Waterborne Acrylate-Based Hybrid Coatings with Enhanced Resistance Properties on Stone Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Application of the Latexes
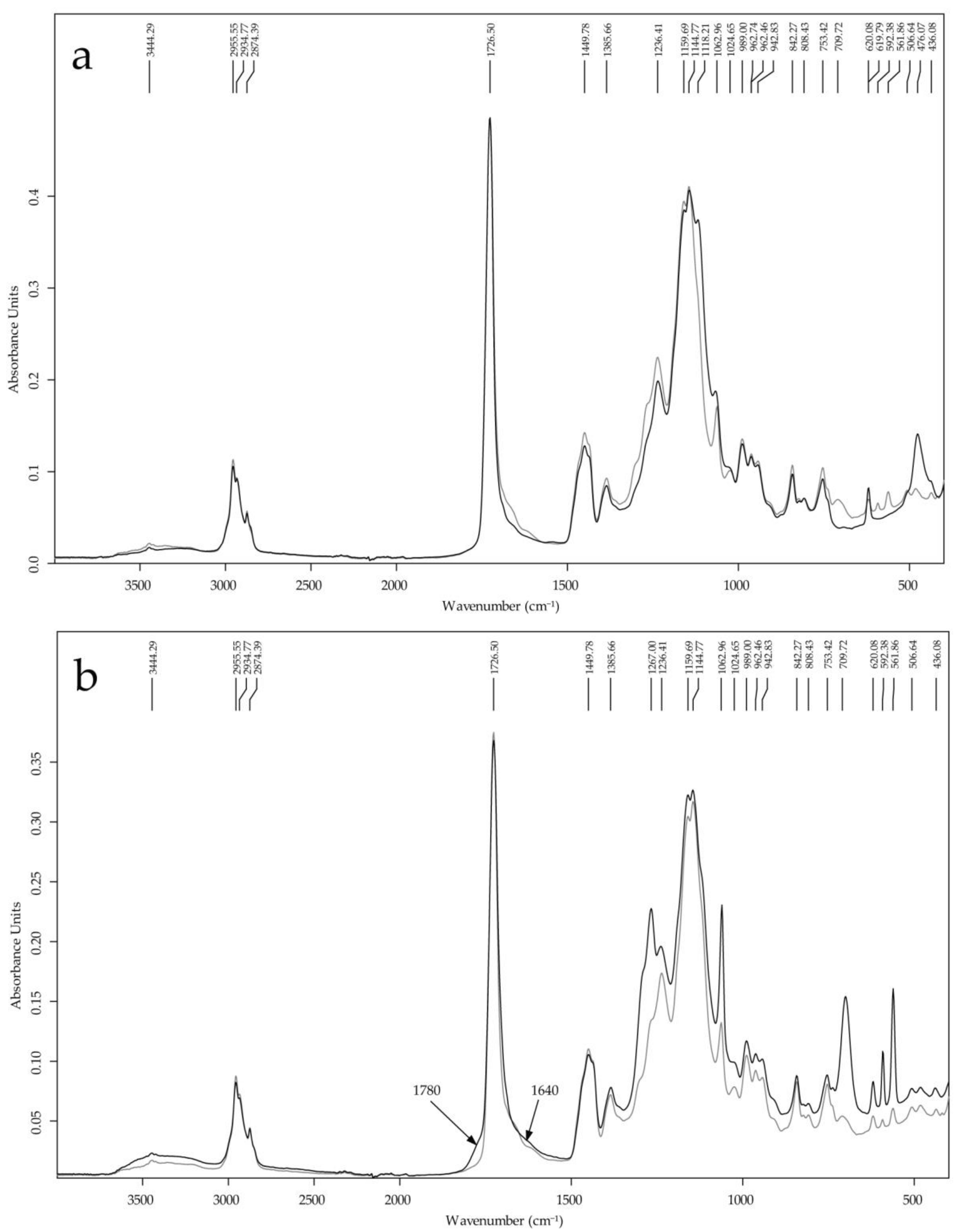
2.3. Characterization
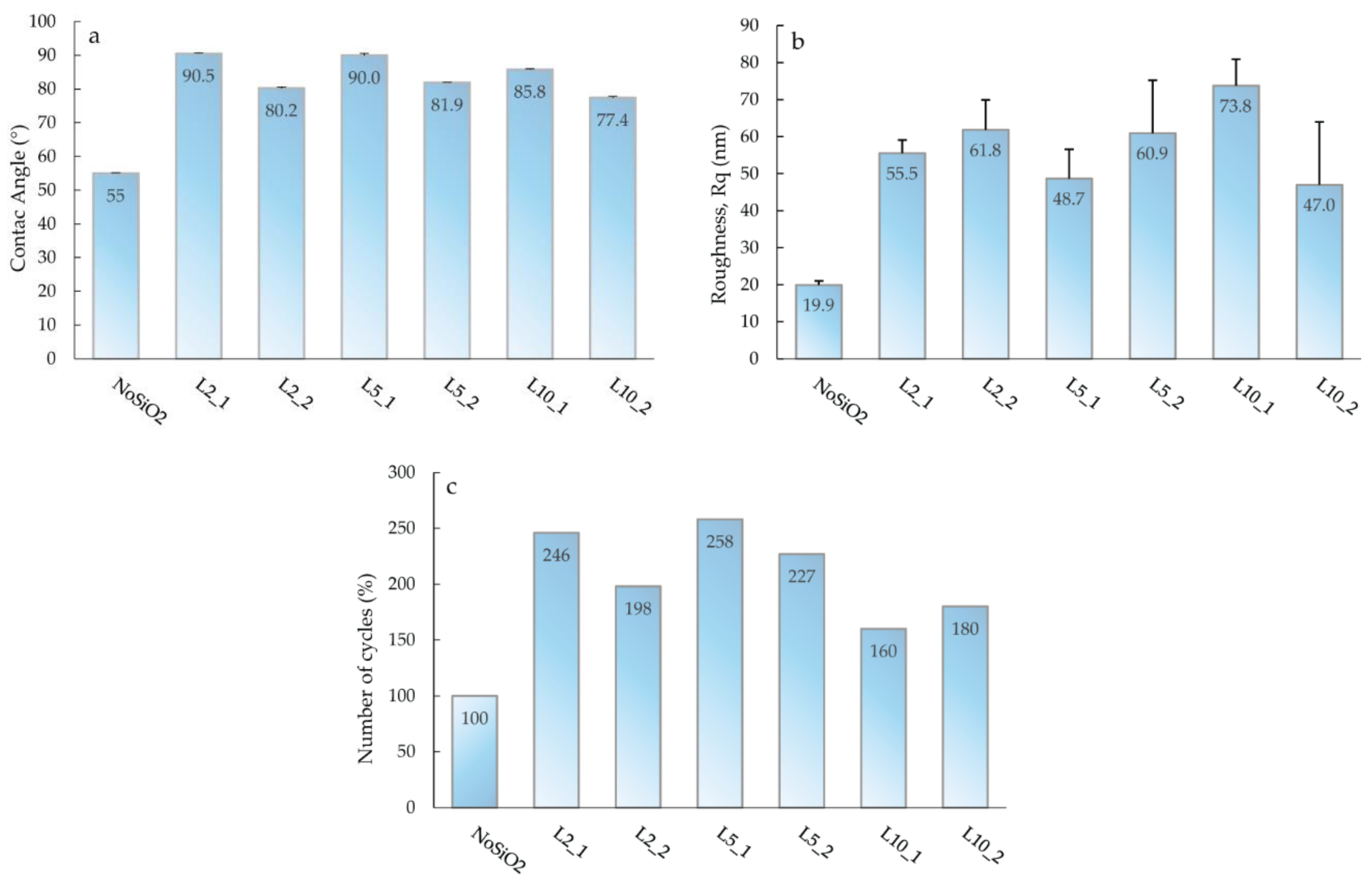
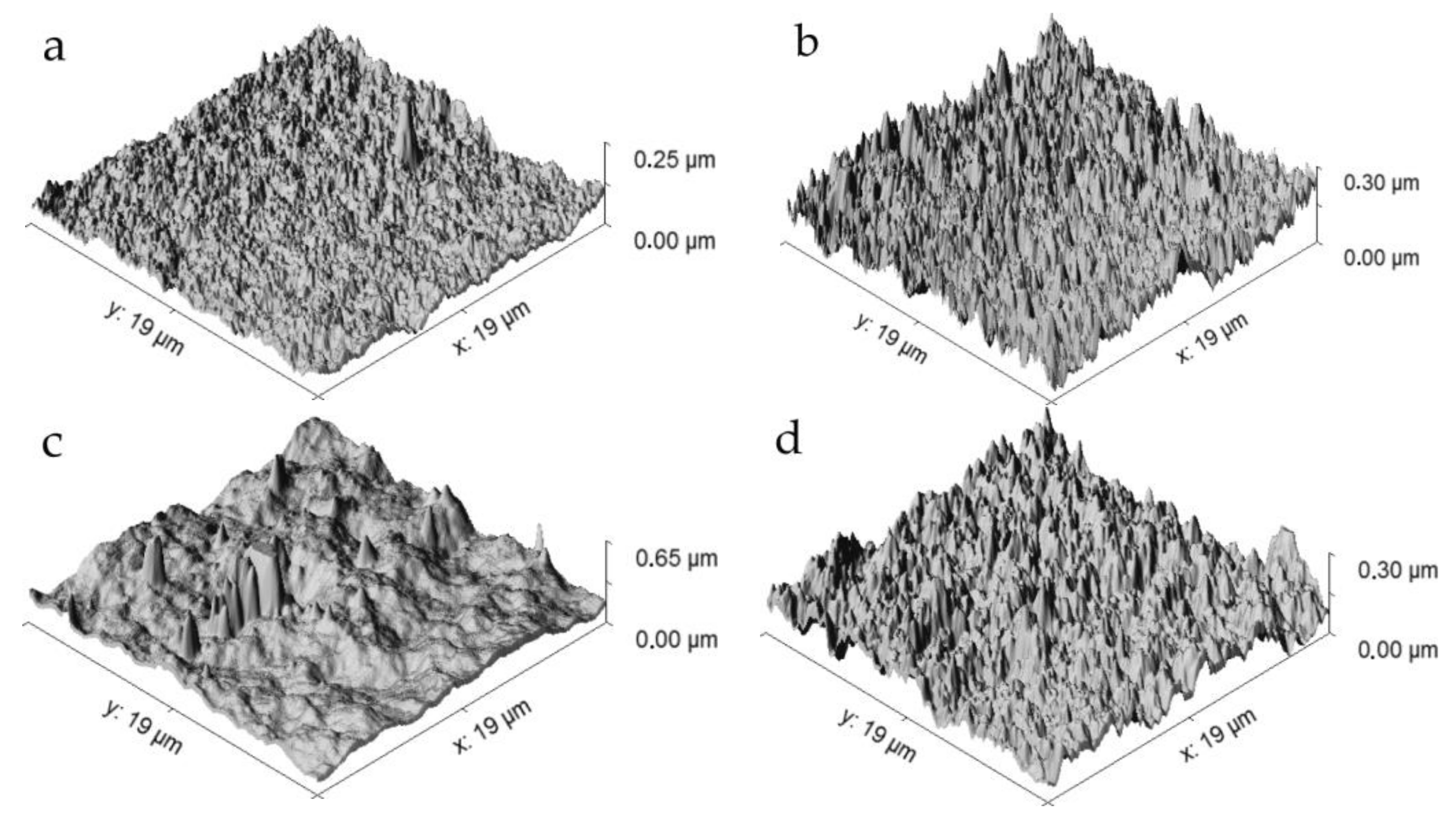
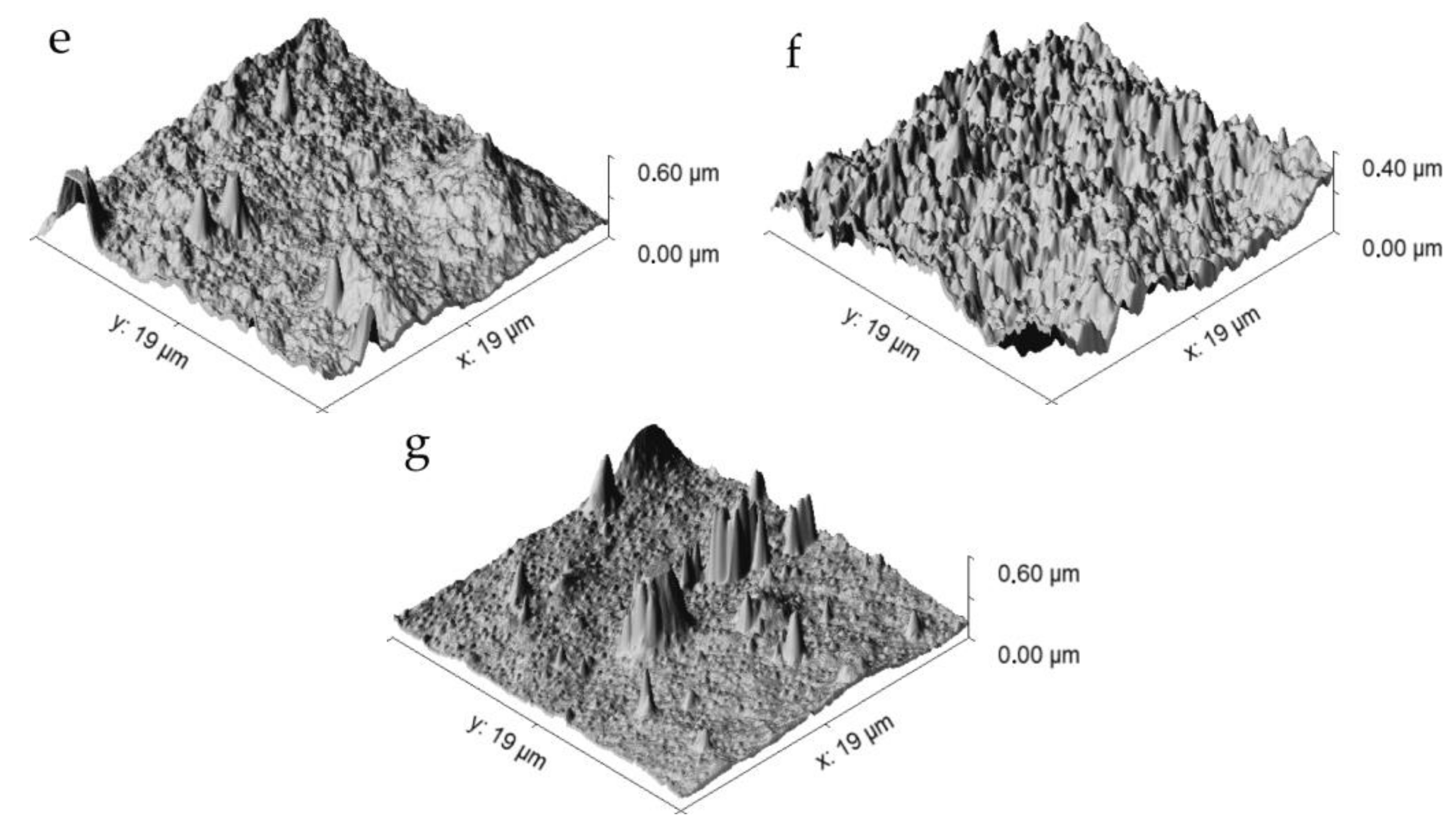
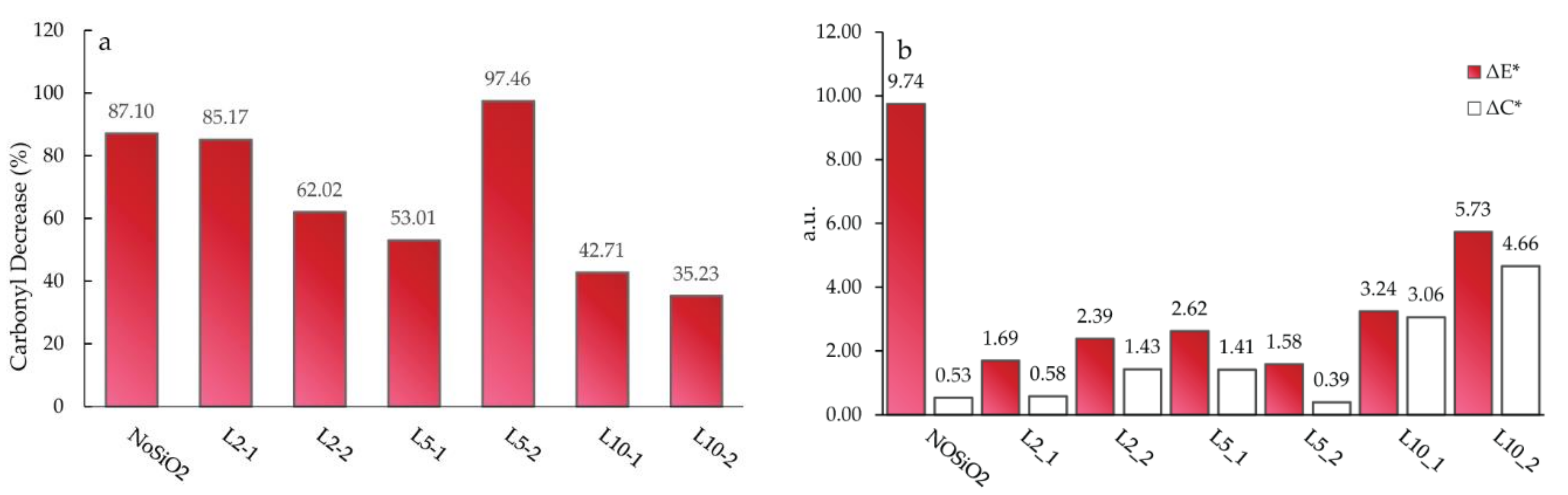
3. Results and Discussion
Accelerated Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doehne, E.F.; Price, C.A. Stone Conservation: An Overview of Current Research; Getty Conservation Institute: Los Angeles, CA, USA, 2010; ISBN 1606060465. [Google Scholar]
- Khallaf, M.K.; El-Midany, A.A.; El-Mofty, S.E. Influence of acrylic coatings on the interfacial, physical, and mechanical properties of stone-based monuments. Prog. Org. Coat. 2011, 72, 592–598. [Google Scholar] [CrossRef]
- López, A.B.; De La Cal, J.C.; Asua, J.M. Highly Hydrophobic Coatings from Waterborne Latexes. Langmuir 2016, 32, 7459–7466. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Irikawa, K.; Yamashita, H. Multifunctional surface designed by nanocomposite coating of polytetrafluoroethylene and TiO2 photocatalyst: Self-cleaning and superhydrophobicity. Sci. Rep. 2017, 7, 13628. