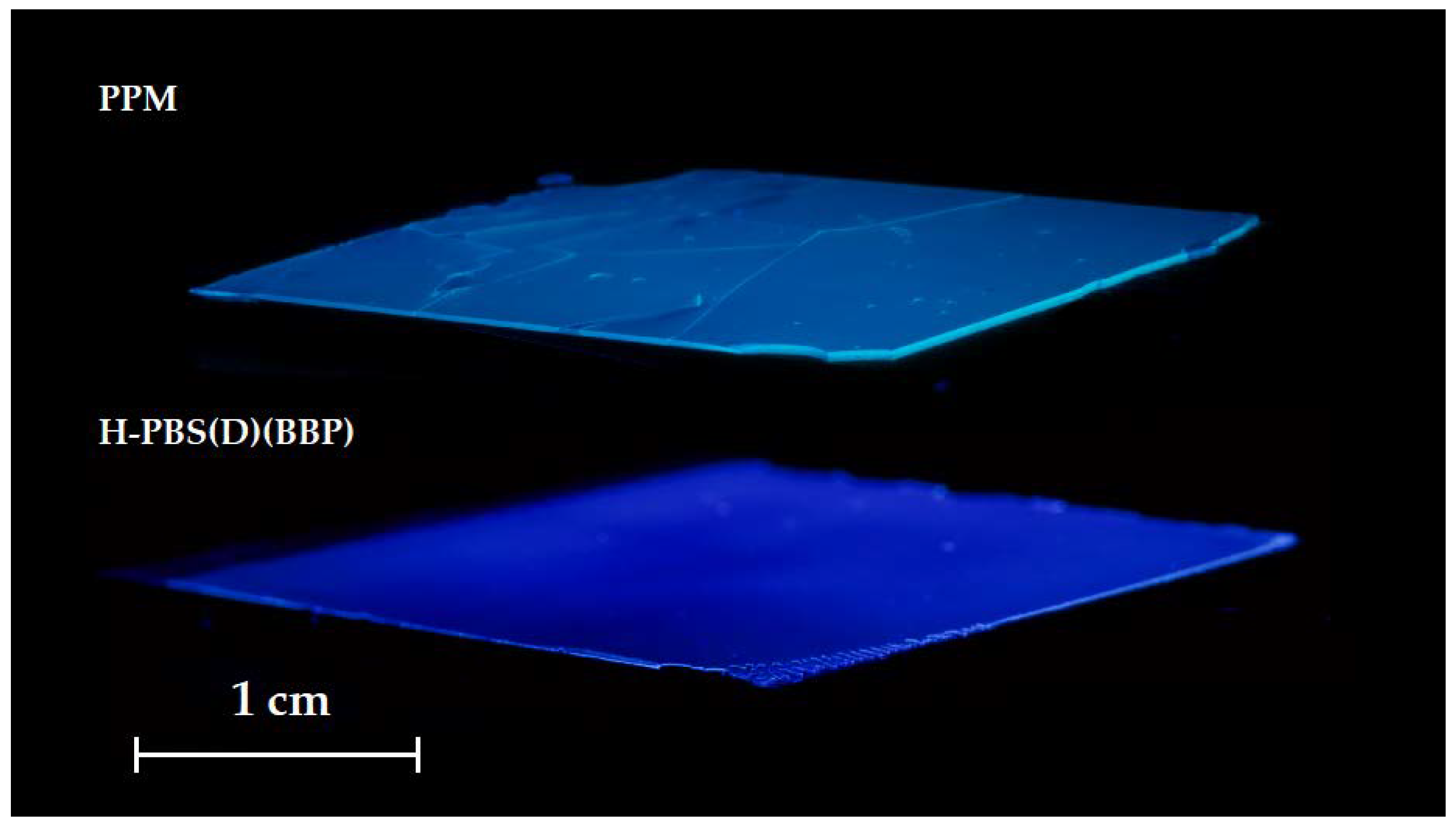
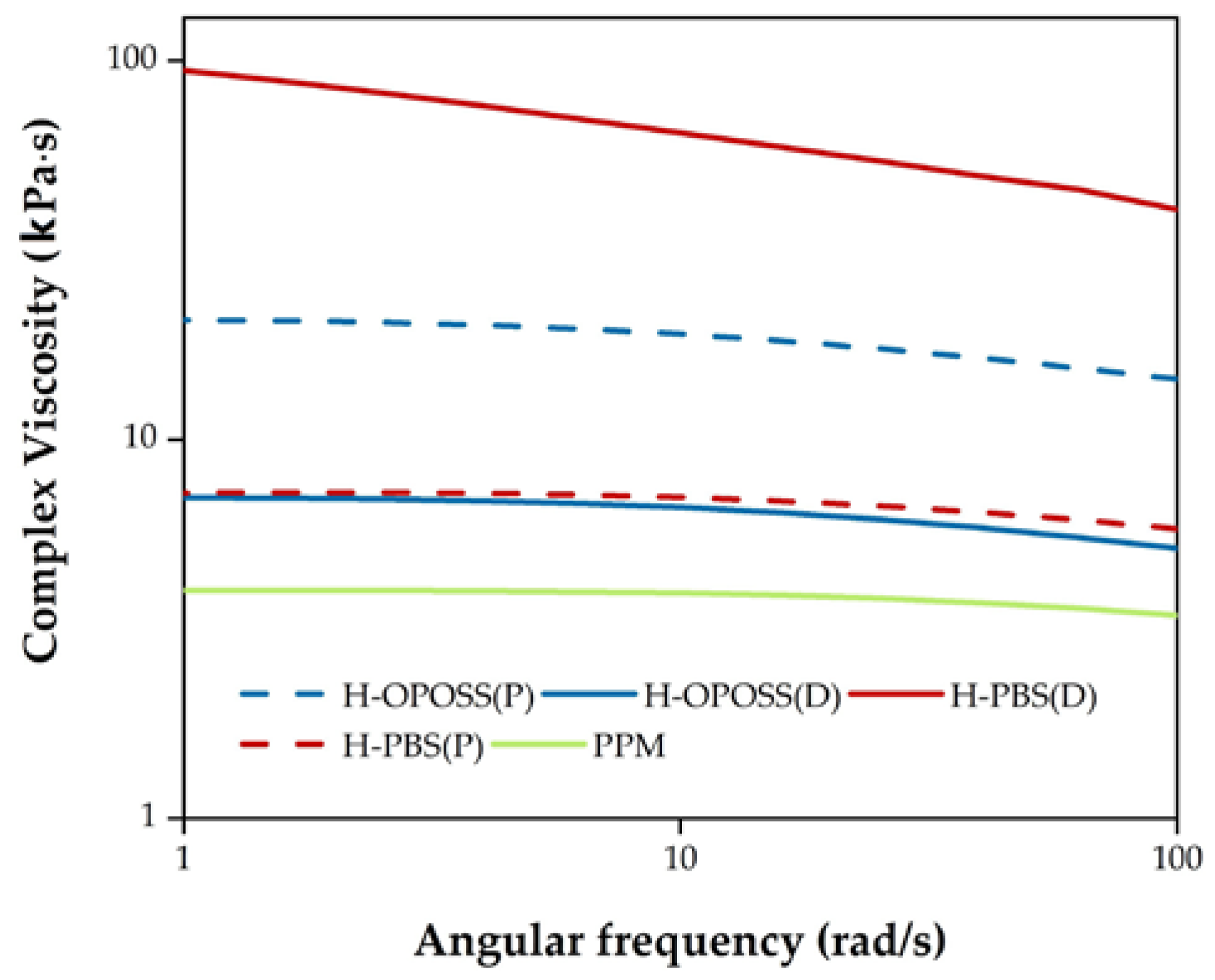
3.2. Coatings
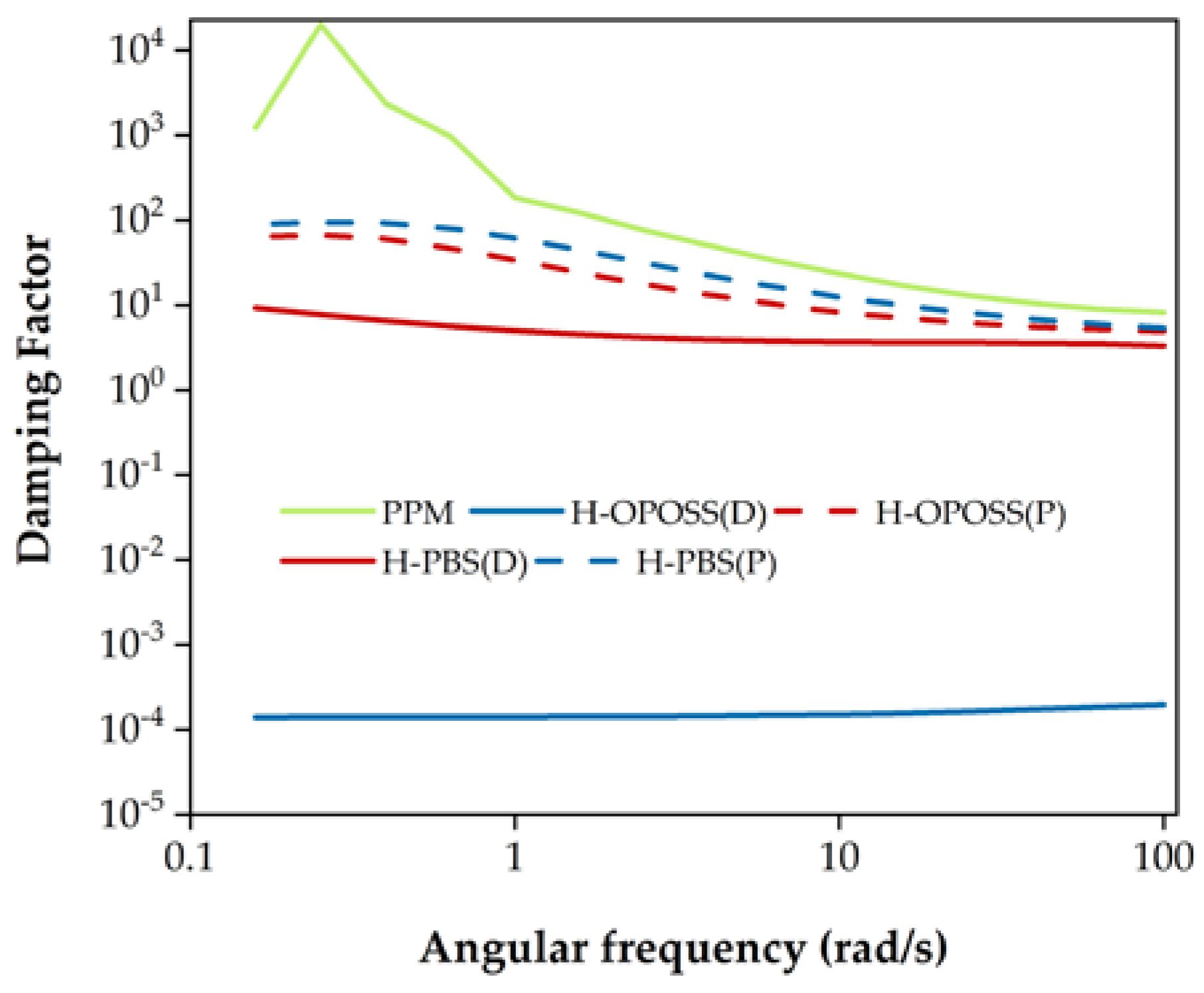
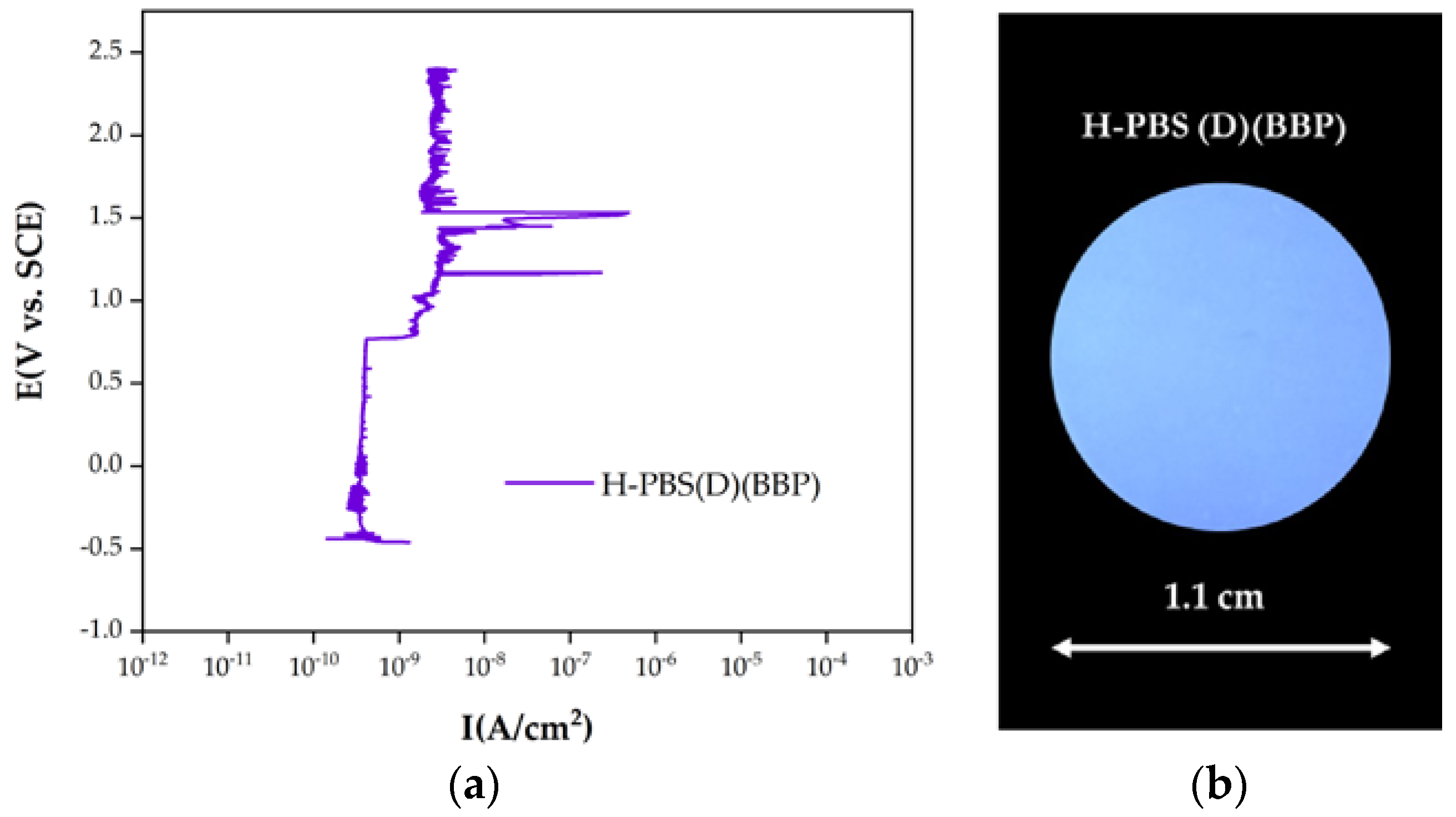
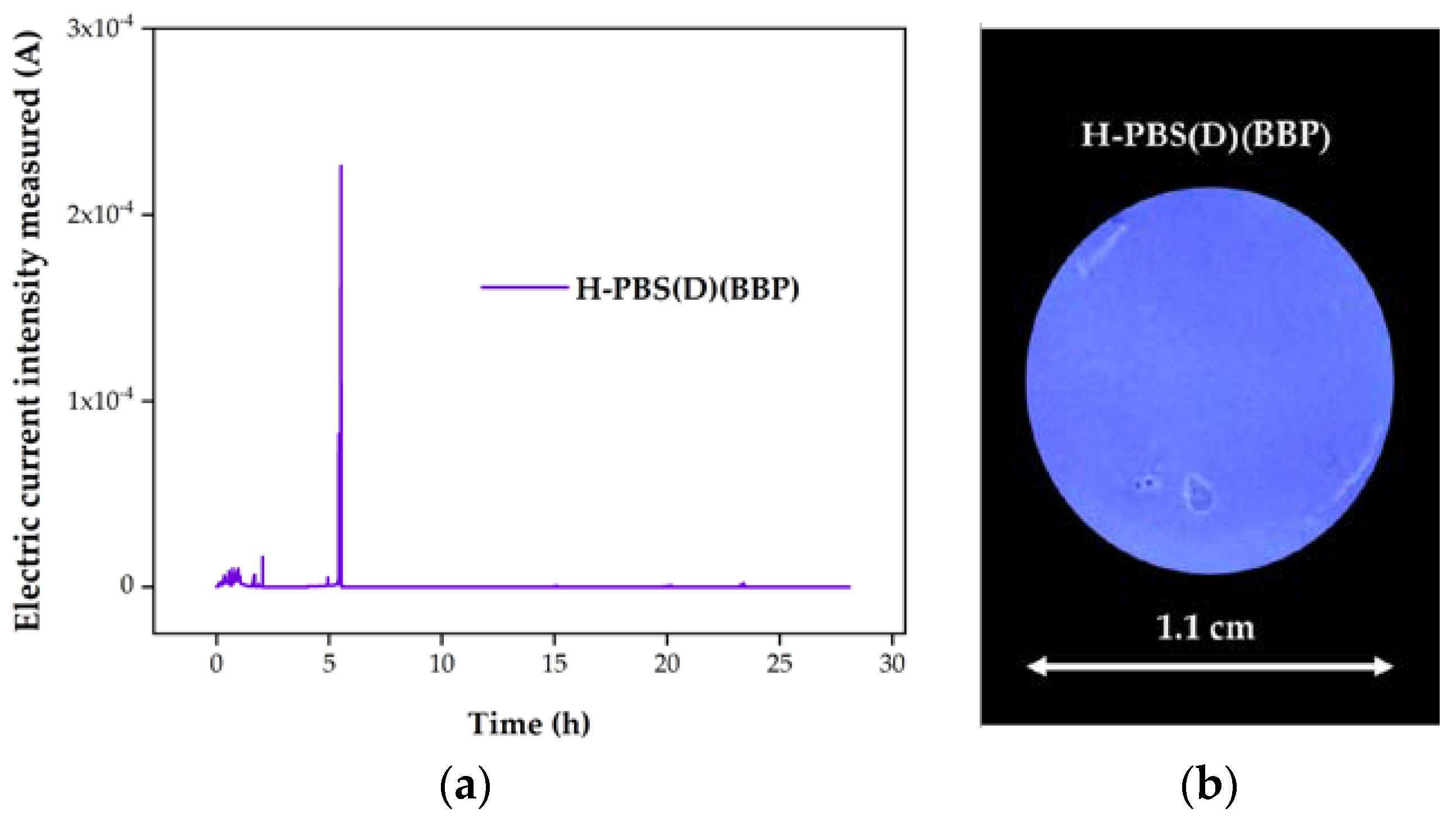
3.3. Corrosion Tests
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blilncow, P.J.; Pritchard, G. Determination of the structure of polyethylarylmethylenes by 13C nuclear magnetic resonance spectroscopy. Polymer 1987, 28, 1824–1828. [Google Scholar] [CrossRef]
- Ul Hasasn, M.; Tsonis, C.P. Structural characterization of polybenzyls by high field 13C-NMR spectroscopy. J. Polym. Sci. A Polym. Chem. 1984, 22, 1349–1355. [Google Scholar] [CrossRef]
- Has, H.C.; Livingston, D.I.; Saunders, M. Polybenzyl type polymers. J. Polym. Sci. A Gen. Pap. 1955, 15, 503–514. [Google Scholar] [CrossRef]
- Braendle, A.; Perevedentsev, A.; Cheetham, N.J.; Stavrinou, P.N.; Schachner, J.A.; Möosch-Zanetti, N.C.; Niederberger, M.; Caseri, W.R. Homoconjugation in poly(phenylene methylene)s: A case study of non-π-conjugated polymers with unexpected fluorescent properties. J. Polym. Sci. B Polym. Phys. 2017, 55, 707–720. [Google Scholar] [CrossRef]
