Abstract
Considerable research has focused on the control of the physiological activity of fruits and vegetables in postharvest conditions as well as microbial decay. The use of edible coatings (ECs) carrying active compounds (e.g., antimicrobials) represents an alternative preservation technology since they can modify the internal gas composition by creating a modified atmosphere through the regulation of the gas exchange (oxygen, carbon dioxide, volatiles) while also limiting water transfer. Of the edible polymers able to form coating films, starch exhibits several advantages, such as its ready availability, low cost and good filmogenic capacity, forming colourless and tasteless films with high oxygen barrier capacity. Nevertheless, starch films are highly water sensitive and exhibit limited water vapour barrier properties and mechanical resistance. Different compounds, such as plasticizers, surfactants, lipids or other polymers, have been incorporated to improve the functional properties of starch-based films/coatings. This paper reviews the starch-based ECs used to preserve the main properties of fruits and vegetables in postharvest conditions as well as the different factors affecting the coating efficiency, such as surface properties or incorporation of antifungal compounds. The great variability in the plant products requires specific studies to optimize the formulation of coating forming products.
Keywords:
edible coating; starch; antifungal; postharvest; preservation; fruit; vegetable; wettability 1. Introduction
Fruits and vegetables are essential in the human diet due to the health and nutritional benefits associated with their intake. However, they are products with a relatively short postharvest life, since they remain as living tissues up until the time they are used for consumption and are prone to physiological and biochemical changes, which can also have physical or pathological origins [1], leading to important economic losses [2,3]. Fruits and vegetables lose weight during postharvest handling and storage by transpiration, resulting in textural changes and surface shrinkage that affects their shelf life. On the other hand, softening of fruit during storage is also attributed to the deterioration of the cell wall components, mainly pectin, due to the activity of various enzymes.
Postharvest treatments with conventional synthetic waxes and/or chemical fungicides have been used for many years to control postharvest decay and extend fruit shelf life. However, the continuous application of these treatments has led to health and environmental issues, associated with chemical residues, or to the proliferation of resistant pathogenic strains. The increasing restrictions on the use of agrochemicals imposed by many countries and the growing consumer demand for high quality, minimally processed fresh food products have intensified the search for new preservation methods and technologies. The use of edible coatings (ECs) has emerged as an effective and environmentally-friendly alternative to extend their shelf life [4] and protect them from harmful environmental effects. Such films, applied as coatings, can create semipermeable barriers to gases and water vapour, reducing respiration and weight loss and maintaining the firmness of the fresh product while providing gloss to the coated products. In addition, coatings are able to act as carriers of a wide variety of functional ingredients, such as antimicrobials, antioxidants, anti-browning agents, nutrients or flavouring and colouring compounds [5,6], enhancing food stability, quality and safety, thus promoting the coatings’ functional performance beyond their barrier properties [7]. Non-toxic antifungal compounds, incorporated in edible coatings, can prevent fungal decay, which is one of the main causes for deterioration of fruits and vegetables.
An EC is a thin layer of edible material coated directly on a food surface, applied in liquid form (film-forming solution/dispersion) on the food, usually by immersing or spraying [8]. The film-forming solution or dispersion contains a polymeric material with filmogenic capacity [9]. The film provides a barrier against water vapour and gases and thus lower levels of O2 and higher levels of CO2 inside the fruit, which helps to control the enzyme activities, contributing to maintain the firmness of the coated product during storage.
The efficiency and stability of ECs depend on their composition. Polysaccharides, including starch, cellulose, pectin, alginates, chitosan and others, are naturally occurring polymers, widely used for this purpose [10], and are compatible with a broad range of functional compounds [11] whose aim is to improve their properties. Starch is a promising polysaccharide for food coating/packaging purposes, when taking into account its filmogenic capacity, ready availability and low cost. The starch world market can mainly be divided into four raw materials: corn, potato, sweet potato and cassava, although the predominant source used to obtain biodegradable plastics has been corn starch. This may be due to corn being the main source of starch produced worldwide (approximately 65%), followed by sweet potato (13%) and cassava (11%) [12].
Starch-based coatings are colourless and have an oil-free appearance, and can be used to increase the shelf life of fruits, vegetables and other products, although due to their hydrophilic nature, they are highly water sensitive and exhibit low water vapour barrier capacity. Other components, such as plasticizers and emulsifiers (or surfactants), may be added to the polymer matrix to improve the flexibility, extensibility and/or the stability of the polymer matrix structure. Blending (with other hydrophobic compounds to limit the hygroscopicity of starch-based materials has become an economical and versatile way to obtain new materials with better properties [13].
This paper reviews the starch-based ECs used to preserve the main properties of fruits and vegetables in postharvest conditions. The different methods used to determine or improve surface properties of coating materials when applied on fruits and vegetables, which greatly affect coating spreading and efficiency, have been analysed. Moreover, recent studies related with the application and/or characterization of mainly starch-based edible coatings, with and without antifungal properties to prevent fruit fungal decay, have been reviewed and their main conclusions summarized.
