Growth of NbC Thin Film Using CH4 as a Carbon Source and Reducing Agent
Abstract
1. Introduction
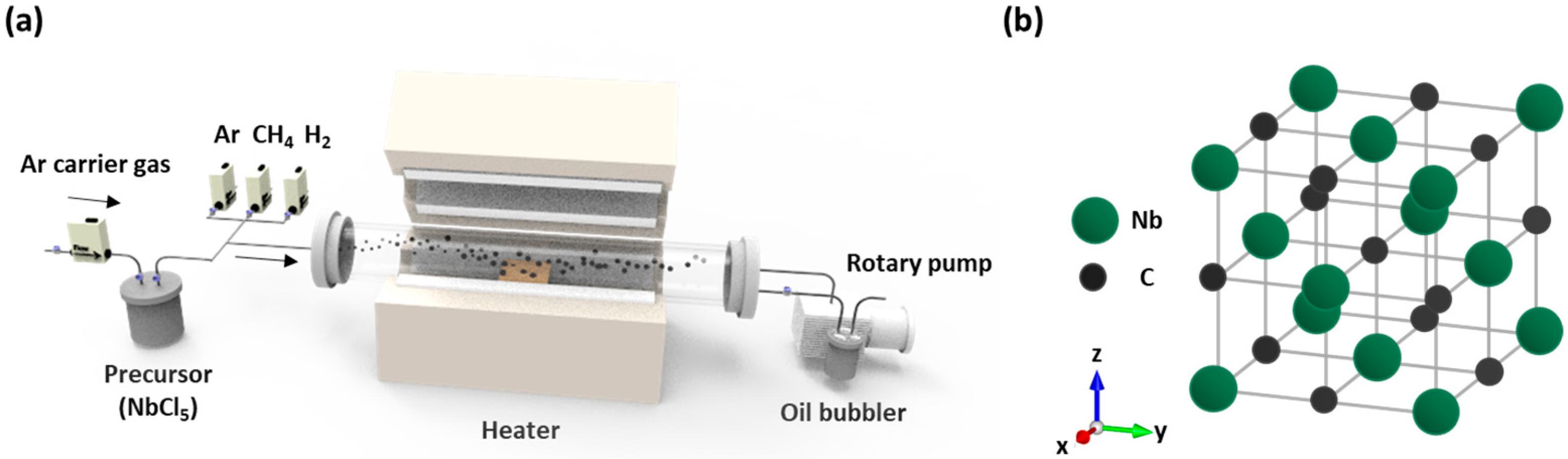
2. Materials and Methods
2.1. Preparation of Coatings
2.2. Structure and Property Characterization
3. Results and Discussion
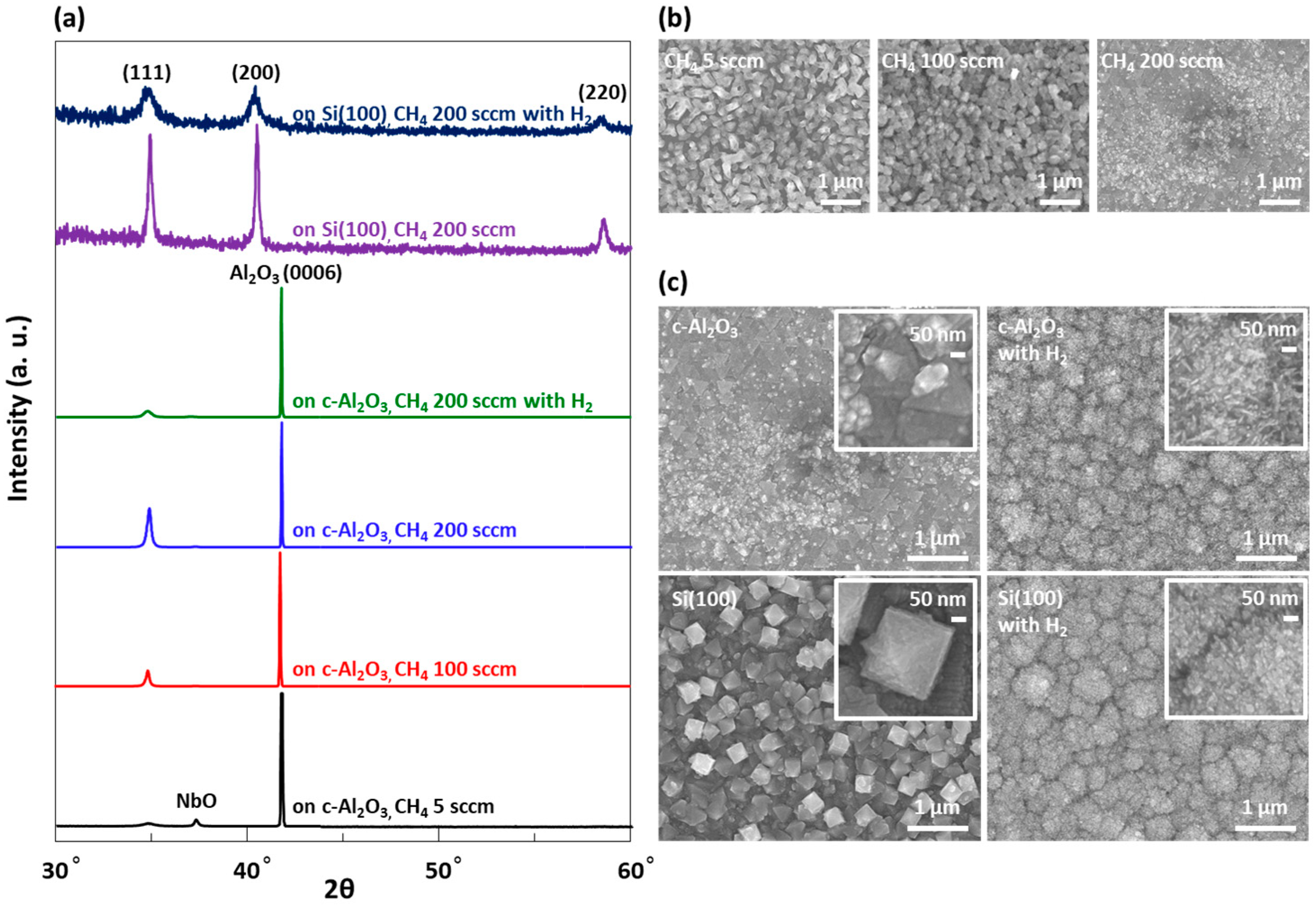
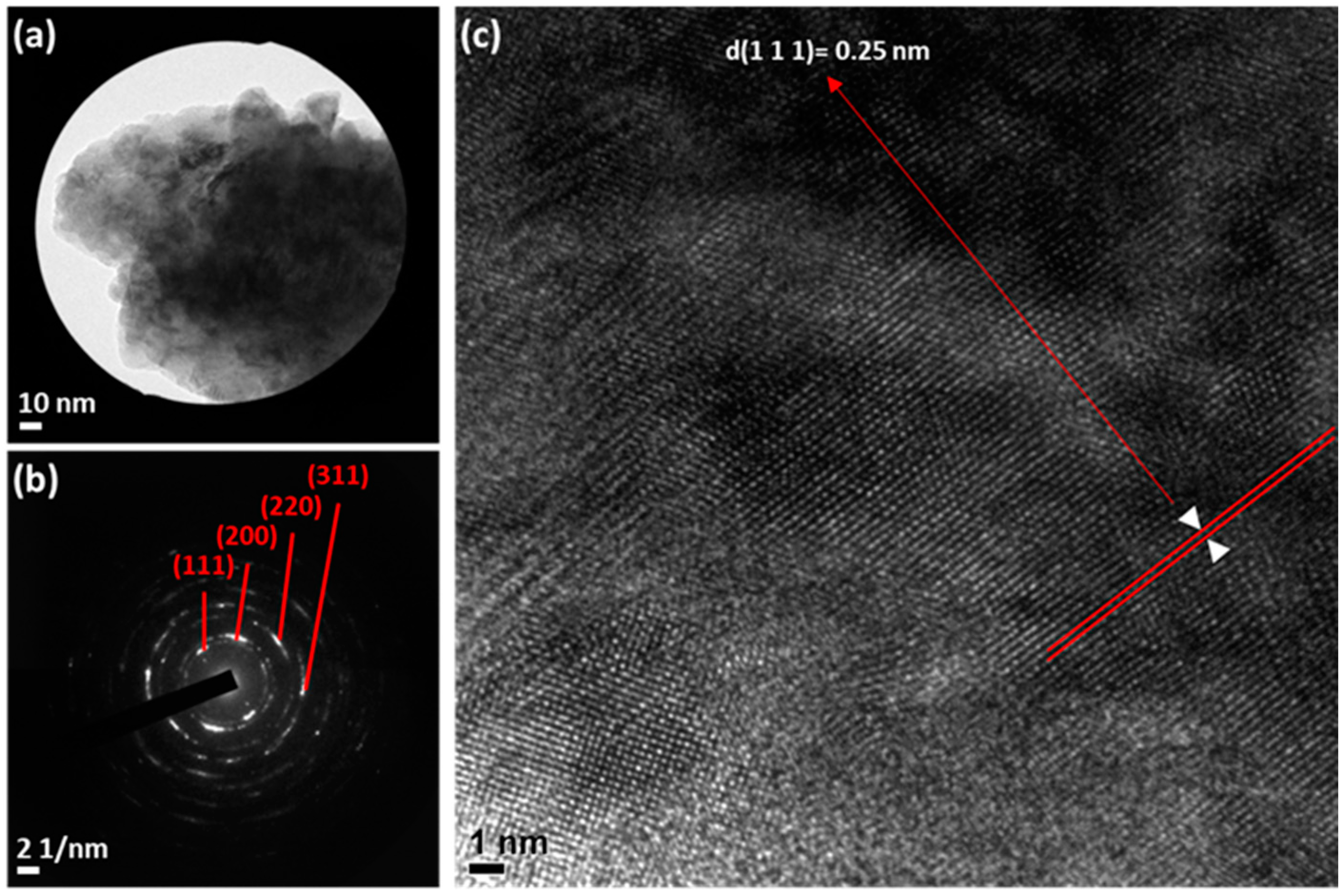
3.1. Morphology and Structural Characterization
3.2. Epitaxial Growth Mechanism
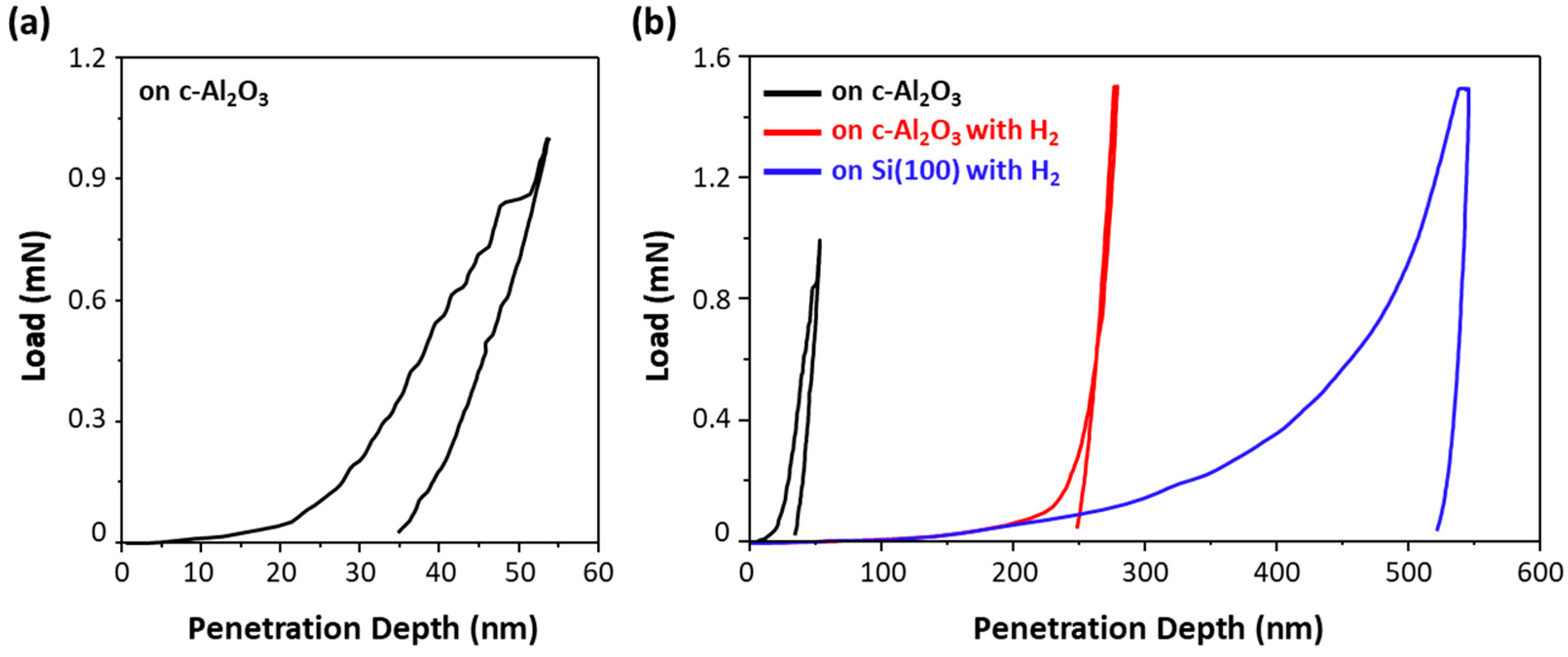
3.3. Mechanical Property Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuppari, M.G.D.V.; Santos, S.F. Physical properties of the NbC carbide. Metals 2016, 6, 250. [Google Scholar] [CrossRef]
- Quesne, M.G.; Roldan, A.; de Leeuw, N.H.; Catlow, C.R.A. Bulk and surface properties of metal carbides: Implications for catalysis. Phys. Chem. Chem. Phys. 2018, 20, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Niu, T. Old materials with new properties II: The metal carbides. Nano Today 2018, 18, 12–14. [Google Scholar] [CrossRef]
- Abolaji, R.S.; Bingxue, Z.; Anbalgam, K.; Thomas, T.; Minghui, Y. Synthesis and application of nano-structured metal nitrides and carbides: A review. Prog. Solid State Chem. 2018, 50, 1–15. [Google Scholar]
- Amaya, A.; Piamba, O.; Olaya, J. Improvement of corrosion resistance for gray cast iron in palm biodiesel application using thermoreactive diffusion niobium carbide (NbC) coating. Coatings 2018, 8, 216. [Google Scholar] [CrossRef]
- Woydt, M.; Huang, S.; Vleugels, J.; Mohrbacher, H.; Cannizza, E. Potentials of niobium carbide (NbC) as cutting tools and for wear protection. Int. J. Refract. Met. Hard Mater. 2018, 72, 380–387. [Google Scholar] [CrossRef]