- Muller, P. Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 306, 1077–1184. [Google Scholar] [CrossRef]
- Ferguson, L.N.; Nnadi, J.C. Electronic interactions between nonconjugated groups. J. Chem. Educ. 1965, 42, 529–535. [Google Scholar] [CrossRef]
- Baumberger, T.R.; Woolsey, N.F. Metal arene complexation of polybenzyl: Preparation and methylation. J. Polym. Sci. A Polym. Chem. 1992, 30, 1717–1723. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jarvis, K.A.; Ferreira, P.J.; Bielawski, C.W. Graphite oxide as a dehydrative polymerization catalyst: A one-step synthesis of carbon-reinforced poly(phenylene methylene) composites. Macromolecules 2011, 44, 7659–7667. [Google Scholar] [CrossRef]
- Grassie, N.; Meldrum, I.G. Friedel-crafts polymers—IX Later stages of the copolymerization of p-di(chloromethyl)benzene with aromatic substances. Eur. Polym. J. 1971, 7, 1253–1273. [Google Scholar] [CrossRef]
- Gunes, D.; Yagci, Y.; Bicak, N. Synthesis of soluble poly(p-phenylene methylene) from tribenzylborate by acid-catalyzed polymerization. Macromolecules 2010, 43, 7993–7997. [Google Scholar] [CrossRef]
- Arata, K.; Fukui, A. Toyoshima High catalytic activity of calcined iron sulphates for the polycondensation of benzyl chloride. J. Chem. Soc. Chem. Commun. 1978, 121–122. [Google Scholar] [CrossRef]
