3.1. Effect of Three Treatment Methods on Adhesion Strength
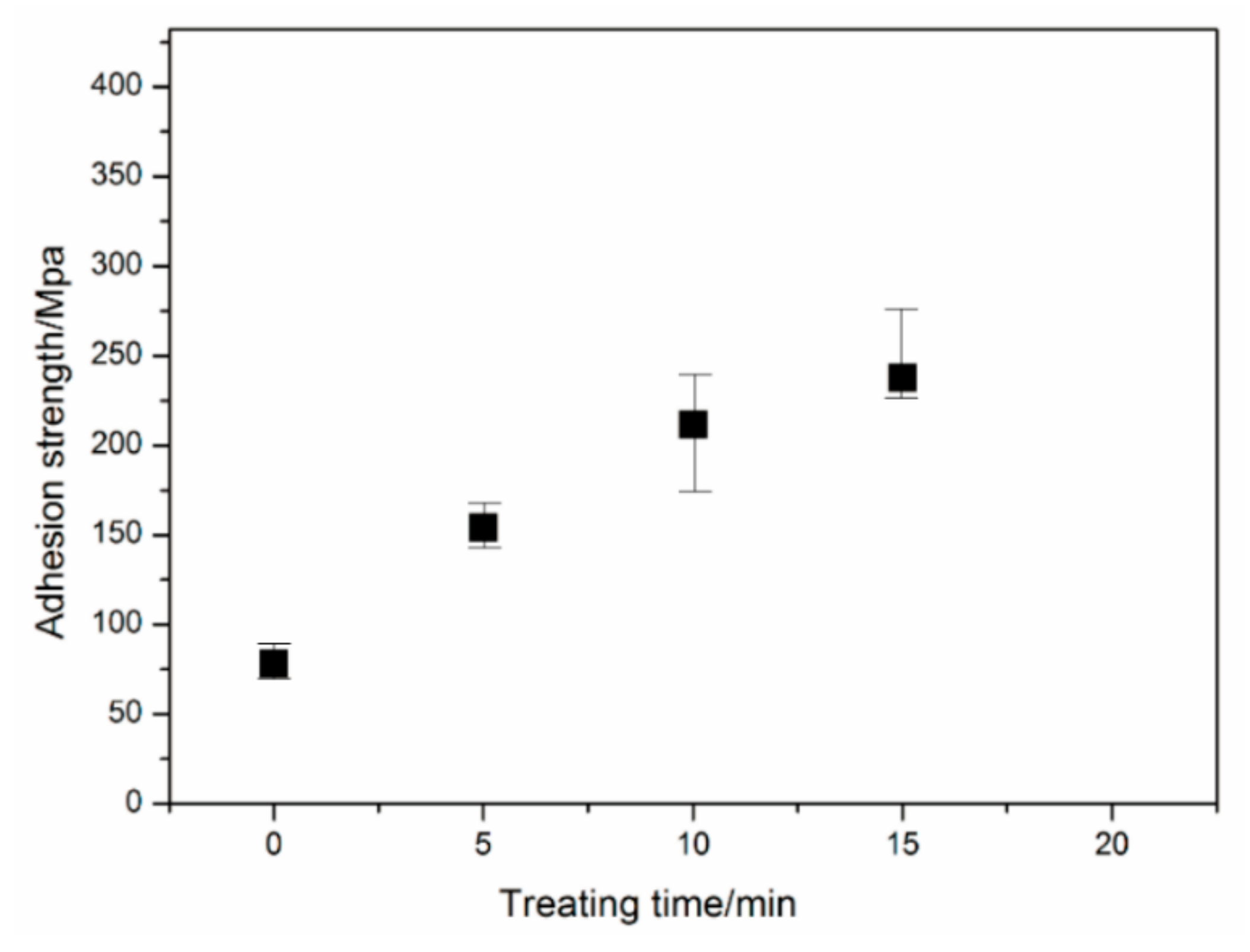
The effect of hydrochloric acid treatment time on the adhesion strength of the lower Ni films is shown in
Figure 1. Three samples are tested for each of the tests. The meaning of the error bar is that the difference between the maximum and the average is the upper limit, the difference between the minimum and the average is the lower limit. The adhesion strength of the Ni laminated films increases linearly with increasing of treating time. In the first ten minutes, the adhesion strength increases significantly with a rising rate of 70 MPa/min, finally up to be 220 MPa. When the treating time is 15 min, the adhesion strength increases to approximately 250 MPa. It is indicated that the longer treating time is beneficial to the adhesion strength. The main reason is due to the increase of surface roughness with treating time.
The effects of pulse reverse current on the adhesion strength of the Ni laminated films have been reported in our previous work [
20]. Here we focus on the comparison of the adhesion strength of the Ni laminated films treated by optimized pulse reverse current (PR). The results indicate that an average shear adhesion strength of approximately 400 MPa is achieved for the Ni laminated films treated by PR method, which is higher than that of the Ni laminated films treated by hydrochloric acid. In this PR method, the increased micro roughness and transition layer are beneficial to improve the adhesion.