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Song, D.; Li, X.; Zhang, D.; Gao, J.; Du, C. Failure Mechanisms of the Coating/Metal Interface in Waterborne Coatings: The Effect of Bonding. Materials 2017, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Diao, X.; Zhang, J.; Kang, P. Corrosion Resistance of Waterborne Epoxy Coatings by Incorporation of Dopamine Treated Mesoporous-TiO2 Particles. Coatings 2018, 8, 209. [Google Scholar] [CrossRef]
- Raditoiu, V.; Raditoiu, A.; Raduly, M.; Amariutei, V.; Gifu, I.; Anastasescu, M. Photocatalytic Behavior of Water-Based Styrene-Acrylic Coatings Containing TiO2 Sensitized with Metal-Phthalocyanine Tetracarboxylic Acids. Coatings 2017, 7, 229. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Calia, A.; Colangiuli, D.; Lettieri, M.; Matera, L. A deep knowledge of the behaviour of multi-component products for stone protection by an integrated analysis approach. Prog. Org. Coat. 2013, 76, 893–899. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; De Simone, N.; Santarelli, M.L.; Frigione, M. Protective properties and durability characteristics of experimental and commercial organic coatings for the preservation of porous stone. Prog. Org. Coat. 2017, 103, 193–203. [Google Scholar] [CrossRef]
- Pia, G.; Esposito Corcione, C.; Striani, R.; Casnedi, L.; Sanna, U. Coating’s influence on water vapour permeability of porous stones typically used in cultural heritage of Mediterranean area: Experimental tests and model controlling procedure. Prog. Org. Coat. 2017, 102, 239–246. [Google Scholar] [CrossRef]