2. Requirements of the Coating-Forming Agents to Preserve Fruits and Vegetables
The most important functional properties of edible films and coatings are their barrier properties to water vapour and gases, compound migration, their ability for physical and mechanical protection and their impact on the product appearance (colour and gloss) [1]. The loss of quality in fresh products occurring during postharvest storage is associated with the biochemical and physiological changes in the live tissue, which is greatly affected by mass transfer phenomena, including moisture or oxygen exchanges, flavour loss or undesirable odour absorption [14,15]. Likewise, great losses in post-harvested products are due to microbiological alterations, mainly fungal decay, which shorten their shelf life and increase the risk of foodborne illnesses. Then, one of the main interests in coating design is the inclusion of substances with antimicrobial activity within polymeric matrices.
How effective EC is at protecting fruits and vegetables greatly depends on the product wettability to obtain a uniformly coated surface, which is influenced both by the fruit/vegetable surface properties and by the chemical composition and structure of the coating-forming polymers: the presence of different compounds, such as plasticizers, surfactants, antimicrobials or antioxidants [16]. The possible loss of these molecules during coating formation can affect the thickness of the film [3]. The EC effectiveness is also closely related with other factors, such as tensile properties. Mechanical resistance is important for two reasons: to prevent the film/coating fracture and to protect fruits and vegetables from mechanical factors and the physical damage caused by impact, pressure or vibrations during storage.
As regards the mass transfer properties of the coatings, the challenges include decreasing the water vapour permeability values in order to prevent both the moisture loss in the products (weight loss) and any changes in texture, flavour and appearance [17]. Both loss and gain of water are nearly always considered undesirable. The coating must also provide an adequate gas barrier (low oxygen permeability values), since this respiration process accelerates the consumption of sugars and other compounds, thus increasing the ethylene production and causing senescence [14]. In terms of their oxygen barrier properties, starch-based coatings usually stand out compared to other coating materials such as other polysaccharides or proteins [18]. However, in order to prevent anaerobic respiration, moderate barriers with a certain degree of oxygen and carbon dioxide permeability are needed for the respiration of living tissues [19]. In this sense, depending on the different respiration rates of the fruit or vegetable, a different minimum oxygen transfer rate may be needed to avoid unwanted metabolic changes. A sufficient gas barrier could also prevent fruits and vegetables from losing volatile flavour compounds or acquiring foreign odours. The good adherence and extensibility of the coating are key factors for the enhancement of the coating functions while also improving the appearance and attractiveness of the coated fruit or vegetable. Other considerations to take into account when formulating ECs are that some active ingredients might change the organoleptic profile of the coated product, causing undesirable odours or modifications in the functional properties. Some active compounds, such as essential oils, may cause toxicity in plant cells at high concentrations, or lose their functionality when reacting with external factors or food components [20]. Figure 1 shows the main relationships between the coating properties and the quality factors that are preserved in the fruit and vegetable. The interfacial interaction between the coating-forming agents, affected by its surface properties, and the product surface energy determine how effective the product coating is at exerting adequate protection.
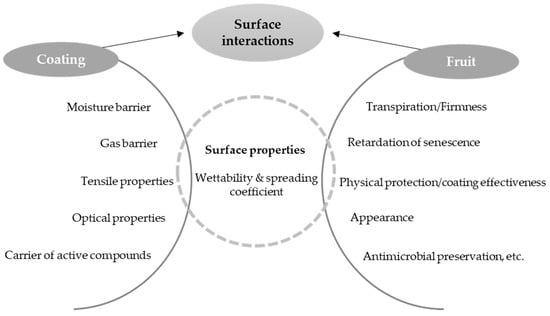
Figure 1.
Relationship between coating properties and quality attributes of fruits and vegetables.
Factors Affecting the Coating Spreadability
The wettability of the product by a coating solution is of particular importance, as it is crucial when defining the ability of a coating to wet and spread uniformly on the surface of the fruit or vegetable. Wettability is studied by determining the values of the spreading coefficient (Ws) as a function of the works of adhesion (Wa) (Equation (1)) and cohesion (Wc) (Equation (2)). The equilibrium-spreading coefficient can be defined by Equation (3) [21] and can only be negative or zero. The adhesive forces promote the extension of a liquid on a solid surface and the cohesive forces its contraction. Consequently, the wetting behaviour is conditioned by the balance between these forces, and it is important to optimize the coating formulations in terms of biopolymer, plasticizer, surfactant, antimicrobial, antioxidant or other compound concentration in order to promote their spreading coefficient on a determined surface. In practical terms, the closer the Ws values are to zero, the better a surface will be coated.
The surface tension of the coating solution, as well as its contact angle (θ) on the target solid surface can be measured and then used to determine the Ws, through the estimation of the vapour-solid-liquid interaction. The contact angle of a liquid drop on the solid surface is defined by the mechanical equilibrium established under the action of the three interfacial tensions: solid-vapour (γSV), liquid-vapour (γLV) and solid-liquid (γSL) (Equation (4)).