Table 1 lists the surface roughness (
Ra) and the adhesion strength of the Ni laminated films treated by anodic current method with different current density and treating time. When the current density keeps a constant, the surface roughness and the adhesion strength increases with the increasing of treating time. Vice versa, when the treating time is fixed a value, the surface roughness and the adhesion strength increase with the increasing of the current density. Meanwhile, it can be seen that the value of the anodic current density is more than 20 mA/cm
2 even with treating time of 5 min, the adhesion strength of the Ni laminated films is higher than the maximum of the Ni laminated films treated by hydrochloric acid (shown in
Figure 1). It indicates that the adhesion strength of the Ni laminated films is improved significantly when the lower Ni films are treated by anodic current in the nickel chloride solution. One thing that cannot be denied is that the higher the surface roughness, then the higher the adhesion strength of the Ni films. So the surface roughness is responsible for the improvement of the adhesion strength. Moreover, these results may be related to removing the oxygen between the interfaces and increasing the surface roughness, thus significantly improving the adhesion strength.
3.2. Surface Morphology
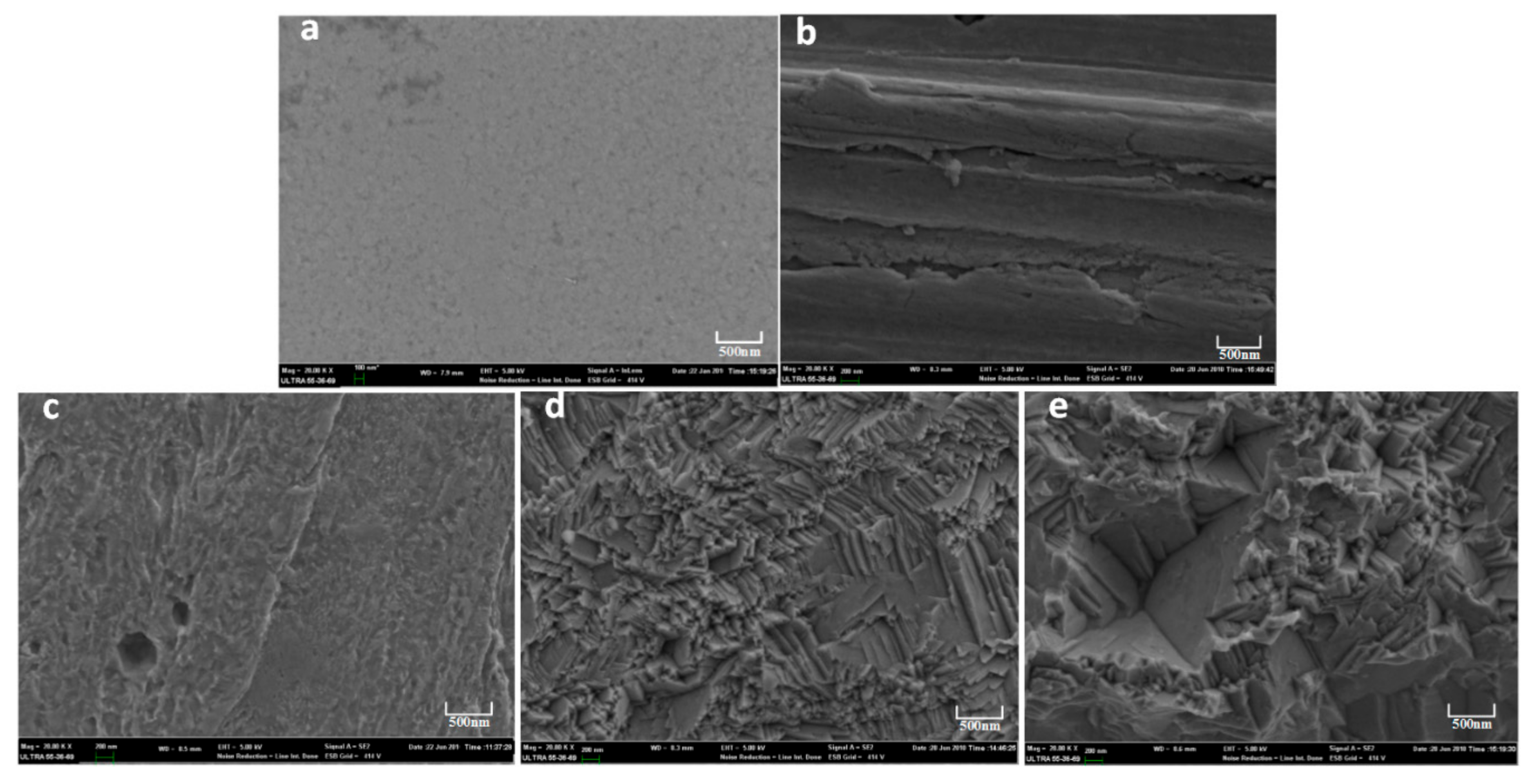
The surface morphologies of the Ni laminated films treated with different methods are shown in
Figure 2. The untreated Ni film presents a smooth surface, shown in
Figure 2a. When the Ni films are treated with a 5 vol % hydrochloric acid solution for 10 min, the surface of the Ni laminated films show significant roughness, accompanied by some groove strips, as shown in
Figure 2b.
Figure 2c corresponds to the surface morphology of the Ni films treated by anodic current with 10 mA for 10 min. It can be seen that there is a certain corrosion on the surface of Ni films. Compared with
Figure 2c, surface corrosion is more obvious in
Figure 2d with 20 mA for 10 min.