- Finocchiaro, P. Frazionamento di polimeri benzilici ottenuti a bassa temperature. Boll. Sci. Fac. Chim. Ind. Bologna 1968, 26, 255. [Google Scholar]
- Hino, M.; Arata, K. Iron oxide as an effective catalyst for the polycondensation of benzyl chloride, the formation of para-substituted polybenzyl. Chem. Lett. 1979, 8, 1141–1144. [Google Scholar] [CrossRef]
- Grassie, N.; Meldrum, I.G. Friedel-crafts polymers—2 Initial stages of the copolymerization of p-di(chloromethyl) benzene with benzene. Eur. Polym. J. 1969, 5, 195–209. [Google Scholar] [CrossRef]
- Tsonis, C.P. Homogeneous catalytic polymerization of benzyl chloride leading to linear high molecular weight polymers: An elusive goal. J. Mol. Catal. 1990, 57, 313–323. [Google Scholar] [CrossRef]
- Banks, R.E.; François, P.-Y.; Preston, P.N. Polymerization of benzyl alcohol in anhydrous hydrogen fluoride: An efficient synthesis of poly(phenylenemethylene). Polymer 1992, 33, 3974–3975. [Google Scholar] [CrossRef]
- Klärner, C.; Greiner, A. Synthesis of polybenzyls by Suzuki Pd-catalyzed crosscoupling of boronic acids and benzyl bromides: Model reactions and polyreactions. Macromol. Rapid Commun. 1998, 19, 605–608. [Google Scholar] [CrossRef]