Likewise, the interfacial tension can be separated into polar and dispersive components (Equation (5)) and, for pure liquids, the polar and dispersive components are known. If the surface contact angle between those liquids and the solid is obtained, the interaction can be described by Equation (6). This can be used to estimate the dispersive () and polar () components of the solid surface tension if at least three pure liquids are used and the dependent variable is plotted vs. the independent variable , from the intercept and slope of the fitted straight line, respectively.
Then, the determination of the solid surface energy or surface tension, which is a controlling factor in the wetting processes, involves the measurement of the contact angle of several standard liquids on the product surface in order to estimate the dispersive and polar contributions of the surface tension.
The estimation of the critical surface tension (γc) can be carried out from the Zisman plots [22] by plotting the cosine of the contact angle of these pure liquids against their surface tension. From the fitted straight line, the intercept with cos θ = 1 corresponds to the critical surface tension, which is an imaginary point of the γSV, frequently used to describe the wettability of the solid surface. It represents the value of γLV of the liquid above which its spreading on the solid surface is complete (Equation (7)).
The reported values of the critical surface tension are generally lower than the solid surface tension values [23]. In Table 1, the surface and the critical surface tension of some fruits and vegetables are summarized. All of the fruit surfaces were found to be of low-energy and had the ability to participate in non-polar interactions, as a consequence of the higher values of the dispersive component of the surface tension.

Table 1.
Surface properties of fruit and vegetable skins/peels (mN·m−1).
How the composition of coating solutions and the addition of surfactants affect the wetting properties of different fruits and vegetables has been evaluated by several authors (Table 2). It has been reported that Tween 80 was effective at reducing the surface tension of different coating solutions, through a reduction in the cohesion forces, which improved the compatibility between the solution and the surface of the fruit skin, enhancing its wettability [25,29]. The authors of [27] found that increasing chitosan and glycerol concentrations in the coating solutions reduced both the Ws and Wa, but increased the Wc. On the other hand, different formulations of galactomannans and glycerol coatings showed good values of Ws when applied on different tropical fruits [26]. Ribeiro [28] obtained good wettability with a coating based on 2% starch and 2% sorbitol as a plasticizer applied on strawberry.

Table 2.
Recent studies into the effect of different components and concentrations on the surface properties of coatings and their spreading coefficient (Ws) on fruits.
Upon drying, a coating with adequate cohesion and adhesion must be obtained, directly affecting its performance as a preservation agent [17,25]. Coating integrity also is a critical factor which depends on the film flexibility, surface tension and adhesion to the food product. Plasticizer-free matrices are too brittle and rigid because of strong interactions between the polymer chains and are incompatible with irregular surfaces, such as that of some fruits [32].
3. Starch-Based Coatings for the Preservation of Fruits and Vegetables
Starch, the reserve polysaccharide of most plants, can be obtained from different sources: cereals (corn, wheat or rice), legumes (pea) and tubers (cassava or potato) and is one of the most abundant natural polysaccharides used as a food hydrocolloid. This is because of its wide-ranging functionality, relatively low cost and great ability to form transparent, tasteless, odourless films, with very good oxygen barrier properties, which is very useful for food preservation purposes [33,34]. It has a granular structure and is composed of two macromolecules: amylose and amylopectin. Amylose is a linear polymer formed by glucose units linked by α-(1,4) whereas amylopectin is a highly branched polymer of glucose units with ramifications in α-(1,6) [35,36]. However, due to their hydrophilicity starch-based films/coatings exhibit water solubility and poor water vapour barrier properties [10]. It has been particularly studied in the context of the postharvest preservation of a variety of fresh horticultural products, including apples, oranges, strawberries and tomatoes [37].
To better control the weight loss during postharvest handling and storage caused by transpiration, one of the alternatives is to incorporate hydrophobic substances into the coating formulation. The authors of [38] reported a moisture loss restriction in pea starch-guar gum coatings with oleic acid and shellac, as well as a decrease in the respiration rates of treated fruit. Similar observations were described by [39] in oranges coated with polysaccharide-based coatings. In the same way, different plasticizers, especially sorbitol, had a significant effect on the delay in the change of weight and firmness of tomatoes coated with a non-conventional starch [40].
Starch-based coating formulations (2 wt % starch) with 2% added sorbitol exhibited good wettability properties on the surface of strawberries. However, they were more permeable to O2, which was associated with the high concentration of plasticizer in the dry coating. In fact, plasticizers are used to decrease the intermolecular attractions between adjacent polymeric chains, which in turn facilitates the molecular mobility and diffusion of the gas molecules through the polymer network [28,42].