Figure 2e shows the surface morphology of the Ni films treated by anodic current with 30 mA with 10 min. The surface of the Ni films is completely corroded. The surface shows some corrosion spits and steps resulting in a significant roughness. With the same treating time, the surface roughness changed significantly with the increase of anodic current.
The morphology with uniform and big roughness is considered to be positive on the improvement of adhesion strength of the Ni laminated films. It not only improves the metal bonding between Ni laminated films but also enhances the mechanical occlusion property. Therefore, rough surface is responsible for the further improvement of the adhesion strength of the Ni laminated films.
In the previous paper, the Ni films were treated by the pulse reverse current. The uniform corrosion morphology on the Ni films is also achieved [
20]. The Ni substrate has been coarsened owing to the presence of deposition current intermittent treatment. The adhesion strength of the Ni laminated films is improved, because the coarsened substrate increases the micro-surface area and the effect of physical interlocking between Ni films.
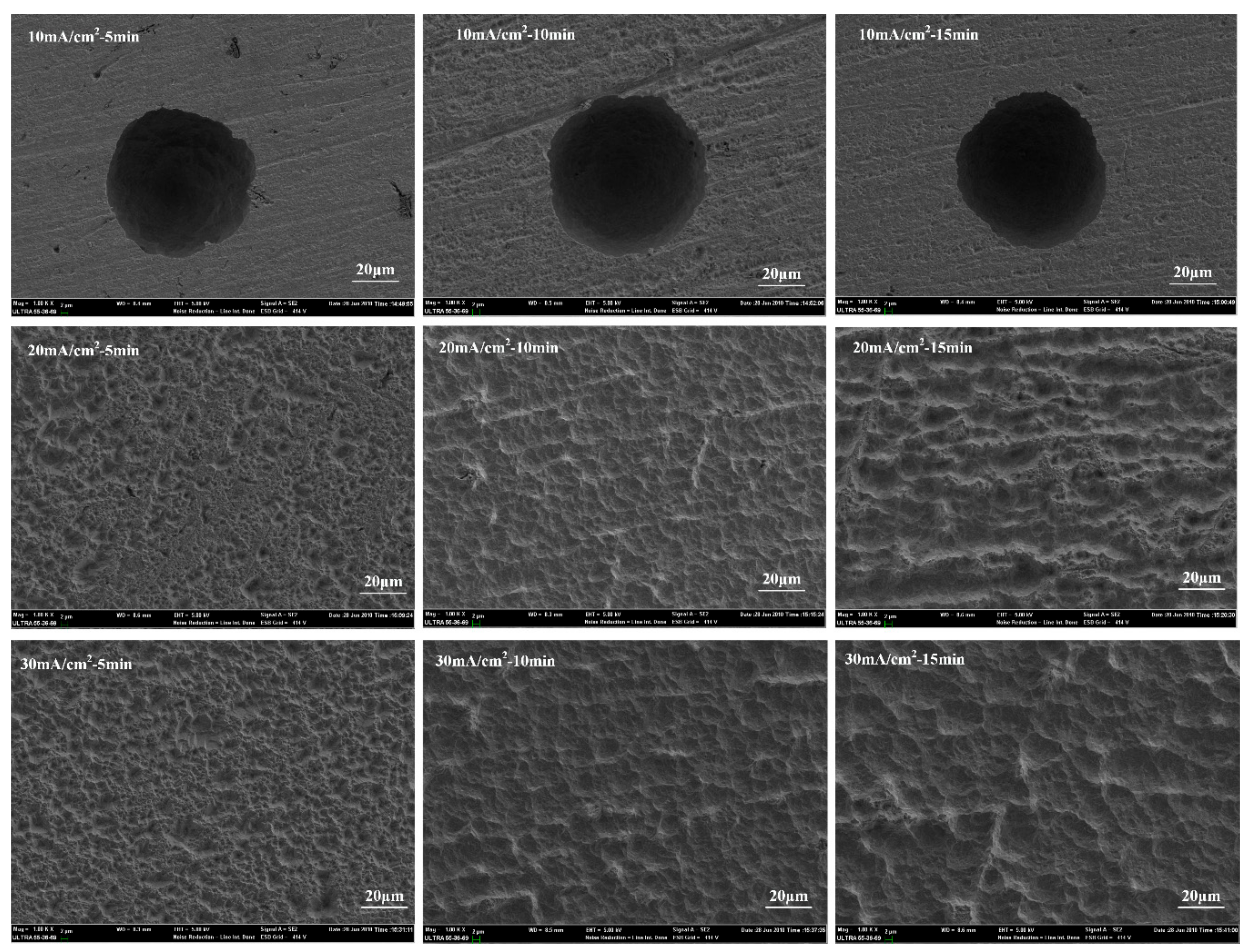
In order to obtain the influence of anodic current treatment on the surface morphology, the surface of the Ni films is treated with different current densities and treating time. The results are shown in
Figure 3. As can be seen that the surface of the Ni films is strongly corroded with a dot when the anodic current density is 10 mA/cm
2. However, the whole surface roughness of the Ni films treated with different treating time presents no significant change.