- Montenegro, P.; Gomes, J.; Rego, R.; Borille, A. Potential of niobium carbide application as the hard phase in cutting tool substrate. Int. J. Refract. Met. Hard Mater. 2018, 70, 116–123. [Google Scholar] [CrossRef]
- Woydt, M.; Mohrbacher, H.; Vleugels, J.; Huang, S. Niobium carbide for wear protection–tailoring its properties by processing and stoichiometry. Met. Powder Rep. 2016, 71, 265–272. [Google Scholar] [CrossRef]
- Nedfors, N.; Tengstrand, O.; Lewin, E.; Furlan, A.; Eklund, P.; Hultman, L.; Jansson, U. Structural, mechanical and electrical-contact properties of nanocrystalline-NbC/amorphous-C coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2011, 206, 354–359. [Google Scholar] [CrossRef]
- Liu, Y.; Kelly, T.G.; Chen, J.G.; Mustain, W.E. Metal carbides as alternative electrocatalyst supports. ACS Catal. 2013, 3, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Nabil-Moreau, Y.; Cavaliere, S.; Harkness, I.; Hards, G.; Sharman, J.; Jones, D.J.; Rozière, J. Corrosion resistant electrospun niobium carbide nanotube supports for PEMFC cathodes. ECS Trans. 2015, 69, 1221–1226. [Google Scholar] [CrossRef]
- Meyer, S.; Nikiforov, A.V.; Petrushina, I.M.; Köhler, K.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Transition metal carbides (WC, Mo2C, TaC, NbC) as potential electrocatalysts for the hydrogen evolution reaction (HER) at medium temperatures. Int. J. Hydrogen Energy 2015, 40, 2905–2911. [Google Scholar] [CrossRef]
- Coy, E.; Yate, L.; Valencia, D.P.; Aperador, W.; Siuzdak, K.; Torruella, P.; Azanza, E.; Estrade, S.; Iatsunskyi, I.; Peiro, F.; et al. High electrocatalytic response of a mechanically enhanced NbC nanocomposite electrode toward hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 30872–30879. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, P.; Peng, F.; Liu, J.; He, D. Hardness and compression behavior of niobium carbide. High Press. Res. 2017, 37, 244–255. [Google Scholar] [CrossRef]