A representative summary of recent studies highlighting the positive effect of active edible coatings on the shelf life of fruit and vegetables is shown in Table 3, giving an overview of other compounds commonly used to improve the properties of starch-based coatings. Different results can be obtained and these not only depend on the kind of fruit or vegetable but also on the coating composition. In general, the incorporation of lipids into starch or polysaccharide-based coatings contribute to the reduction in the amount of water lost in the coated product and can also affect the gas exchanges, depending on the lipid ratio and its physical state. Solid lipids generally offer better resistance to the mass transfer of water or gas molecules than liquid lipids [43]. The lipid-polymer ratio is also an important factor, since lipids provoke interruptions in the polymer matrix which have a great impact on the coating performance. In this sense, the adequate compatibilization of lipids and polymers and the final size distribution of lipid particles in the film also affect the coating’s functional properties [44]. As concerns starch coatings, the amylose–amylopectin ratio affected their functionality due to the different structure of the films generated with high-amylose starch, where linear amylose, with regions of helical conformation, exhibited better packed domains in the matrix that is more crystalline in nature [36]. The authors of [37] found that coatings made with high-amylose starch better preserved the weight losses and firmness of strawberries for longer periods than coatings of medium-amylose starch. Likewise, these authors observed that coatings plasticized with sorbitol exhibited a better water vapour barrier capacity than those containing glycerol. In this sense, the authors of [45] report the use of amylose-only starch obtained from transgenic plants to obtain starch materials with improved properties.

Table 3.
Starch-based coatings applied to fruits and vegetables (polymer and additives concentration in the coating-forming dispersion, except when indicated with respect to the total polymer).
In general, coatings help to better retain different active compounds incorporated into their formulation, such as antimicrobials, on the product surface for longer storage times, thus enhancing their effectiveness. The authors of [11] observed a better retention of potassium sorbate and antifungal activity during the refrigerated storage of apple, tomato and cucumber coated with starch-based formulations.
In fresh-cut products, the effects of starch-based coatings can also be beneficial for the maintenance of quality and safety during storage. Coatings with a starch:protein ratio of 15:85, with an added 6% (v/w) of pink pepper phenolic compounds, prevented enzymatic browning in fresh-cut apples for 12 days [46] and cassava starch coatings decreased the respiration rate, preserving the mechanical properties and colour characteristics, of fresh-cut mango that was pre-treated with citric acid (0.5% w/v) and peracetic acid (0.05% w/v) [47]. In general, a good performance could be achieved with starch-based coatings in different fruits or vegetables, forming edible barriers at a competitive cost. However, other components, such as plasticizers, surfactants, lipids or other more hydrophobic polymers, must be incorporated into the coating-forming formulation in order to obtain a good adherence on the target product surface, an adequate mechanical resistance of the film and optimal barrier properties against water vapour or gas. Likewise, taking into account the different physiology and surface properties of the plant products, an optimal formulation must be developed for the different products; this must include active compounds permitting the control of fungal or bacterial decay which limits the product shelf life. In this sense, new approaches must make use of compounds that are innocuous for human health and effective at controlling microbial growth or physiological processes. The following sections analyse the new tendencies in this field.
4. Antifungal Coatings for Fruit Preservation
During storage, fruits are often subjected to different levels of microbial decay, mainly due to phytopathogenic fungi, which usually infect the host through wounds sustained during harvesting, handling and/or processing [55]. Fungal disease is mainly controlled chemically, but the use of synthetic fungicides is limited due to undesirable aspects, including the toxicological hazard to human health and slow degradation periods, which could lead to environmental problems [56]. The negative public perception of industrially-synthesized food antimicrobials has generated interest in the use of more naturally occurring compounds.
As reported by [1], there are three categories of antifungal agents that can be incorporated into ECs: (a) synthetic food preservatives or GRAS (Generally recognized as safe) compounds with antimicrobial activity, including some organic and inorganic acids and their salts (benzoates, sorbates, carbonates, propionates, etc.) and parabens (ethyl and methyl parabens) and their salts, (b) natural compounds, such as essential oils (EOs) or plant extracts (thyme oil, carvacrol, cinnamon, cinnamaldehyde, citral, eugenol, lemongrass, oregano, rosemary, etc.); and (c) antimicrobial antagonists (yeasts or bacteria).
The use of antifungal compounds, such as organic acids and various plant extracts or EOs, are among those mostly antimicrobial substances studied as a possible means of controlling the growth of phytopathogens in fruits and vegetables during postharvest shelf life [57]. There are a great number of in vitro and in vivo (inoculated fruits and vegetables) studies dealing with this topic, in which different active compounds have been tested against different fungi through their direct application or incorporated into film-forming formulations. In Table 4, different studies analysing the in vitro antifungal activity of different natural extracts or essential oils are summarized. Likewise, Table 5 shows several in vitro studies on the antifungal effect of these kinds of products incorporated into different polymer matrices to obtain active films. Studies into the application of these kinds of coatings to fruits or vegetables so as to extend their shelf-life are shown in Table 6, where the main findings of the authors are remarked on.

Table 4.
Recent studies into natural antifungal compounds with potential use in fruits and vegetables.

Table 5.
Recent studies on natural antifungal compounds incorporated into coating-forming formulations (in vitro tests) with potential use in fruits and vegetables.

Table 6.
Natural antifungal compounds applied to coatings (in vivo tests) to preserve fruits and vegetables.