When the anodic current density is 20 mA/cm
2, the whole surface is completely corroded and produces a significant roughness. In addition, with the increase of treating time, the surface roughness has been improving. Especially when the treating time reaches 15 min, the surface roughness is improved obviously. The adhesion strength of Ni sample also reached 500 MPa (
Table 1). When the anodic current density goes up to be 30 mA/cm
2, the further corrosion of the Ni films occurred. When the treating time reaches 10 min, the surface roughness is clearly changed. Moreover, when the treatment time reaches 15 min, the surface roughness has no obvious change, as compared with 10 min. The corresponding adhesion strength is also consistent with this result in
Table 1. Therefore, the increase of surface roughness with increase of current densities and treating time is helpful for the enhancement of the physical occlusion between the Ni laminated films. It clearly explains the increase of adhesion strength of Ni films.
The surface morphology of electrodeposited Ni film is shown in
Figure 4. It can be seen that the surface of electroplated nickel has a certain roughness. This phenomenon may be related to current density and deposition speed in electrodeposited process.
The seed layer is treated with 1% diluted hydrochloric acid for 5 s to remove the oxide layer on the surface. Previous experiments show that this method is suitable for electroplating process. The seed layer has little influence on the morphology of electrodeposited films. Therefore, in this paper, all seed layers of sample are treated before electrodepositing.