- Som, A.; Ramakrishnan, S. Linear soluble polybenzyls. J. Polym. Sci. A Polym. Chem. 2003, 41, 2345–2353. [Google Scholar] [CrossRef]
- Saliba, P.A.; Mansus, A.A.; Santos, D.B.; Mansur, H.S. Fusion-bonded epoxy composite coatings on chemically functionalized API steel surfaces for potential deep-water petroleum exploration. Appl. Adhes. Sci. 2015, 3, 22. [Google Scholar] [CrossRef]
- Choi, Y.K.; Hyeong, J.K.; Sung, R.K.; Young, M.C.; Dong, J.A. Enhanced Thermal Stability of Polyaniline with Polymerizable Dopants. Macromolecules 2017, 50, 3164–3170. [Google Scholar] [CrossRef]
- Braendle, A.; Schwendimann, P.; Niederberger, M.; Caseri, W.R. Synthesis and fractionation of poly(phenylene methylene). J. Polym. Sci. A Polym. Chem. 2018, 56, 309–318. [Google Scholar] [CrossRef]
- Twite, R.L.; Bioerwagen, G.P. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Witucki, G.L. A Silane Primer: Chemistry and applications of alkoxy silanes. J. Coat. Technol. 1993, 65, 57–60. [Google Scholar]
- Plueddemann, E.P. Chemistry of Silane Coupling Agents. In Silane Coupling Agents, 2nd ed.; Plueddemann, E.P., Ed.; Springer: Boston, MA, USA, 1991; pp. 31–54. [Google Scholar]