The antifungal activity of some plant extracts, such as Aloe vera, can be attributed to the presence of bioactive compounds, such as quinones and phenol compounds (flavonoids), which can be more or less active against different fungi. Moringa plant extracts had a significant effect on the growth rate of C. gloeosporiodes, A. alternata and L. theobromae, a fact that can be closely linked to the high concentration of phenolic compounds in the tissue [58]. B. cinerea and P. expansum were better inhibited than A. niger in the presence of pulp or liquid fractions of A. vera [41]. Aloe vera gel was also effective at controlling the growth of several fungi, exhibiting the greatest efficacy against F. oxysporum. When incorporated into corn starch matrices in a Aloe vera solids–starch ratio of up to 1:1, using glycerol as plasticizer, these coatings were effective at controlling fungal decay in cherry tomatoes and were a natural, non-toxic alternative to synthetic fungicides [48].
EOs extracted from plants are rich sources of bioactive compounds (terpenoids, phenolic compounds) which have been recognized as antifungal agents [55]. In order to achieve effective antimicrobial activity, high concentrations of essential oils are generally needed. The incorporation of essential oils into biopolymer matrices (active coatings) can be a useful strategy to improve coating functionality in terms of the enhancement of the antimicrobial properties of the coatings, while reducing the matrix’s hygroscopic character. Likewise, the incorporation of EOs into the coating matrix allows for a reduction in the cost and minimizes their intense aroma perception [59]. The authors of [60] observed an enhanced in vitro antifungal activity against B. cinerea of chitosan films after the incorporation of lemon essential oil, although a decrease in the inhibition capacity was reported after two days of storage. This decrease was explained by considering the changes in the availability of the antimicrobials, controlled by the rate of diffusion of the active compounds into the agar medium throughout the storage period and the progressive evaporation of volatiles.
Edible composite coatings based on hydroxypropyl methylcellulose, hydrophobic components (beeswax and shellac) and food preservatives with antifungal properties (potassium sorbate, sodium benzoate and sodium propionate), were effective at reducing the incidence of P. digitatum and P. italicum during the long-term cold storage of mandarins [61]. Lemongrass essential oil has been observed to be effective as a treatment for the control of anthracnose (C. capsici) of bell pepper in vitro, due to the presence of numerous secondary compounds. This was attributed to the ability of the oil to penetrate through the cell membrane, resulting in deteriorative biological processes. However, the antimicrobial activity of the EO was higher in the in vitro tests than that observed in in vivo applications on bell pepper, which was attributed to the inability of the oil to adhere to the bell pepper surface [62].
Biological control is also a promising alternative to unpopular synthetic fungicides, and research into postharvest biocontrol has increased in recent decades [63,64]. The main characteristics of an ideal biocontrol agent (BCA) are related to its biosafety, activity in a range of environments and against a variety of pathogens, and ease of management and use [65]. Extensive research has been developed to understand the mechanisms by which BCAs exert their action against pathogens. Nevertheless, in many cases, the suggested modes of action whereby antagonists wield their biocontrol effect have not been totally elucidated. Competition for nutrients and space between the pathogen and the antagonist is considered to be the main mode of action, but other mechanisms, such as parasitism or the production of secondary metabolites, have also been reported. Several microorganisms, which can act as microbial antagonists, have received considerable attention as controlling agents of diseases in fruits. Table 7 shows some examples of BCA applied to the preservation of different fruits.

Table 7.
Representative antagonistic fungi and yeasts used as biocontrol agents for the preservation of fruits.
The combined application of BCAs and edible coatings or films offers many possibilities, both because of the wide variety of matrices which can be used and their potential benefits for the survival and retention of the antagonists. In this sense, the coating-forming formulations should contain components which not only allow for coating formation, but are also compatible with the cells and provide them with an adequate substrate for nutrition and growth [64]. Marín [66] evaluated the effect of different coating-forming systems containing C. sake CPA-1 as BCA, based on different biopolymers (corn starch, hydroxypropylmethylcellulose, sodium caseinate, or pea protein) with and without surfactants, on the adherence, viability and survival of C. sake cells, as well as on their biocontrol efficacy against B. cinerea infections of coated grapes. Taking into account the relative increase in the survival and efficacy of C. sake, and the cost of ingredients, sodium caseinate or corn starch were the most suitable coatings with which to obtain formulated biocontrol C. sake products. Likewise, the authors of [67] developed dried formulations of C. sake CPA-1 based on starch derivatives, which exhibit good stability at a very low moisture content. These formulates can be used as antifungal coating agents when applied on different fruits that are susceptible to B. cinerea infections, after their dispersion in water at the adequate concentration.
5. Final Remarks
Consumer demand for minimally processed, additive-free foods and products has led to the development of new packaging/coating materials with active properties. Starch-based edible films and coatings are an environmentally friendly alternative to synthetic polymers due to their low cost, availability, biodegradability and food contact properties. Starch can be used in combination with other polymers or compounds to improve the functional properties of the polymeric matrix, which can also carry active compounds to better control the product shelf-life. Edible coatings can be applied to fruits and vegetables to extend the product shelf-life, decrease water loss, slow down the colour change, pH and titratable acidity during storage and modify the internal atmosphere. Despite the significant benefits gained from using edible coatings for the purposes of extending the product shelf-life and enhancing the quality and microbial safety of fresh or minimally-processed fruits and vegetables, commercial applications are still very limited.
