3.1. Structural and Compositional Analysis
Structural analysis and phase purity of pure Ni-B and Ni-B-Y
2O
3 composite coatings in their as synthesized state are shown in
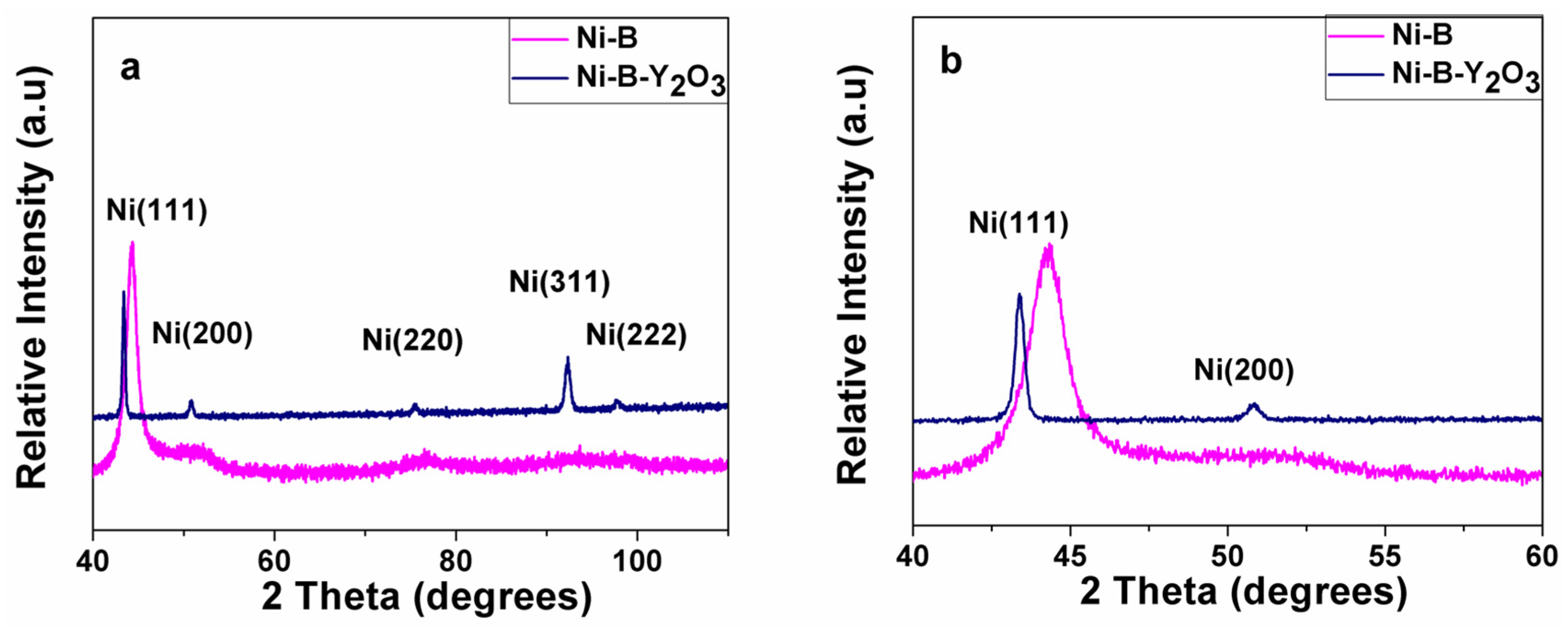
Figure 1. The large scale XRD scans with 2θ range (40°–110°) for Ni-B and Ni-B-Y
2O
3 composite coatings have been presented in
Figure 1a, whereas the small scale scan with 2θ range (40°–60°) is presented in
Figure 1b for a clear comparison. The absence of any undesired peaks indicates the synthesis of phase pure coatings.
The small scale XRD pattern i.e., 2θ range (40°–60°) clearly demonstrates the peak shifting and broadening of peak (111) in the XRD scan. As illustrated, the XRD spectra of Ni-B show a single broad Ni (111) peak at 2θ (~45°) depicting its semi amorphous crystal structure. However, additional reflections of Ni (200), Ni (220), Ni (222), and Ni (311) are clearly visible in the XRD spectrum of Ni-B-Y
2O
3 composite coatings. Unlike Ni-B coating, sharp peaks in Ni-B-Y
2O
3 composite coatings reveal the crystalline nature of the composite layer. This result supports the earlier reported findings that the co-deposition of hard ceramic particles improve the crystallinity of the Ni-B matrix [
1,
2]. Furthermore, shifting of Ni (111) main peak towards lower 2θ value (~43°) indicates the expansion of the unit cell confirming the co-deposition of Y
2O
3 particles into Ni-B matrix. It is anticipated that incorporation of Y
2O
3 particles having relatively large size results in misfit into the matrix. This mechanism creates strain and causes expansion of the matrix. The expansion leads to the shifting of 2θ values towards lower range. The crystallinity of nickel is improved because of the reason that Y
2O
3 particles itself are of crystalline phase. The incorporation of high crystalline phase results in high accumulative intensity of nickel phase. The grain size of the synthesized coatings was calculated by the following Scherrer equation [
5,
15]. The diffraction peak of Ni (111) was used to calculate the grain size (see Equation (1)).