- Goldschmidt, P.D.A.; Streitberger, D.H.-J. Basics of Coating Technology; Vincentz: Hannover, Germany, 2007. [Google Scholar]
- Gilleo, K.B. Rheology and Surface Chemistry. In Coating Technology, Fundamentals, Testing and Processing Techniques; Tracton, A.A., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 1-1–1-12. [Google Scholar]
- Chan, C.-M.; Venkatraman, S. Coating Rheology. In Coating Technology, Fundamentals, Testing and Processing Techniques; Tracton, A.A., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 2-1–2-14. [Google Scholar]
- Kickelbick, G. Introduction to hybrid materials. In Hybrid Materials. Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–46. [Google Scholar]
- Trdan, U.; Grum, J. Evaluation of corrosion resistance of AA6082-T651 aluminium alloy after laser shockpeening by means of cyclic polarisation and ElS methods. Corros. Sci. 2012, 59, 324–333. [Google Scholar] [CrossRef]
- Bain, C.D.; Troughton, E.B.; Tao, Y.-T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Troughton, E.B.; Bain, C.D.; Whitesides, G.M.; Nuzzo, R.G.; Allara, D.L.; Porter, M.D. Monolayer films prepared by the spontaneous self-assembly of symmetrical and unsymmetrical dialkyl sulfides from solution onto gold substrates: Structure, properties, and reactivity of constituent functional groups. Langmuir 1988, 4, 365–385. [Google Scholar] [CrossRef]