Knowledge of the surface interactions between coating- forming dispersions and the product skin is essential if film adhesion is to be understood and the coating performance optimized in terms of barrier or mechanical properties. Good surface wettability and the proper adhesion of the coating are required to ensure its functionality. Coating adhesion and durability are important for the preservation of food quality during storage. In order to truly receive the benefit of edible coatings on fruits and vegetables in commercial applications, the coating must adhere to the food surface during processing, storage, and transportation.
Given the variability in both the surface characteristics of the different fruits and vegetables, as well as in their physiological behaviour, further studies should be carried out optimizing the coating formulation for specific applications. Starch-based formulations have the advantage of their food contact properties since starch is edible and low cost and has good carrying properties for different actives which can protect fruits and vegetable from microbial decay or physiological disorders. Most of the studies into food applications have been conducted on a laboratory scale; thus, research into cost reduction and large-scale production is required.
Author Contributions
Both authors organised, wrote and revised the review.
Acknowledgments
The authors acknowledge the financial support from the Ministerio de Economía y Competitividad (MINECO) of Spain, through the projects and AGL2016-76699-R and RTA2015-00037-C02. Mayra Sapper thanks the Conselleria de Educación, Investigación, Cultura y Deporte de la Comunitat Valenciana for the Santiago Grisolía grant GRISOLIA/2015/001.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palou, L.; Valencia-Chamorro, S.; Pérez-Gago, M. antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Olivas, G.I.I.; Barbosa-Cánovas, G. Edible films and coatings for fruits and vegetables. In Edible Films and Coatings for Food Applications, 1st ed.; Embuscado, M.E., Huber, K.C., Eds.; Springer Science + Business Media: New York, NY, USA, 2009; pp. 211–244. ISBN 978-0-387-92823-4. [Google Scholar]
- Park, H.J. Development of advanced edible coatings for fruits. Trends Food Sci. Technol. 1999, 10, 254–260. [Google Scholar] [CrossRef]
- Karaca, H.; Pérez-Gago, M.B.; Taberner, V.; Palou, L. Evaluating food additives as antifungal agents against monilinia fructicola in vitro and in hydroxypropyl methylcellulose-lipid composite edible coatings for plums. Int. J. Food Microbiol. 2014, 179, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Hydroxypropyl methylcellulose-beeswax edible coatings formulated with antifungal food additives to reduce alternaria black spot and maintain postharvest quality of cold-stored cherry tomatoes. Sci. Hortic. 2015, 193, 249–257. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Combinational edible antimicrobial films and coatings. Antimicrob. Food Packag. 2016, 633–646. [Google Scholar] [CrossRef]
- Mariniello, L.; Giosafatto, C.V.L.; Di Pierro, P.; Sorrentino, A.; Porta, R. Swelling, mechanical, and barrier properties of albedo-based films prepared in the presence of phaseolin cross-linked or not by transglutaminase. Biomacromolecules 2010, 11, 2394–2398. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kim, S.J.; You, Y.S.; Lacroix, M.; Han, J. Inhibitory effect of soy protein coating formulations on walnut (Juglans regia L.) kernels against lipid oxidation. LWT Food Sci. Technol. 2013, 51, 393–396. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2017, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Mehyar, G.F.; Al-Qadiri, H.M.; Swanson, B.G. Edible coatings and retention of potassium sorbate on apples, tomatoes and cucumbers to improve antifungal activity during refrigerated storage. J. Food Process. Preserv. 2014, 38, 175–182. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch content affects physicochemical properties of corn and cassava starch-based films. Ind. Crops Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Miller, K.S.; Upadhyaya, S.K.; Krochta, J.M. Permeability of D-Limonene in whey protein films. J. Food Sci. 1998, 63, 244–247. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coating for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Kramer, M.E. Structure and function of starch-based edible films and coatings. In Edible Films and Coatings for Food Applications, 1st ed.; Embuscado, M.E., Huber, K.C., Eds.; Springer Science + Business Media: New York, NY, USA, 2009; pp. 113–134. ISBN 978-0-387-92823-4. [Google Scholar]
- Rojas-Graü, M.A.; Tapia, M.S.; Rodríguez, F.J.; Carmona, A.J.; Martin-Belloso, O. alginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples. Food Hydrocoll. 2007, 21, 118–127. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanoemulsions as edible coatings. Curr. Opin. Food Sci. 2017, 15, 43–49. [Google Scholar] [CrossRef]
- Rulon, J.; Robert, H. Wetting of low-energy surfaces. In Wettability; Berg, J.C., Ed.; Marcel Deckker: New York, NY, USA, 1993; pp. 4–73. ISBN 0824790464. [Google Scholar]