In this equation,
L is the mean grain size of the phase and
K is the shape factor which is a dimensionless quantity. The shape factor has a usual value of about 0.9 and changes with the real shape of the crystallites, X-ray wavelength is shown with λ (1.54060 Å), line broadening at half of the maximum intensity (FWHM) is β, and θ is the Bragg angle (in degrees). A comparison of grain size measurement indicates that Ni-B coatings (~10 nm) have large grain size compared to Ni-B-Y
2O
3 composite coatings (~6 nm) and concludes that the incorporation of Y
2O
3 particles into the Ni-B matrix refines its grain. The refinement in grain size might be attributed to the hard and insoluble Y
2O
3 particles. Incorporation of Y
2O
3 particles provide numerous heterogeneous nucleation sites throughout the Ni-B matrix and blocks the grain growth [
16,
17].
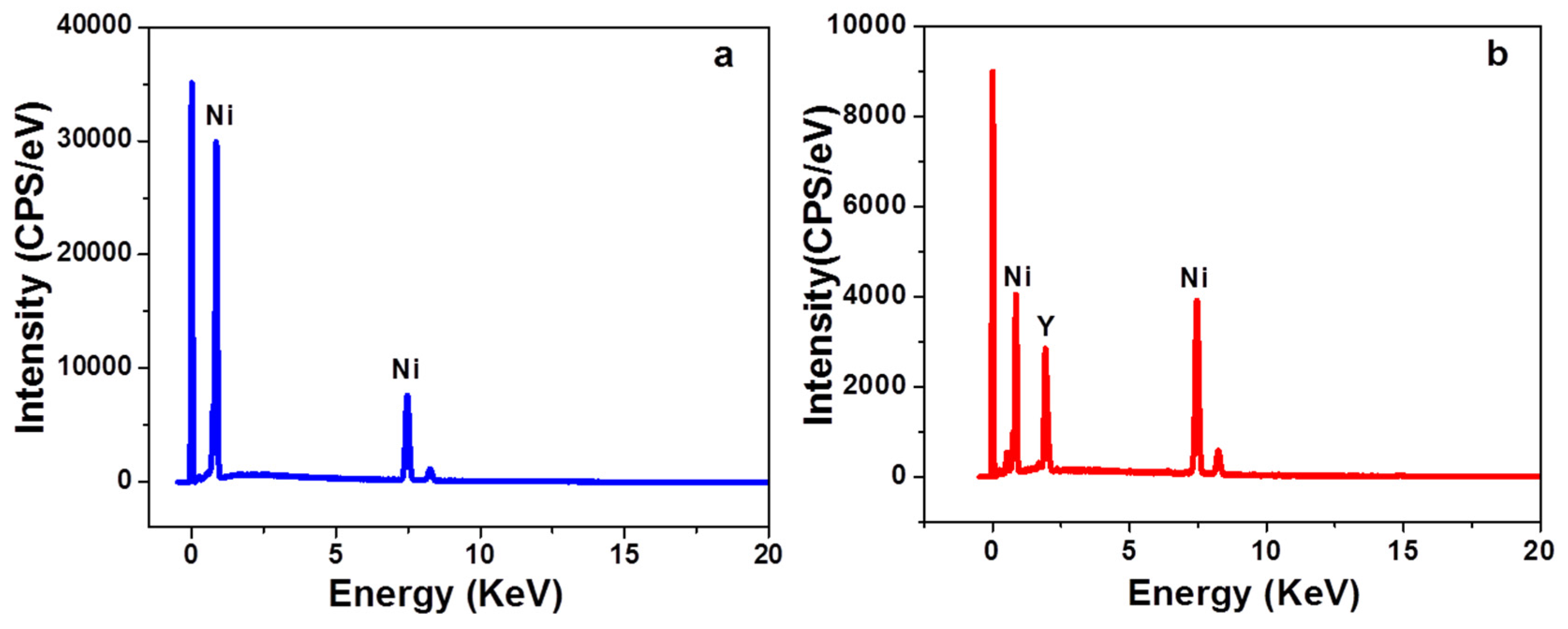
The co-deposition of both boron (B) and yttria (Y
2O
3) into nickel (Ni) matrix was also confirmed with EDX analysis and the results are presented in
Figure 2.