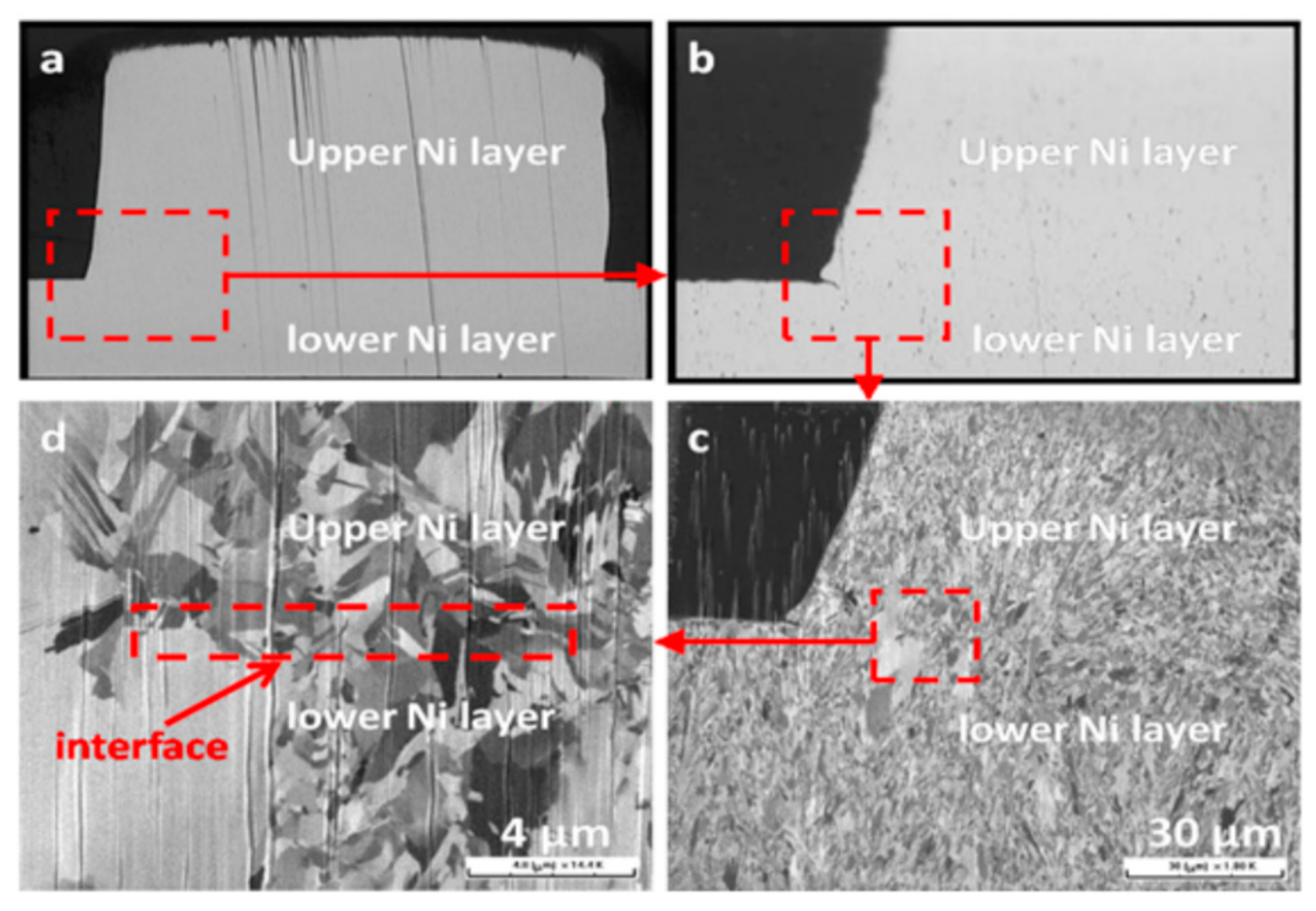
3.3. Fracture Morphology
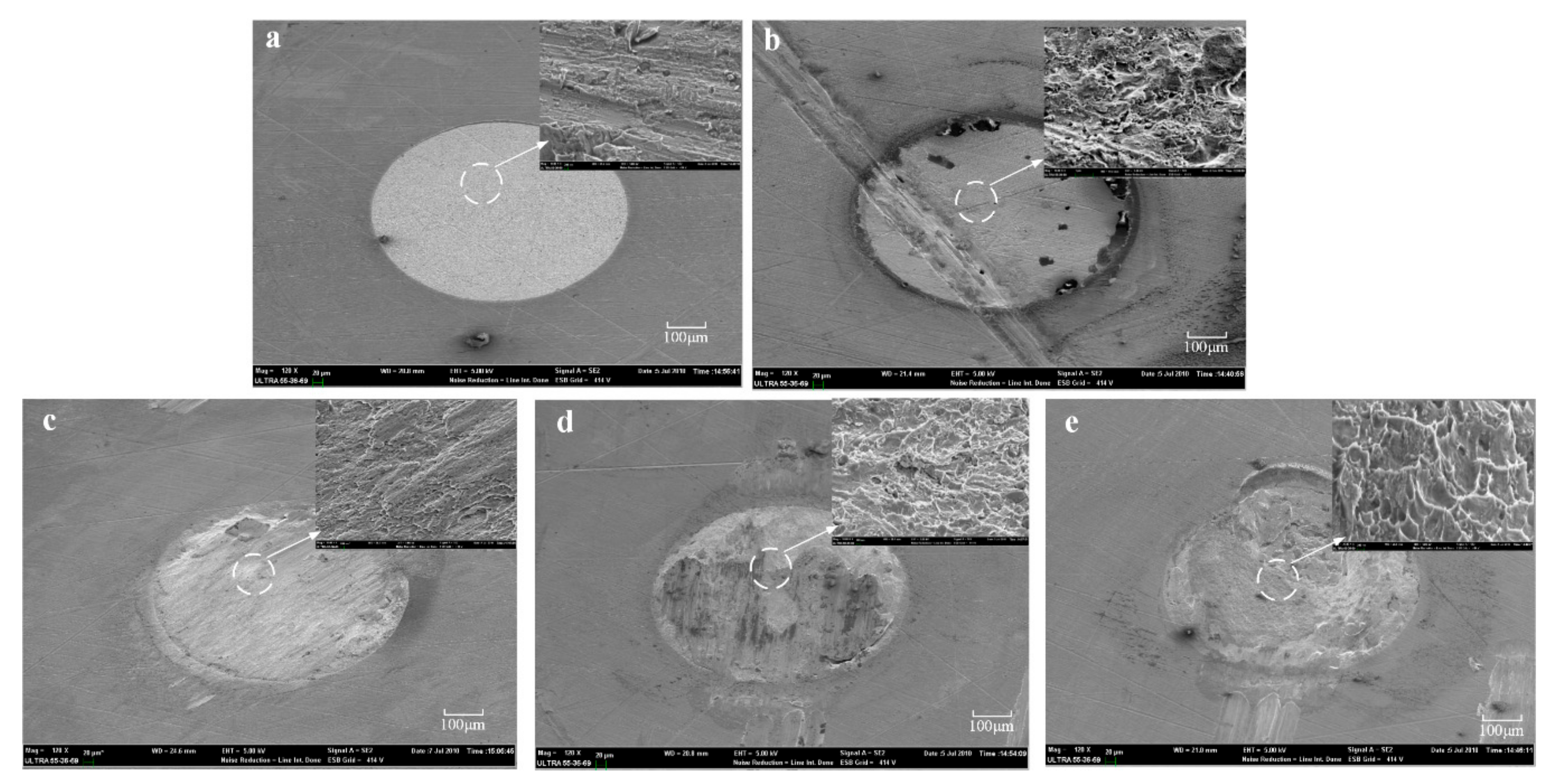
Figure 5 presents the fracture morphologies of the Ni films: untreated, treated by 5 vol % HCl with 10 min and different anodic current treatment with 10 min. The location and morphologies of the fracture shows significant difference after the measurement of the adhesion strength of the Ni laminated films. For both of the Ni films without treated and treated with 5 vol % HCl with 10 min, the fracture behavior takes place at interfaces between the first Ni layer and the second Ni layers, shown in
Figure 5a,b. The adhesion strength of the Ni films treated with 5 vol % HCl with 10 min is two times that of the untreated Ni films. It is shown that the roughness caused by acid treatment may increase the adhesion strength of the Ni films to a limited extent.
However, for the Ni films treated with HCl solution, no visible dimples in the part of fracture and the fracture takes place at interfaces between Ni film layers. Concerning the Ni films treated by the anodic treatment method, the interface can be seen clearly and the dimples are clearer than that of the Ni films treated by HCl solution, as shown in
Figure 5c–e.
Figure 5c corresponds to the fracture morphology of the Ni films treated by anodic current with 10 mA for 10 min. A part of the dimples appeared in
Figure 5c. Compared with
Figure 5c, there are more dimples in
Figure 5d which is treated with 20 mA for 10 min.