- Zisman, W.A. Relation of the equilibrium contact angle to liquid and solid constitution. In Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1964; pp. 1–51. [Google Scholar] [CrossRef]
- Dann, J.R. Forces involved in the adhesive process: I. critical surface tensions of polymeric solids as determined with polar liquids. J. Colloid Interface Sci. 1970, 32, 302–320. [Google Scholar] [CrossRef]
- Lima, Á.M.; Cerqueira, M.A.; Souza, B.W.S.; Santos, E.C.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. New edible coatings composed of galactomannans and collagen blends to improve the postharvest quality of fruits—Influence on fruits gas transfer rate. J. Food Eng. 2010, 97, 101–109. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Souza, M.P.; Teixeira, J.A.; Vicente, A.A. Physical properties of edible coatings and films made with a polysaccharide from Anacardium occidentale L. J. Food Eng. 2009, 95, 379–385. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Lima, Á.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. Suitability of novel galactomannans as edible coatings for tropical fruits. J. Food Eng. 2009, 94, 372–378. [Google Scholar] [CrossRef]
- Casariego, A.; Souza, B.W.S.; Vicente, A.A.; Teixeira, J.A.; Cruz, L.; Díaz, R. Chitosan coating surface properties as affected by plasticizer, surfactant and polymer concentrations in relation to the surface properties of tomato and carrot. Food Hydrocoll. 2008, 22, 1452–1459. [Google Scholar] [CrossRef]
- Ribeiro, C.; Vicente, A.A.; Teixeira, J.A.; Miranda, C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol. Technol. 2007, 44, 63–70. [Google Scholar] [CrossRef]
- Choi, W.Y.; Park, H.J.; Ahn, D.J.; Lee, J.; Lee, C.Y. Wettability of chitosan coating solution on ‘Fuji’ apple skin. J. Food Sci. 2002, 67, 2668–2672. [Google Scholar] [CrossRef]
- Hershko, V.; Nussinovitch, A. The behavior of hydrocolloid coatings on vegetative materials. Biotechnol. Prog. 1998, 14, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Hagenmaier, R.D.; Baker, R.A. Reduction in gas exchange of citrus fruit by wax coatings. J. Agric. Food Chem. 1993, 41, 283–287. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch-Staerke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Acosta, S.; Jiménez, A.; Cháfer, M.; González-Martínez, C.; Chiralt, A. Physical properties and stability of starch-gelatin based films as affected by the addition of esters of fatty acids. Food Hydrocoll. 2015, 49, 135–143. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan-tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Brigham, C. Biopolymers: Biodegradable alternatives to traditional plastics. In Green Chemistry; Elsevier Inc.: New York, NY, USA, 2018; pp. 753–770. [Google Scholar] [CrossRef]
- Cano, A.; Jiménez, A.; Cháfer, M.; Gónzalez, C.; Chiralt, A. Effect of amylose: amylopectin ratio and rice bran addition on starch films properties. Carbohydr. Polym. 2014, 111, 543–555. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Plasticized starch-based coatings to improve strawberry (Fragaria × ananassa) quality and stability. J. Agric. Food Chem. 1998, 46, 3758–3767. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B.; Marques, J.R.; Pristijono, P.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of “Valencia” oranges. Postharvest Biol. Technol. 2018, 137, 9–20. [Google Scholar] [CrossRef]
- Cháfer, M.; Sánchez-González, L.; González-Martínez, C.; Chiralt, A. Fungal decay and shelf life of oranges coated with chitosan and bergamot, thyme, and tea tree essential oils. J. Food Sci. 2012, 77, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Alam, F.; Hasnain, A. Mango kernel starch as a novel edible coating for enhancing shelf-life of tomato (Solanum lycopersicum) fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-Aloe vera coating on postharvest quality of blueberry (vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierro, P.; Giosafatto, C.V.L.; Esposito, M.; Mariniello, L.; Regalado-Gonzales, C.; Porta, R. Plasticizing effects of polyamines in protein-based films. Int. J. Mol. Sci. 2017, 18, 1026. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Talens, P.; Gavara, R.; Chiralt, A. Barrier properties of sodium caseinate films as affected by lipid composition and moisture content. J. Food Eng. 2012, 109, 372–379. [Google Scholar] [CrossRef]
- Perdones, Á.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Sagnelli, D.; Hooshmand, K.; Kemmer, G.C.; Kirkensgaard, J.J.K.; Mortensen, K.; Giosafatto, C.V.L.; Holse, M.; Hebelstrup, K.H.; Bao, J.; Stelte, W.; et al. Cross-linked amylose bio-plastic: A transgenic-based compostable plastic alternative. Int. J. Mol. Sci. 2017, 18, 2075. [Google Scholar] [CrossRef] [PubMed]
- Romani, V.P.; Hernández, C.P.; Martins, V.G. Pink pepper phenolic compounds incorporation in starch/protein blends and its potential to inhibit apple browning. Food Packag. Shelf Life 2018, 15, 151–158. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Pereira, L.M.; Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Hubinger, M.D. Cassava starch coating and citric acid to preserve quality parameters of fresh-cut “Tommy Atkins” mango. J. Food Sci. 2010, 75, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Roselló, J.; Santamarina, P.; Chiralt, A. Antifungal starch-based edible films containing Aloe vera. Food Hydrocoll. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Botelho, L.N.S.; Rocha, D.A.; Braga, M.A.; Silva, A.; de Abreu, C.M.P. Quality of guava cv. “Pedro Sato” treated with cassava starch and cinnamon essential oil. Sci. Hortic. 2016, 209, 214–220. [Google Scholar] [CrossRef]