Incorporation of both boron (B) and Y
2O
3 particles into nickel matrix is clearly recognized, resulting in the formation of Ni-B and Ni-B-Y
2O
3 composite coatings. It can be observed that nickel, boron, and yttrium peaks are detected in the Ni-B and Ni-B-Y
2O
3 coatings. The presence of yttrium (Y) peaks in Ni-B-Y
2O
3 composite spectrum clearly confirms the successful incorporation of particles in the nickel matrix. Furthermore, change in the metallic luster of Ni-B coatings is also noticed by the incorporation of Y
2O
3 particles. Although, the phenomenon of co-deposition of boron in the Ni matrix through the electrodeposition process is not yet well understood, it is anticipated that co-deposition of boron into Ni matrix takes place because of adsorption of dimethylamine borane complex (DMAB) used as a source of boron in this study. The addition of Y
2O
3 into Ni-B matrix is resulted firstly because of nickel ions absorbing at the Y
2O
3 particle surface. These particles diffuse in the second step due to their ionic cloud which is close to the cathode and a poor adhesion of Y
2O
3 particles on the surface of the substrate (cathode) is possible with the diffusion layer. Towards the completion of the incorporation process, nickel ions are discharged at the substrate surface and the already adhered Y
2O
3 particles are entrenched into the Ni-B matrix [
10].
3.2. Surface Morphology
Surface analysis of electrodeposited Ni-B and Ni-B-Y
2O
3 coatings has been carried out through FESEM and AFM. Morphology of the coatings significantly affects the performance of the coatings.
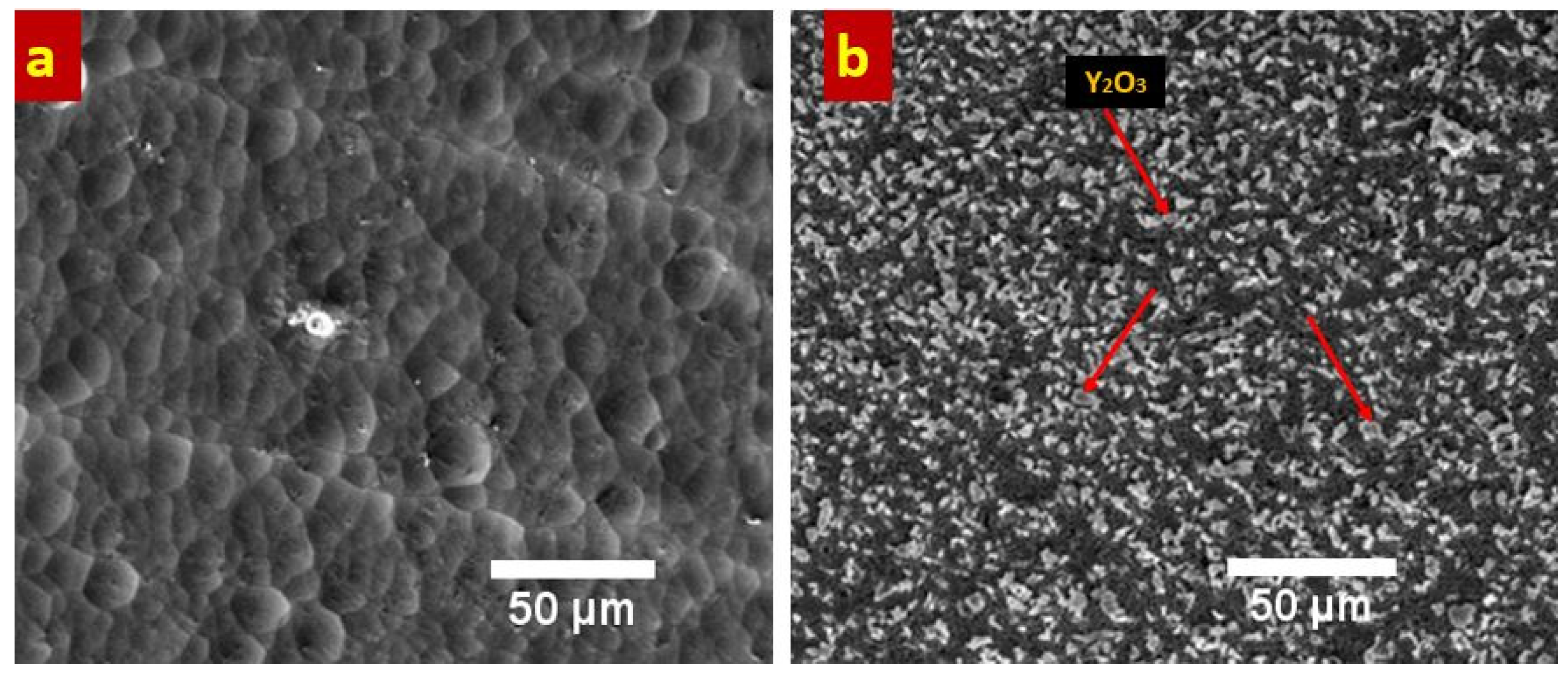
Figure 3 presents the FESEM analysis of Ni-B (a) and Ni-B-Y
2O
3 composite coatings (b) in their as deposited state.