- Kim, B.R.; Woo, K.D.; Yoon, J.K.; Doh, J.M.; Shon, I.J. Mechanical properties and rapid consolidation of binderless niobium carbide. J. Alloys Compd. 2009, 481, 573–576. [Google Scholar] [CrossRef]
- Yate, L.; Coy, L.E.; Wang, G.; Beltrán, M.; Díaz-Barriga, E.; Saucedo, E.M.; Ceniceros, M.A.; Załęski, K.; Llarena, I.; Möller, M.; et al. Tailoring mechanical properties and electrical conductivity of flexible niobium carbide nanocomposite thin films. RSC Adv. 2014, 4, 61355–61362. [Google Scholar] [CrossRef]
- Li, H.; Song, Q.; Xu, Q.; Chen, Y.; Xu, L.; Man, T. Electrochemical synthesis of core-shell-structured NbC-Fe composite powder for enforcement in low-carbon steel. Materials 2017, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Klug, J.A.; Proslier, T.; Elam, J.W.; Cook, R.E.; Hiller, J.M.; Claus, H.; Becker, N.G.; Pellin, M.J. Atomic layer deposition of amorphous niobium carbide-based thin film superconductors. J. Phys. Chem. C 2011, 115, 25063–25071. [Google Scholar] [CrossRef]
- Woydt, M.; Mohrbacher, H. Friction and wear of binder-less niobium carbide. Wear 2013, 306, 126–130. [Google Scholar] [CrossRef]
- Agte, C.; Moers, K. Methoden zur reindarstellung hochschmelzender carbide, nitride und boride und beschreibung einiger ihrer eigenschaften. Z. Anorg. Allg. Chem. 1931, 198, 233–275. (In German) [Google Scholar] [CrossRef]