- De Aquino, A.B.; Blank, A.F.; De Aquino Santana, L.C.L. Impact of edible chitosan-cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 2015, 171, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated red crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Razak, A.S.; Lazim, A.M. Starch-based edible film with gum arabic for fruits coating. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2015; Volume 1678, p. 050020. [Google Scholar] [CrossRef]
- Das, D.K.; Dutta, H.; Mahanta, C.L. Development of a rice starch-based coating with antioxidant and microbe-barrier properties and study of its effect on tomatoes stored at room temperature. LWT Food Sci. Technol. 2013, 50, 272–278. [Google Scholar] [CrossRef]
- Garcia, L.C.; Pereira, L.M.; de Luca Sarantópoulos, C.I.G.; Hubinger, M.D. Selection of an edible starch coating for minimally processed strawberry. Food Bioprocess Technol. 2010, 3, 834–842. [Google Scholar] [CrossRef]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical characterization and antifungal activities of four thymus species essential oils against postharvest fungal pathogens of citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Faleiro, M.L. The mode of antibacterial action of essential oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1143–1156. ISBN 13 978-84-939843-1-1. [Google Scholar]
- Junqueira-Gonçalves, M.P.; Alarcón, E.; Niranjan, K. Development of antifungal packaging for berries extruded from recycled PET. Food Control 2013, 33, 455–460. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S.; Mbili, N.; Mditshwa, A. Carboxyl methylcellulose (CMC) containing Moringa plant extracts as new postharvest organic edible coating for avocado (Persea americana mill.) fruit. Sci. Hortic. 2017, 226, 201–207. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Pérez-Gago, M.B.; Del Río, M.A.; Palou, L. Effect of antifungal hydroxypropyl methylcellulose-lipid edible composite coatings on penicillium decay development and postharvest quality of cold-stored “ortanique” mandarins. J. Food Sci. 2010, 75, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Noh, N.M.; Mustafa, M.A. antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packag. Shelf Life 2015, 3, 56–61. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Marín, A.; Atarés, L.; Chiralt, A. improving function of biocontrol agents incorporated in antifungal fruit coatings: A review. Biocontrol Sci. Technol. 2017, 27, 1220–1241. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Villalobos, M.C.; Calle, A.; Serradilla, M.J.; Córdoba, M.G.; Hernández, A. Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol. 2016, 57, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.; Cháfer, M.; Atarés, L.; Chiralt, A.; Torres, R.; Usall, J.; Teixidó, N. Effect of different coating-forming agents on the efficacy of the biocontrol agent candida sake CPA-1 for control of Botrytis cinerea on grapes. Biol. Control 2016, 96, 108–119. [Google Scholar] [CrossRef]
- Marín, A.; Atarés, L.; Chiralt, A. Stability of biocontrol products carrying Candida sake CPA-1 in starch derivatives as a function of water activity. Biocontrol Sci. Technol. 2017, 27, 268–287. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant and antimicrobial properties of chitosan-cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Palou, E.; Jiménez Munguía, M.T.; Nevárez-Moorillón, G.V.; Navarro Cruz, A.R.; López-Malo, A. Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. Int. J. Food Microbiol. 2012, 153, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Xu, W.; Zheng, X.; Zhang, X.; Abdelhai, M.H.; Zhao, L.; Li, H.; Diao, J.; Zhang, H. Exploring the effect of β-glucan on the biocontrol activity of Cryptococcus podzolicus against postharvest decay of apples and the possible mechanisms involved. Biol. Control 2018, 121, 14–22. [Google Scholar] [CrossRef]
- De Paiva, E.; Serradilla, M.J.; Ruiz-Moyano, S.; Córdoba, M.G.; Villalobos, M.C.; Casquete, R.; Hernández, A. Combined effect of antagonistic yeast and modified atmosphere to control Penicillium expansum Infection in sweet cherries cv. Ambrunés. Int. J. Food Microbiol. 2017, 241, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Zeng, K. Efficacy of Pichia membranaefaciens combined with chitosan against Colletotrichum gloeosporioides in citrus fruits and possible modes of action. Biol. Control 2016, 96, 39–47. [Google Scholar] [CrossRef]
- Gava, C.A.T.; Pinto, J.M. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol. Control 2016, 97, 13–20. [Google Scholar] [CrossRef]
- Zeng, L.; Yu, C.; Fu, D.; Lu, H.; Zhu, R.; Lu, L.; Zheng, X.; Yu, T. Improvement in the effectiveness of Cryptococcus laurentii to control postharvest blue mold of pear by its culture in β-glucan amended nutrient broth. Postharvest Biol. Technol. 2015, 104, 26–32. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).