Figure 5e show that the fracture morphology has a large number of dimples in the part of fracture. The interfaces between the Ni film layers cannot be distinguished. In addition, the fracture morphology indicates that the interfaces between the Ni film layers mixed. Moreover, the fracture morphology shows a tear shape. This indicates that the interfaces inside the Ni films fused well together, which is helpful for the improvement of the adhesion strength of the Ni laminated films.
To further obtain the detailed interface information of the electrodeposited Ni films treated by anodic current of 30 mA/cm
2 with 10 min, the Electron Backscattered Diffraction (EBSD) morphologies were measured and shown in
Figure 6. The interfaces between the Ni films totally mixed, indicating that both Ni layers grew well together. This explains well the reason of good performance of the electrodeposited Ni films with the adhesion strength value of 629.8 MPa.
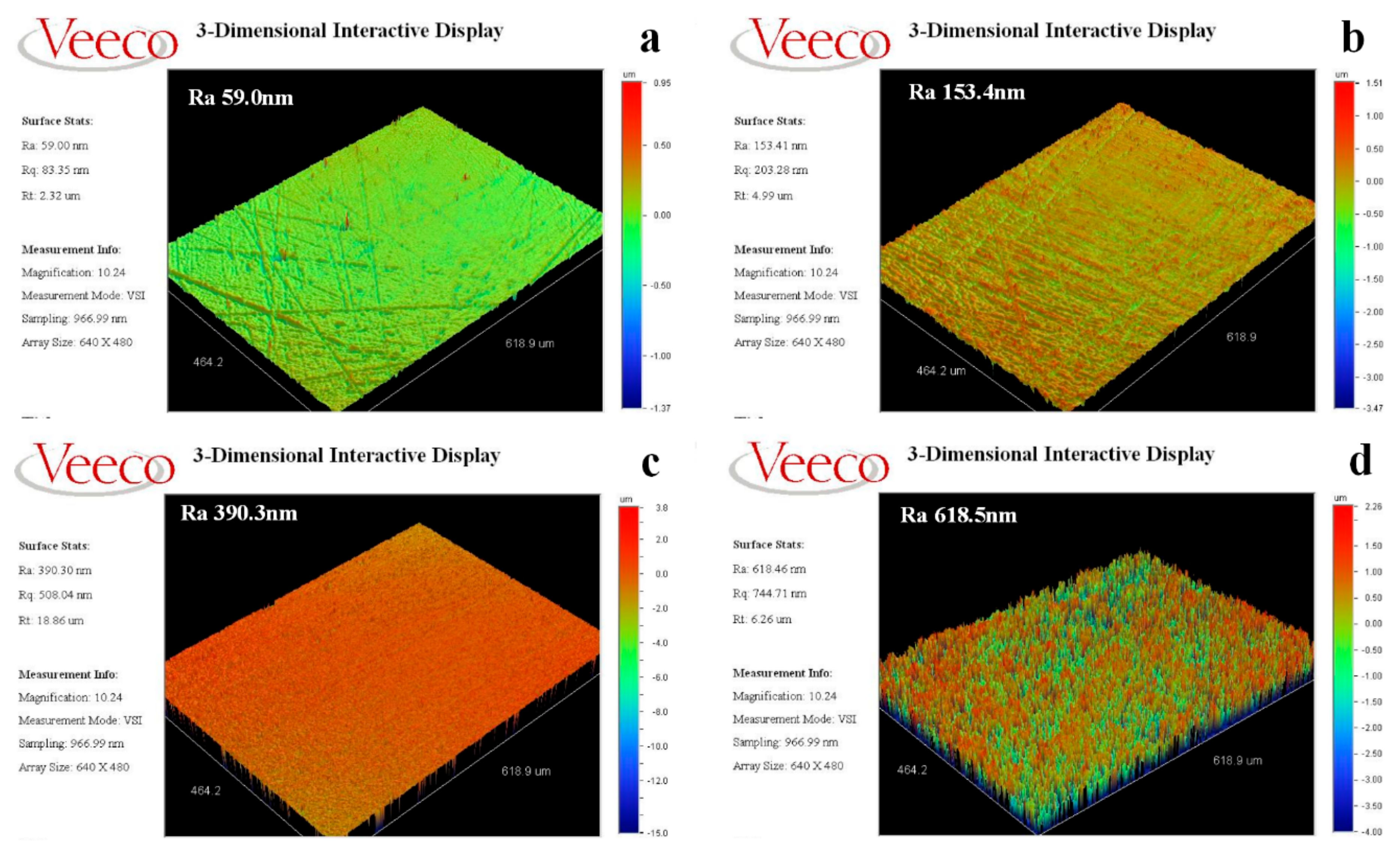
Figure 7 shows the results of the surface roughness of the metal Ni films treated with different methods: untreated, 5 vol % HCl with 10 min, pulse reverse current, anodic current for 30 mA with 10 min. It can be seen that the surface roughness of the metal Ni films presents significant difference.
The roughness of the untreated metal Ni film is small, with a value of 59.0 nm, and the value raises up to be 153.4 nm when the metal Ni films are treated with 5 vol % HCl with 10 min. However, when the metal Ni films are treated with pulse reverse current and anodic current, the roughness significantly increased up to be 390.3 nm and 618.5 nm, respectively.