- Naumenko, V.Y. Poluchenie karbidov perekhodnykh metallov IV-V grupp v oblastyakh ikh gomogennosti [preparation carbides of transition metals of IV-V groups in their areas homogeneity]. Poroshkovaya Metal. Sov. Powder Metall. Met. Ceram. 1970, 10, 20–22. [Google Scholar]
- Powell, R.M.; Skocpol, W.J.; Tinkham, M. Preparation and superconducting properties of ultrafine powders and sintered compacts of NbC and NbN. J. Appl. Phys. 1977, 48, 788–794. [Google Scholar] [CrossRef]
- Crayton, P.H.; Gridly, M.C. Vapour-phase synthesis of submicron tantalum carbide. Powder Metall. 1971, 14, 78–85. [Google Scholar] [CrossRef]
- Da Silva, V.T.; Schmal, M.; Oyama, S.T. Niobium carbide synthesis from niobium oxide: Study of the synthesis conditions, kinetics, and solid-state transformation mechanism. J. Solid State Chem. 1996, 123, 168–182. [Google Scholar] [CrossRef]
- Xu, G.Y.; Li, J.B.; Huang, Y.; Xie, Z.P. Fabrication and morphology of different color NbCx whiskers. J. Cryst. Growth 1999, 200, 143–147. [Google Scholar] [CrossRef]
- Oyama, S.T.; Schlatter, J.C.; Metcalfe, J.E., III; Lambert, J.M., Jr. Preparation and characterization of early transition metal carbides and nitrides. Ind. Eng. Chem. Res. 1988, 27, 1639–1648. [Google Scholar] [CrossRef]
- Xu, Y.X.; Lu, J.T.; Li, W.Y.; Yang, X.W. Oxidation behaviour of Nb-rich Ni-Cr-Fe alloys: Role and effect of carbides precipitates. Corros. Sci. 2018, 140, 252–259. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren struktur und der größe von kolloidteilchen mittels röntgenstrahlen. In Kolloidchemie ein Lehrbuch; Springer: Berlin, Germany, 1912; pp. 387–409. (In German) [Google Scholar]