The results show that electrodeposition has produced intact coatings which are free of surface defects including pores and cracks. It also reveals that very homogenous, and dense coatings were developed. Such morphological features are highly desirable as the corrosion phenomenon is highly reduced by restricting the permeation of water and other solvents towards the underlying metal substrate. The presence of Y
2O
3 particles can be clearly noticed in the Ni-B matrix,
Figure 3b. The particles are uniformly and evenly distributed throughout the Ni-B matrix which will ensure stable and homogeneous properties throughout the entire span of coatings. It is also observed that no agglomeration of particles happened suggesting that the selected stirring rate was sufficient to maintain the uniform dispersion of particles in the salt bath during the coating deposition. Addition of ceramic particles in the Ni-B matrix usually leads to improve their mechanical characteristics [
1,
4,
11,
13]. Interestingly, addition of insoluble, hard Y
2O
3 particles have resulted in significant grain refinement of the Ni-B matrix which also leads to enhance the mechanical and electrochemical behavior of the coatings. The incorporation of Y
2O
3 particles is believed to facilitate the prevention of grain growth at the surface of cathode which ultimately results in the grain size refinement of the nickel matrix [
4]. This observation is in agreement with our grain size calculations based on XRD analysis presented in
Figure 1.
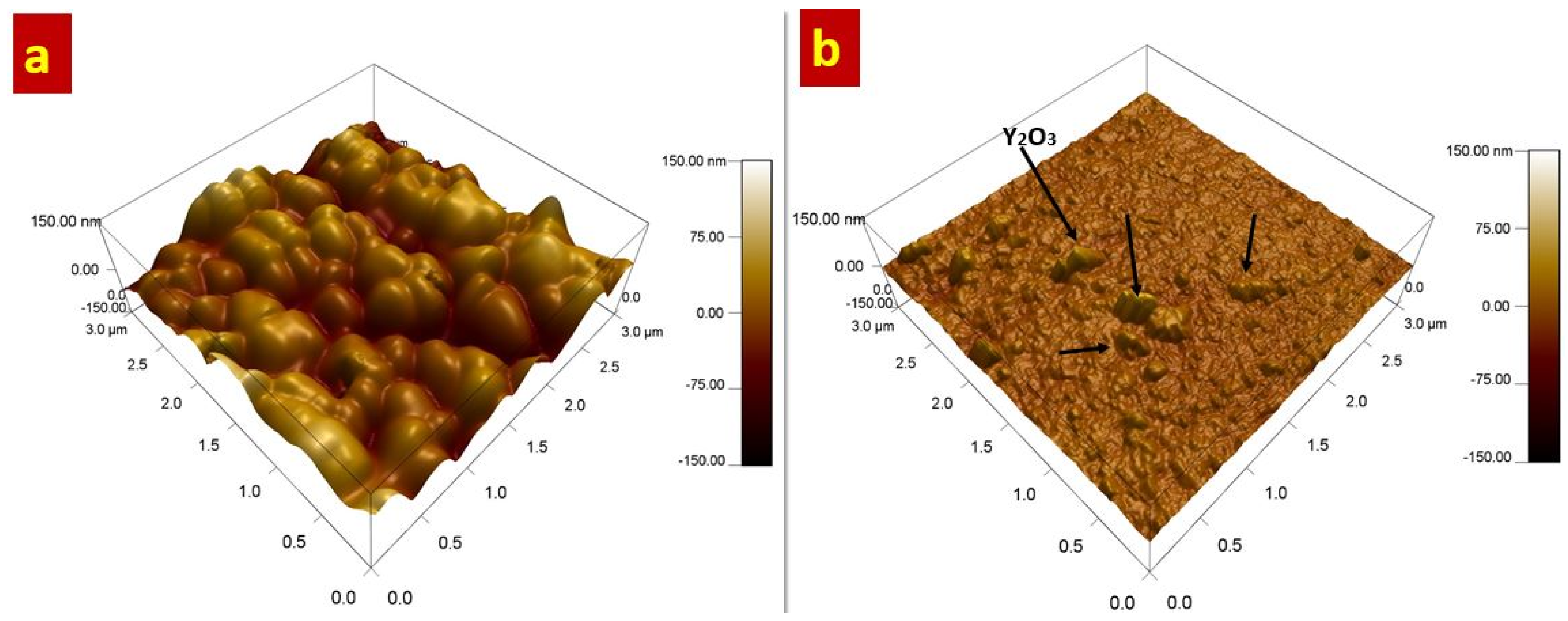
Morphology of the developed coatings was further studied through Atomic Force Microscopy (AFM) and 3D images are presented in
Figure 4. The current results strongly support the FESEM analysis confirming that the grain size has been refined with introduction of Y
2O
3 particles into the coating bath for Ni-B-Y
2O
3 composite coatings. It can be figured out that Ni-B coatings have larger nodular structure when compared to Ni-B-Y
2O
3 composite coatings. Moreover, the presence of Y
2O
3 particles can be clearly seen in the Ni-B-Y
2O
3 coatings (
Figure 4b). This outcome reveals that incorporation of Y
2O
3 ceramic particles into the Ni-B matrix have resulted in significant alteration of coating morphology. The surface morphology was also studied quantitatively in terms of root-mean-square roughness (RMS) which is described as the average height deviation from the reference line. The surface roughness analysis of Ni-B and Ni-B-Y
2O
3 composite coatings indicates that Ni-B coatings have higher RMS value (27.872 nm) when compared to the Ni-B-Y
2O
3 composite coatings (7.834 nm). The alteration of surface roughness presumably can be considered the effect of grain refinement by the addition of Y
2O
3 into Ni-B matrix. Furthermore, the AFM analysis confirms that the developed coatings are free of defects such as cracks and pores, etc. supporting the observations made through FESEM.