- Tabasso, M.L. Acrylic Polymers for the Conservation of Stone: Advantages and Drawbacks. APT Bull. J. Preserv. Technol. 1995, 26, 17–21. [Google Scholar] [CrossRef]
- Siegesmund, S.; Snethlage, R. Stone in Architecture: Properties, Durability; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 3642451551. [Google Scholar]
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The preservation damage of hydrophobic polymer coating materials in conservation of stone relics. Prog. Org. Coat. 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Paulis, M.; Asua, J.M. Knowledge-Based Production of Waterborne Hybrid Polymer Materials. Macromol. React. Eng. 2016, 10, 8–21. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C. Hybrid organic/inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Gagliardi, S.; Rondino, F.; D’erme, C.; Persia, F.; Menchini, F.; Santarelli, M.L.; Paulke, B.-R.; Enayati, L. Preparation and characterization of polymeric nanocomposite films for application as protective coatings. In Proceedings of the AIP Conference Proceedings, Rome, Italy, 20–23 September 2016; Volume 1873, pp. 20007–20009. [Google Scholar]
- Kim, E.K.; Won, J.; Do, J.; Kim, S.D.; Kang, Y.S. Effects of silica nanoparticle and GPTMS addition on TEOS-based stone consolidants. J. Cult. Herit. 2009, 10, 214–221. [Google Scholar] [CrossRef]
- Dei, L.; Salvadori, B. Nanotechnology in cultural heritage conservation: Nanometric slaked lime saves architectonic and artistic surfaces from decay. J. Cult. Herit. 2006, 7, 110–115. [Google Scholar] [CrossRef]