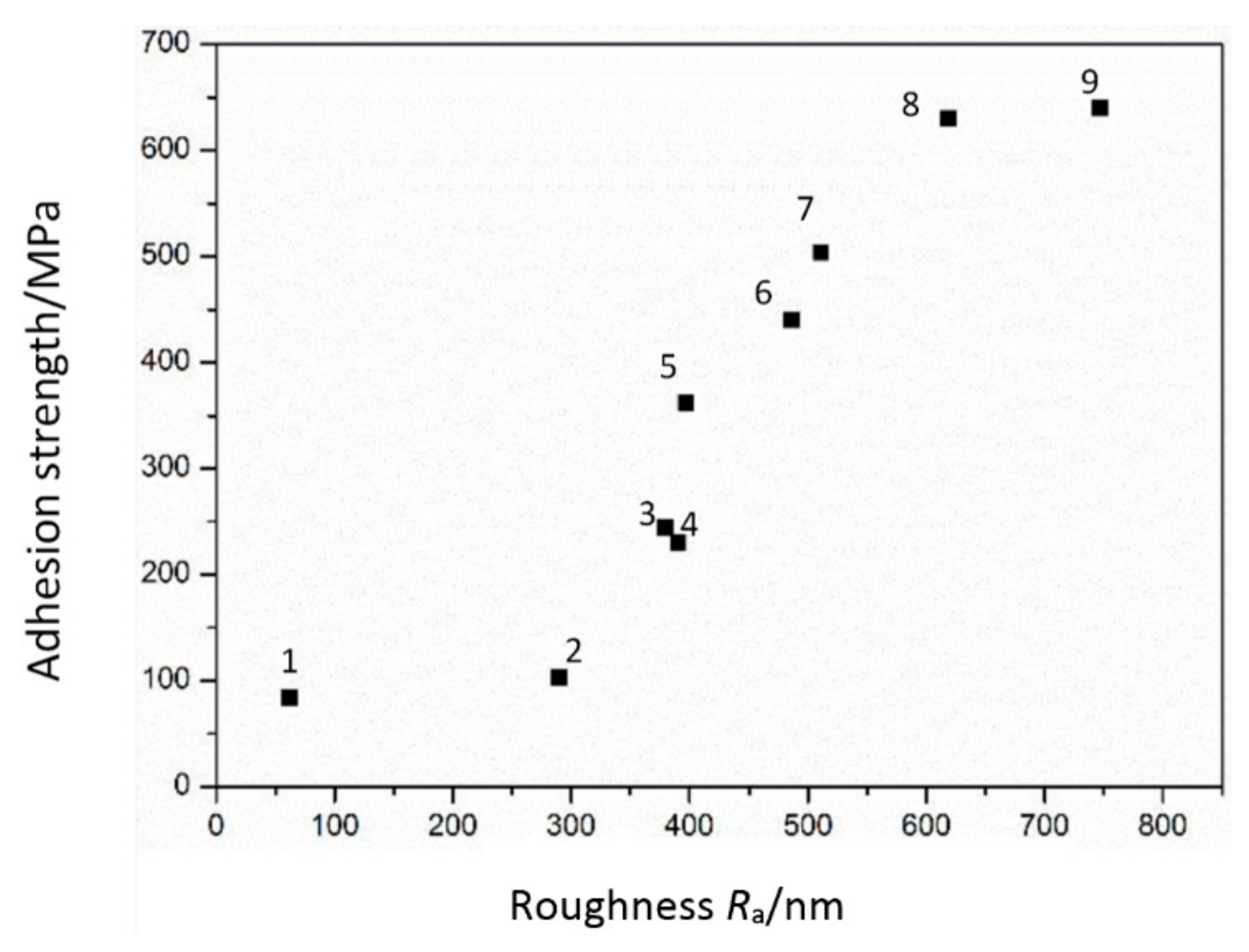
Figure 8 shows the relationship between adhesion strength and surface roughness of the metal Ni films. In these experiments, the seed layers of all sample are treated with 1% diluted hydrochloric acid for 5 s to remove the oxide layer on the surface firstly. Then, the lower Ni layer is electrodeposited with certain condition. After that, the lower Ni layer is treated by different conditions with anodic current treatment. Finally, the upper Ni films is electrodeposited on the lower Ni layer. We focus on adhesion strength between the lower Ni layer and upper layer in this paper. From the
Figure 7, it indicates that the adhesion strength increases with the increasing of the surface roughness. The number 1, 2 and 3 are treated by anodic current density of 10 mA/cm
2 with 5, 10 and 15 min, respectively. Three samples were tested for each value. The number 4, 5 and 6 are treated by anodic current density of 20 mA/cm
2 with 5, 10 and 15 min. The number 7, 8 and 9 are treated by anodic current density of 30 mA/cm
2 with 5, 10 and 15 min. Compared with the results of
Figure 1, the adhesion strength of the Ni films is highly related to the surface roughness. This is due to the reason that the rough surface improves the physical occlusion effect between Ni/Ni layers. The same reason explains the improvement of adhesion strength of the metal Ni films treated by anodic current density of 30 mA/cm
2 with 10 min.