- Talo, A.; Passiniemi, P.; Forsén, O.; Yläsaari, S. Polyaniline/Epoxy Coatings with Good Anti-Corrosion Properties. Synth. Met. 1997, 85, 1333–1334. [Google Scholar] [CrossRef]
- Rodošek, M.; Rauter, A.; Perše, L.S.; Kek, D.M.; Vuk, A.S. Vibrational and corrosion properties of poly(dimethylsiloxane)-based protective coatings for AA 2024 modified with nanosized polyhedral oligomeric silsesquioxane. Corros. Sci. 2014, 85, 193–203. [Google Scholar] [CrossRef]
- Kreta, A.; Rodošek, M.; Perše, L.S.; Orel, B.; Gaberšček, M.; Vuk, A.Š. In situ electrochemical AFM, ex situ IR reflection–absorption and confocal Raman studies of corrosion processes of AA 2024-T3. Corros. Sci. 2016, 104, 290–309. [Google Scholar] [CrossRef]
- Jin, K.; Li, L.; Torkelson, J.M. Bulk physical aging behavior of cross-linked polystyrene compared to its linear precursor: Effects of cross-linking and aging temperature. Polymer 2017, 115, 197–203. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, M.F.; Braendle, A.; Schweizer, T.B.; Ortenzi, M.A.; Trasatti, S.P.M.; Niederberger, M.; Caseri, W. Poly(Phenylene Methylene): A Multifunctional Material for Thermally Stable, Hydrophobic, Fluorescent, Corrosion-Protective Coatings. Coatings 2018, 8, 274. https://doi.org/10.3390/coatings8080274
D’Elia MF, Braendle A, Schweizer TB, Ortenzi MA, Trasatti SPM, Niederberger M, Caseri W. Poly(Phenylene Methylene): A Multifunctional Material for Thermally Stable, Hydrophobic, Fluorescent, Corrosion-Protective Coatings. Coatings. 2018; 8(8):274. https://doi.org/10.3390/coatings8080274
Chicago/Turabian StyleD’Elia, Marco F., Andreas Braendle, Thomas B. Schweizer, Marco A. Ortenzi, Stefano P. M. Trasatti, Markus Niederberger, and Walter Caseri. 2018. "Poly(Phenylene Methylene): A Multifunctional Material for Thermally Stable, Hydrophobic, Fluorescent, Corrosion-Protective Coatings" Coatings 8, no. 8: 274. https://doi.org/10.3390/coatings8080274
APA StyleD’Elia, M. F., Braendle, A., Schweizer, T. B., Ortenzi, M. A., Trasatti, S. P. M., Niederberger, M., & Caseri, W. (2018). Poly(Phenylene Methylene): A Multifunctional Material for Thermally Stable, Hydrophobic, Fluorescent, Corrosion-Protective Coatings. Coatings, 8(8), 274. https://doi.org/10.3390/coatings8080274