- Xu, F.; Wang, C.; Li, D.; Wang, M.; Xu, F.; Deng, X. Preparation of modified epoxy-SiO2 hybrid materials and their application in the stone protection. Prog. Org. Coat. 2015, 81, 58–65. [Google Scholar] [CrossRef]
- Illescas, J.F.; Mosquera, M.J. Producing Surfactant-Synthesized Nanomaterials In Situ on a Building Substrate, without Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2012, 4, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Kugler, S.; Kowalczyk, K.; Spychaj, T. Influence of dielectric nanoparticles addition on electroconductivity and other properties of carbon nanotubes-based acrylic coatings. Prog. Org. Coat. 2016, 92, 66–72. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-Z.; Hu, J.; Zhang, Z.-J. Polyacrylate/silica nanocomposite materials prepared by sol–gel process. Eur. Polym. J. 2007, 43, 4169–4177. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Hsieh, C.C.; Lin, Y.M.; Li, A.K. Preparation of transparent silica-PMMA nanocomposite hard coatings. Prog. Org. Coat. 2008, 62, 436–439. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizão, C.; Farinha, J.P.S. Functional films from silica/polymer nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheng, L.; Zhang, X.; Chen, X.; Yang, B. Silica-PMMA core-shell and hollow nanospheres. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 145–150. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Zhang, X.; Shi, C. Recent advances in the modification of polyacrylate latexes. J. Mater. Sci. 2015, 50, 6839–6863. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Arcos-Casarrubias, J.A.; Hernandez-Vargas, M.L.; Reyes-Mayer, A.; Aguilar-Franco, M.; Bagdhachi, J. Acrylate hybrid nanocomposite coatings based on SiO2 nanoparticles by in-situ batch emulsion polymerization. Prog. Org. Coat. 2016, 97, 288–300. [Google Scholar] [CrossRef]
- Mahaling, R.N.; Kumar, S.; Rath, T.; Das, C.K. Acrylic elastomer/filler nanocomposite: Effect of silica nanofiller on thermal, mechanical and interfacial adhesion. Plast. Rubber Compos. 2007, 36, 267–273. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Bhowmick, A.K.; De Sarkar, M. Synthesis and characterization of acrylic rubber/silica hybrid composites prepared by sol-gel technique. J. Appl. Polym. Sci. 2004, 93, 2579–2589. [Google Scholar] [CrossRef]
- Arai, K.; Mizutani, T.; Kimura, Y.; Miyamoto, M. Unique structure and properties of inorganic-organic hybrid films prepared from acryl/silica nano-composite emulsions. Prog. Org. Coat. 2016, 93, 109–117. [Google Scholar] [CrossRef]
- Ramos-Fernández, J.M.; Beleña, I.; Romero-Sánchez, M.D.; Fuensanta, M.; Guillem, C.; López-Buendía, Á.M. Study of the film formation and mechanical properties of the latexes obtained by miniemulsion co-polymerization of butyl acrylate, methyl acrylate and 3-methacryloxypropyltrimethoxysilane. Prog. Org. Coat. 2012, 75, 86–91. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, L. Transparent Organic-Inorganic Nanocomposite Coatings. In Functional Polymer Coatings: Principles, Methods, and Applications; Wu, L., Baghdachi, J., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–71. [Google Scholar]
- Krasia-Christoforou, T. Organic-inorganic polymer hybrids: Synthetic strategies and applications. In Hybrid and Hierarchical Composite Materials; Springer International Publishing: Cham, Switzerland, 2015; pp. 11–63. ISBN 9783319128689. [Google Scholar]
- Kickelbick, G. Hybrid Materials—Past, Present and Future. Hybrid Mater. 2014, 1, 39–51. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Lansalot, M. Organic/Inorganic Composite Latexes: The Marriage of Emulsion Polymerization and Inorganic Chemistry. In Hybrid Latex Particles: Preparation with (Mini) Emulsion Polymerization; Van Herk, A.M., Landfester, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 233, pp. 53–123. ISBN 978-3-642-16060-8. [Google Scholar]
- Hamzehlou, S.; Aguirre, M.; Leiza, J.R.; Asua, J.M. Dynamics of the Particle Morphology during the Synthesis of Waterborne Polymer-Inorganic Hybrids. Macromolecules 2017, 50, 7190–7201. [Google Scholar] [CrossRef]
- UNI 10921:2001 Cultural Heritage—Natural and Artificial Stones—Water Repellents—Application on Samples and Determination of their Properties in Laboratory; UNI: Milan, Italy, 2001.