3.4. Surface Residual Oxides
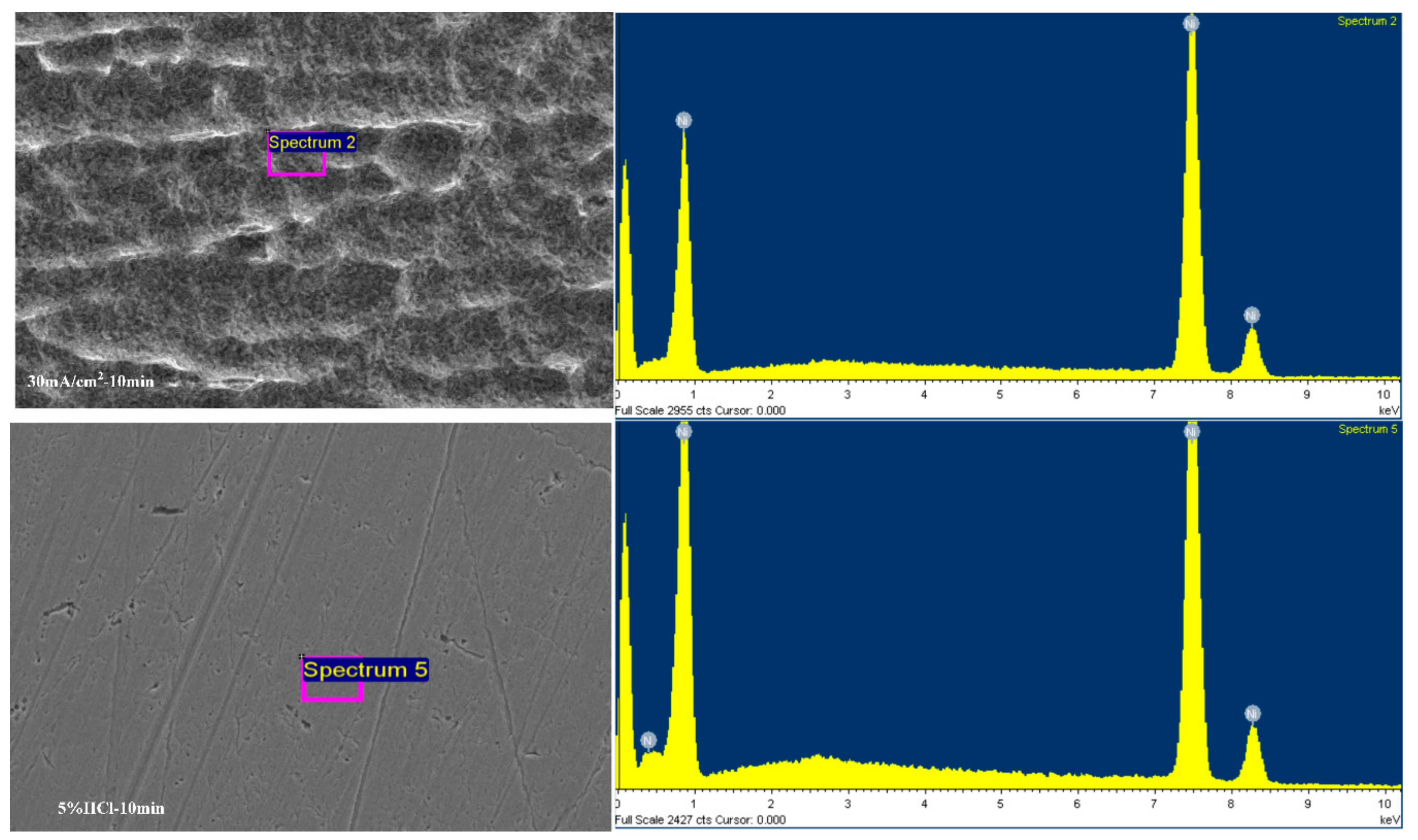
To obtain the effect of surface residual oxides of the metal Ni films on the adhesion, element analysis are measured on the metal Ni films treated by anodic current density of 30 mA/cm
2 with 10 min and 5 vol % HCl with 10 min. As can be seen from
Figure 9, no oxygen was found on the surface of the metal Ni films treated by the anodic current density. The metal Ni films treated by 5 vol % HCl with 10 min shows the presence of oxygen with content of 2.67 wt %. It indicates that the presence of a large amount of chloride ions in the solution accelerates the removal of the nickel oxides, but the dense oxides cannot be removed completely only by using small current density or bath. The presence of the nickel oxides reduces the adhesion strength [
21].
It is mentioned, in
Figure 3, that the surface morphology of the sample treated by anodic current density with 10 mA/cm
2 differs from that of the sample treated by anodic current density of 20 mA/cm
2. The sample shows inhomogeneous corrosion spots and does not change with treating time. Compared the results of
Figure 1, it is found that when the current density is set as 10 mA/cm
2, the increasing of the roughness is ascribed to the depth of corrosion spot. Excepting the big spots, the surface morphology of both samples treated by 5 vol % HCl with 10 min and anodic current density of 10 mA/cm
2 are extremely similar but show no obvious improvement of interface adhesion strength. This phenomenon is mainly due to the presence of the nickel oxides on the top of the metal Ni films when it explores to the air atmosphere. Moreover, the inhomogeneous corrosion spot indicates that the oxides protect the parts without corrosion spots. The same phenomena occur on the metal Ni films treated by 5 vol % HCl with 10 min.