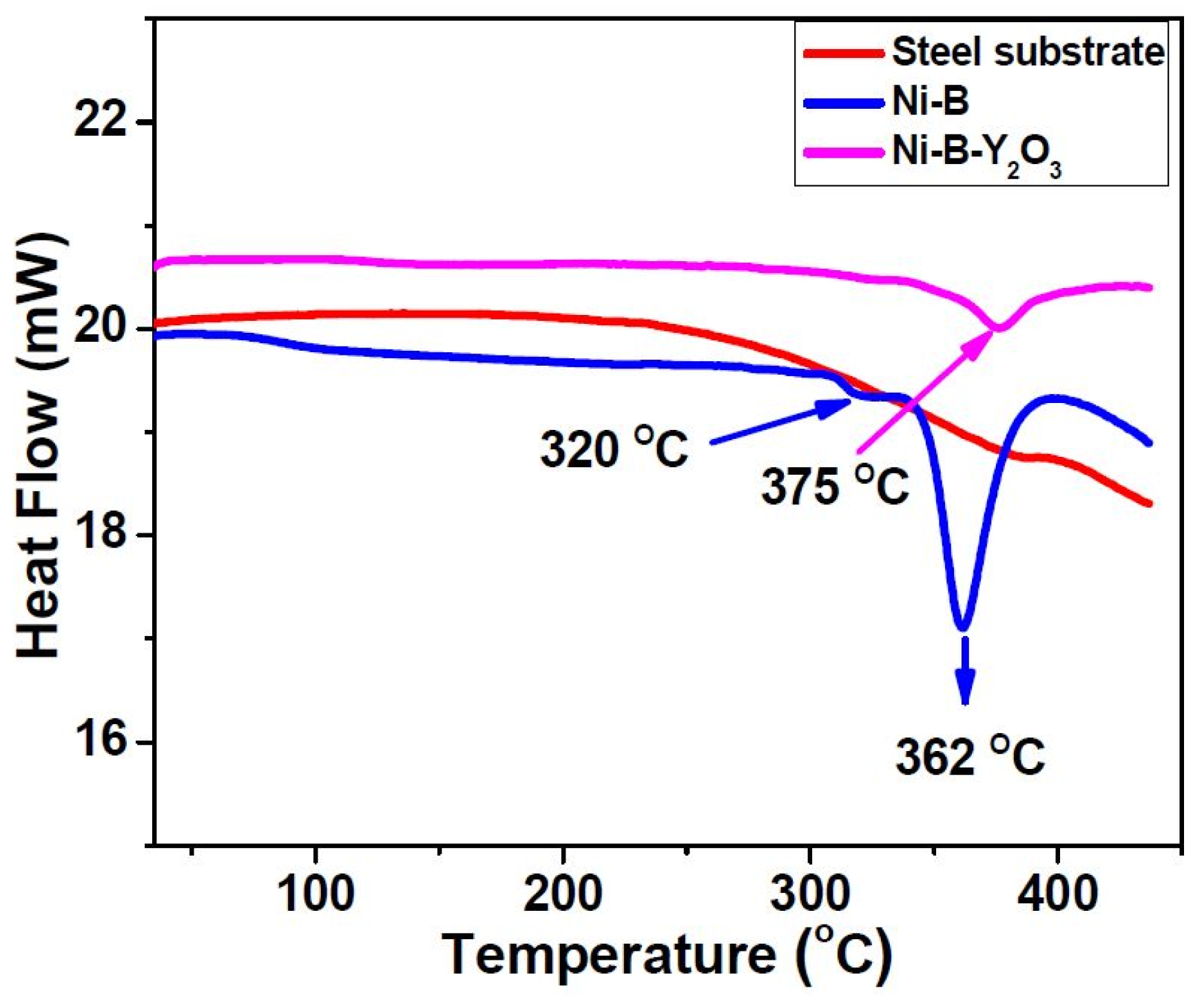
3.3. Thermal Properties
Thermal properties of Ni-B and Ni-B-Y
2O
3 composite coatings were studied by using DSC analysis and the results are shown in
Figure 5.