- Cui, Q.; Wu, J.; Xie, D.; Wu, X.; Huang, Y.; Li, X. Effect of nanosized NbC precipitates on hydrogen diffusion in ×80 pipeline steel. Materials 2017, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.; Eliezer, D.; Boellinghaus, T. Hydrogen-trapping mechanisms of TIG-welded 316L austenitic stainless steels. J. Mater. Sci. 2018, 53, 10457–10468. [Google Scholar] [CrossRef]
- Saitoh, K.I.; Kuramitsu, K.; Sato, T.; Takuma, M.; Takahashi, Y. Molecular dynamics study on deformation mechanism of grain boundaries in magnesium crystal: Based on coincidence site lattice theory. J. Mater. Sci. 2018, 2018, 4153464. [Google Scholar] [CrossRef]
- Trampert, A.; Brandt, O.; Ploog, K.H. Crystal structure of group iii nitrides. In Semiconductors and Semimetals; Elsevier: Amsterdam, The Netherlands, 1997; Volume 50, pp. 167–192. [Google Scholar]
- Sun, C.J.; Kung, P.; Saxler, A.; Ohsato, H.; Haritos, K.; Razeghi, M. A crystallographic model of (00·1) aluminum nitride epitaxial thin film growth on (00·1) sapphire substrate. J. Appl. Phys. 1994, 75, 3964–3967. [Google Scholar] [CrossRef]
- Mykura, H.; Bansal, P.S.; Lewis, M.H. Coincidence-site-lattice relations for MgO-CdO interfaces. Philos. Mag. A 1980, 42, 225–233. [Google Scholar] [CrossRef]
- Sánchez, F.; Bachelet, R.; de Coux, P.; Warot-Fonrose, B.; Skumryev, V.; Tarnawska, L.; Zaumseil, P.; Schroeder, T.; Fontcuberta, J. Domain matching epitaxy of ferrimagnetic CoFe2O4 thin films on Sc2O3/Si (111). Appl. Phys. Lett. 2011, 99, 211910. [Google Scholar] [CrossRef]
- Liu, W.R.; Li, Y.H.; Hsieh, W.F.; Hsu, C.H.; Lee, W.; Lee, Y.J.; Hong, M.; Kwo, J. Domain matching epitaxial growth of high-quality ZnO film using a Y2O3 buffer layer on Si (111). Cryst. Growth Des. 2008, 9, 239–242. [Google Scholar] [CrossRef]