- Bergamonti, L.; Bondioli, F.; Alfieri, I.; Alinovi, S.; Lorenzi, A.; Predieri, G.; Lottici, P.P. Weathering resistance of PMMA/SiO2/ZrO2 hybrid coatings for sandstone conservation. Polym. Degrad. Stab. 2018, 147, 274–283. [Google Scholar] [CrossRef]
- ASTM D2486-00 Standard Test Methods for Scrub Resistance of Wall Paints; ASTM International: West Conshohocken, PA, USA, 2000.
- ISO 16474-3:2013 Paints and Varnishes—Methods of Exposure to Laboratory Light Sources—Part 3: Fluorescent UV Lamps 2013; ISO: Geneva, Switzerland, 2013.
- Sharma, G.; Bala, R. Digital Color Imaging Handbook, 1st ed.; Sharma, G., Ed.; Electrical Engineering & Applied Signal Processing Series; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-8493-0900-7. [Google Scholar]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of Uniform Color Spaces Developed after the Adoption of CIELAB and CIELUV. Color Res. Appl. 1994, 19, 105–121. [Google Scholar] [CrossRef]
- UNI EN 15801:2010 Conservation of Cultural Heritage—Test Methods—Determination of Water Absorption by Capillarity; UNI: Milan, Italy, 2010.
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Li, J.; Ecco, L.; Delmas, G.; Whitehouse, N.; Collins, P.; Deflorian, F.; Pan, J. In-Situ AFM and EIS Study of Waterborne Acrylic Latex Coatings for Corrosion Protection of Carbon Steel. J. Electrochem. Soc. 2014, 162, C55–C63. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Velázquez, A.; Velasco-Soto, M.; Pérez-García, S.; Licea-Jiménez, L. Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties. Nanomaterials 2018, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Shanti, R.; Hadi, A.N.; Salim, Y.S.; Chee, S.Y.; Ramesh, S.; Ramesh, K. Degradation of ultra-high molecular weight poly(methyl methacrylate-co-butyl acrylate-co-acrylic acid) under ultra violet irradiation. RSC Adv. 2017, 7, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stab. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Allen, N.S.; Regan, C.J.; McIntyre, R.; Johnson, B.W.; Dunk, W.A.E. The photooxidative degradation and stabilisation of water-borne acrylic coating systems. Macromol. Symp. 1997, 115, 1–26. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen Tri, P.; Nguyen, T.D.; El Aidani, R.; Trinh, V.T.; Decker, C. Accelerated degradation of water borne acrylic nanocomposites used in outdoor protective coatings. Polym. Degrad. Stab. 2016, 128, 65–76. [Google Scholar] [CrossRef]
- Scalarone, D.; Lazzari, M.; Castelvetro, V.; Chiantore, O. Surface monitoring of surfactant phase separation and stability in waterborne acrylic coatings. Chem. Mater. 2007, 19, 6107–6113. [Google Scholar] [CrossRef]