It can be clearly observed that the substrate material maintains smooth spectrum up to the examined temperature range, revealing the absence of any phase changes. On the other hand, two exothermic peaks are observed at 593 K (320 °C) and 635 K (362 °C) for Ni-B coatings which indicate two different phase transformations. The phase changes observed at 593 K (320 °C) in Ni-B coatings may correspond to the re-crystallization of nickel and the formation of Ni
3B phases into the nickel matrix. On the other hand, the presence of an exothermic peak at 635 K (362 °C) might be assigned to the nucleation of Ni
3B
4 [
1,
9]. In contrast, a single exothermic peak is observed at 648 K (375 °C) in the Ni-B-Y
2O
3 composite layer, which might correspond to the formation of Ni
3B
4 into the nickel matrix. This results indicate that the addition of Y
2O
3 in to the Ni-B matrix has suppressed the first transformation at 593 K (320 °C) occurring in Ni-B coatings, confirming the improved thermal stability of Ni-B matrix.
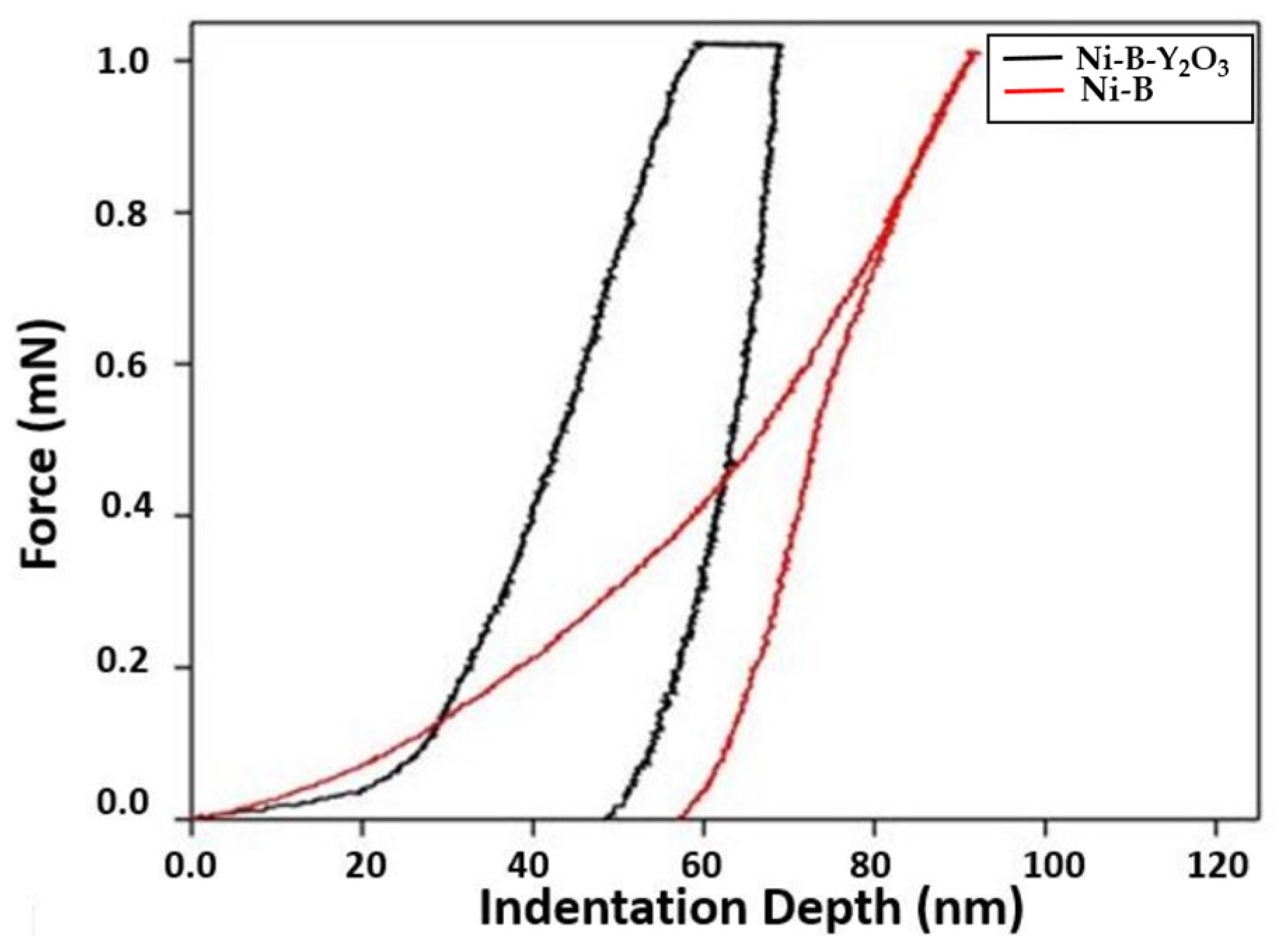
3.4. Mechanical Properties
The mechanical behavior of Ni-B and Ni-B-Y
2O
3 composite coatings was studied through nanoindentation. The loading and unloading profiles of nanoindentation for the comparative analysis of Ni-B and Ni-B-Y
2O
3 coatings are presented in
Figure 6.