- Narayan, J.; Larson, B.C. Domain epitaxy: A unified paradigm for thin film growth. J. Appl. Phys. 2003, 93, 278–285. [Google Scholar] [CrossRef]
- Yu, H.K.; Lee, J.L. Growth mechanism of MgO film on Si (100): Domain matching epitaxy, strain relaxation, preferred orientation formation. Cryst. Growth Des. 2010, 10, 5200–5204. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Doerner, M.F.; Nix, W.D. A method for interpreting the data from depth-sensing indentation instruments. J. Mater. Res. 1986, 1, 601–609. [Google Scholar] [CrossRef]
- Li, X.; Gao, H.; Murphy, C.J.; Caswell, K.K. Nanoindentation of silver nanowires. Nano Lett. 2003, 3, 1495–1498. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-W.; Kim, B.J.; Lee, S.H.; Nasir, T.; Lim, H.K.; Choi, I.J.; Jeong, B.J.; Lee, J.; Yu, H.K.; Choi, J.-Y. Growth of NbC Thin Film Using CH4 as a Carbon Source and Reducing Agent. Coatings 2018, 8, 379. https://doi.org/10.3390/coatings8110379
Kim K-W, Kim BJ, Lee SH, Nasir T, Lim HK, Choi IJ, Jeong BJ, Lee J, Yu HK, Choi J-Y. Growth of NbC Thin Film Using CH4 as a Carbon Source and Reducing Agent. Coatings. 2018; 8(11):379. https://doi.org/10.3390/coatings8110379
Chicago/Turabian StyleKim, Kwan-Woo, Bum Jun Kim, Sang Hoon Lee, Tuqeer Nasir, Hyung Kyu Lim, Ik Jun Choi, Byung Joo Jeong, Jaeyeong Lee, Hak Ki Yu, and Jae-Young Choi. 2018. "Growth of NbC Thin Film Using CH4 as a Carbon Source and Reducing Agent" Coatings 8, no. 11: 379. https://doi.org/10.3390/coatings8110379
APA StyleKim, K.-W., Kim, B. J., Lee, S. H., Nasir, T., Lim, H. K., Choi, I. J., Jeong, B. J., Lee, J., Yu, H. K., & Choi, J.-Y. (2018). Growth of NbC Thin Film Using CH4 as a Carbon Source and Reducing Agent. Coatings, 8(11), 379. https://doi.org/10.3390/coatings8110379