- Winkler, E.M. Stone in Architecture; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 978-3-662-10072-1. [Google Scholar]
| Sample | Composition (wt.%) | LUDOX AS-30 2 (wbm%) | Dowfax 2A1 3 (wbm%) | Conversion Grade (%) | D (nm) | PDI |
|---|---|---|---|---|---|---|
| NoSiO2 | – | 0 | 1 | 81.2 | 142 | 0.022 |
| L2_1 | BA: 47 | 2 | 1 | 100 | 199.7 | 0.017 |
| L2_2 | MMA: 47 | 2 | 96.3 | 156.1 | 0.145 | |
| L5_1 | AM: 1 | 5 | 1 | 91.2 | 212.4 | 0.123 |
| L5_2 | AA: 1 | 2 | 81.5 | 115.4 | 0.029 | |
| L10_1 | SA 1 : 4 | 10 | 1 | 97 | 250 | 0.101 |
| L10_2 | KPS 1 : 2 | 2 | 98.9 | 122.8 | 0.044 |
| Stone | Coating | Contact Angle 1 | Before Aging | After Aging | ||
|---|---|---|---|---|---|---|
| AC 2 | RCI | AC 2 | RCI | |||
| Lecce stone | Untreated | 0 | 108.5 | 1.00 | 108.5 | 1.00 |
| NoSiO2 | 71.08 ± 1.11 | 36.1 | 0.80 | 38.5 | 0.84 | |
| L2_1 | 93.95 ± 0.49 | 10.90 | 0.78 | 11.96 | 0.75 | |
| L2_2 | 91.25 ± 0.67 | 13.38 | 0.85 | 9.87 | 0.78 | |
| L5_1 | 92.69 ± 0.49 | 12.02 | 0.83 | 16.66 | 0.82 | |
| L5_2 | 90.46 ± 0.76 | 14.06 | 0.87 | 10.21 | 0.79 | |
| L10_1 | 89.77 ± 0.65 | 12.57 | 0.84 | 16.67 | 0.83 | |
| L10_2 | 88.93 ± 1.32 | 13.65 | 0.85 | 10.65 | 0.78 | |
| Carrara marble | Untreated | 54.08 ± 0.68 | 0.23 | 1.00 | 0.23 | 1.00 |
| NoSiO2 | 76.85 ± 0.21 | 0.13 | 0.93 | 0.11 | 0.99 | |
| L2_1 | 94.60 ± 0.28 | 0.08 | 0.51 | 0.10 | 0.37 | |
| L2_2 | 91.16 ± 0.45 | 0.10 | 0.63 | 0.08 | 0.35 | |
| L5_1 | 92.82 ± 0.48 | 0.10 | 0.94 | 0.06 | 0.55 | |
| L5_2 | 92.33 ± 0.56 | 0.09 | 0.62 | 0.13 | 0.37 | |
| L10_1 | 92.15 ± 0.88 | 0.10 | 0.92 | 0.07 | 0.55 | |
| L10_2 | 88.28 ± 1.21 | 0.07 | 0.60 | 0.15 | 0.38 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbardella, F.; Pronti, L.; Santarelli, M.L.; Asua Gonzàlez, J.M.; Bracciale, M.P. Waterborne Acrylate-Based Hybrid Coatings with Enhanced Resistance Properties on Stone Surfaces. Coatings 2018, 8, 283. https://doi.org/10.3390/coatings8080283
Sbardella F, Pronti L, Santarelli ML, Asua Gonzàlez JM, Bracciale MP. Waterborne Acrylate-Based Hybrid Coatings with Enhanced Resistance Properties on Stone Surfaces. Coatings. 2018; 8(8):283. https://doi.org/10.3390/coatings8080283
Chicago/Turabian StyleSbardella, Francesca, Lucilla Pronti, Maria Laura Santarelli, José Marìa Asua Gonzàlez, and Maria Paola Bracciale. 2018. "Waterborne Acrylate-Based Hybrid Coatings with Enhanced Resistance Properties on Stone Surfaces" Coatings 8, no. 8: 283. https://doi.org/10.3390/coatings8080283
APA StyleSbardella, F., Pronti, L., Santarelli, M. L., Asua Gonzàlez, J. M., & Bracciale, M. P. (2018). Waterborne Acrylate-Based Hybrid Coatings with Enhanced Resistance Properties on Stone Surfaces. Coatings, 8(8), 283. https://doi.org/10.3390/coatings8080283