Two parameters are important here; the area under the curve for each coating and the depth of penetration. The area under the curve of Ni-B is larger as compared to Ni-B-Y
2O
3 composite coatings. The depth of penetration is also larger in case of Ni-B coatings when compared with Ni-B-Y
2O
3 composite coatings which suggests the lower hardness and strength of Ni-B coatings as compared to Ni-B-Y
2O
3 composite coatings. Higher indentation depth of ~58 nm was achieved for Ni-B coatings, whereas Ni-B-Y
2O
3 composite coatings showed ~50 nm suggesting superior resistance to indentation and thus improved mechanical response. Previously, different research groups have reported similar trends, supporting the idea of mechanical properties enhancement of coatings with incorporation of reinforcing particles [
18]. The graphs also reveal that the Ni-B-Y
2O
3 deposited coatings possess kinks which are not visible for Ni-B coatings. The origination of such kinks are attributed to the presence of pores or multiphase structure of the coatings. When pores are present in the coatings, then a microcollapse might occur underneath the indentor, resulting in an abrupt variation in the stress–strain analysis. Similarly, when a second phase particle is scanned by the indentor, then fluctuation occurs in the slope of the load-displacement profile of nanoindentation [
19]. The mentioned mechanism is well followed by the current results as the Ni-B coatings showed a profile smooth and free of kinks. However, kinks were observed in the nanoindentation profiles of Ni-B-Y
2O
3 composite coatings due to the introduction of second phase reinforcing particles.
The loading and unloading profiles of nanoindentation tests were also utilized to conduct quantitative analysis for the calculation of the mechanical properties listed in
Table 2 (hardness, modulus of elasticity, stiffness, and plasticity).
The hardness of synthesized coatings was determined by the Oliver Pharr method which was explained in detail in our previous published work [
4]. We observed a remarkable improvement in mechanical properties of Ni-B coatings by the incorporation of Y
2O
3 into Ni-B matrix. The hardness of Ni-B matrix was significantly increased by ~79%. Similarly, the modulus of elasticity (~195%) and stiffness (~111%) of Ni-B matrix have been remarkably increased with addition of Y
2O
3 reinforcing particles. The improvement in the modulus of elasticity is attributed to the hard and brittle Y
2O
3 particles. These particles act as strong reinforcements throughout the Ni-B matrix. It leads to producing coatings with characteristics of high hardness/strength and low elongation (strain), thus resulting in the increased modulus of elasticity. The enhancement in modulus of elasticity and hardness is due to the addition of hard ceramic phase leading to dispersion hardening effect as reported in the literature. During the dispersion hardening process, the presence of Y
2O
3 particles into the Ni-B matrix impedes the motion of the dislocations and thus the restraining of dislocations at the Y
2O
3 particles leads to the enhancement in mechanical properties [
1,
10,
11,
13]. The enhancement in hardness of the Ni-B matrix by incorporation of Y
2O
3 particles can also be explained by the rule of mixtures [
20], being presented as Equation (2).
where,
Hm,
Hr, and
Hc are the hardness of the matrix, reinforcement and composite, whereas the
Fr, and
Fm are the volume fraction of reinforcement and the matrix, respectively. Since, Y
2O
3 particles have high hardness so their addition to Ni-B matrix improves its hardness.
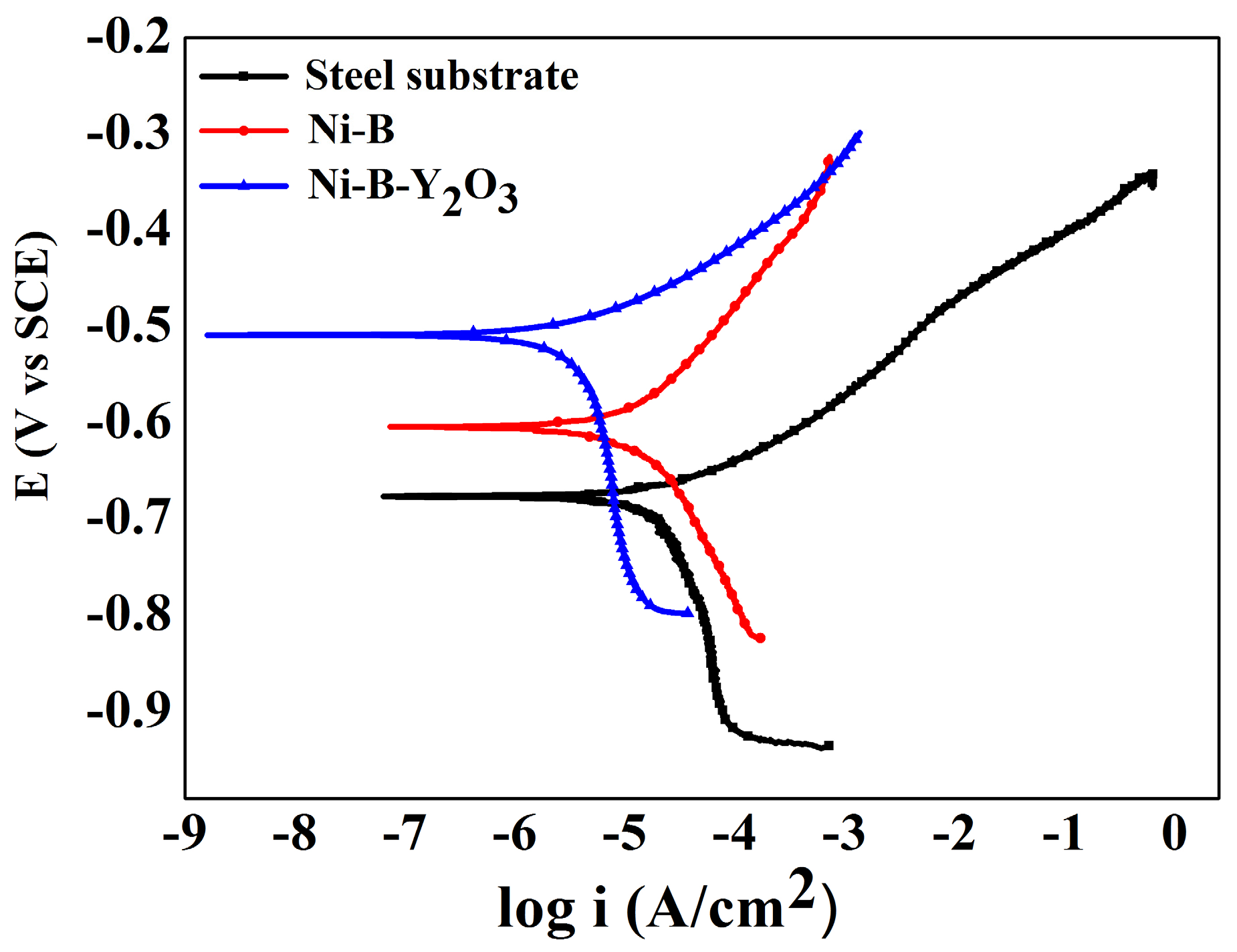
3.5. Corrosion Behavior
The potentiodynamic polarization experiments of the electrodeposited coatings were conducted using a Gamry Reference Eco potentiostat at a scan rate of 10 mV·min
−1 (0.167 mV·s
−1). The potentiodynamic curves of steel substrate, Ni-B and Ni-B-Y
2O
3 composite coatings are presented in
Figure 7. The potentiodynamic curve for steel substrate is included for comparison.
icorr, β
a, β
c,
Ecorr, and protection efficiency has been calculated from the potentiodynamic polarization curves and are presented in
Table 3. The steel substrate and Ni-B showed corrosion current densities of 26.2 μA/cm
2 and 19.3 μA/cm
2, respectively. However, it becomes clearly evident that incorporation of reinforcing Y
2O
3 particles greatly enhances the corrosion resistance of Ni-B matrix. Corrosion current density of Ni-B-Y
2O
3 composite coatings is significantly reduced to 6.9 μA/cm
2. Such a low value of current density confirms decent corrosion resistance of Ni-B-Y
2O
3 composite coatings. The corrosion protection efficiency of coatings was calculated by using Equation (3) [
21].
i1 and
i2 represents the corrosion current densities of the bare substrate and coated samples, respectively. The positive influence of Y
2O
3 particles addition to coatings can be understood by the fact that the corrosion protection efficiency of Ni-B-Y
2O
3 coatings is improved to ~74% which is ~48% higher than that of the Ni-B coatings (26%). The enhanced corrosion efficiency of Ni-B-Y
2O
3 composite coatings is believed to be because of the decrease in the active area of Ni-B matrix. The addition of hard and inert Y
2O
3 particles reduces the active sites on the coating surface for Cl
− ion adsorption, and thus the corrosion resistance is improved [
1,
4,
8].
Although, there is improvement in mechanical and anticorrosion properties of Ni-B coatings by the addition of Y2O3 particles, we believe that the properties of Ni-B-Y2O3 composite coatings can be further improved using Y2O3 nanoparticles and adopting pulse electrodeposition process instead of the conventional electrodeposition process.