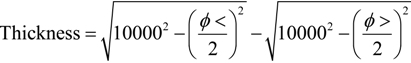3.6. Potentiodynamic Polarizations in NaCl 0.001 M
According to
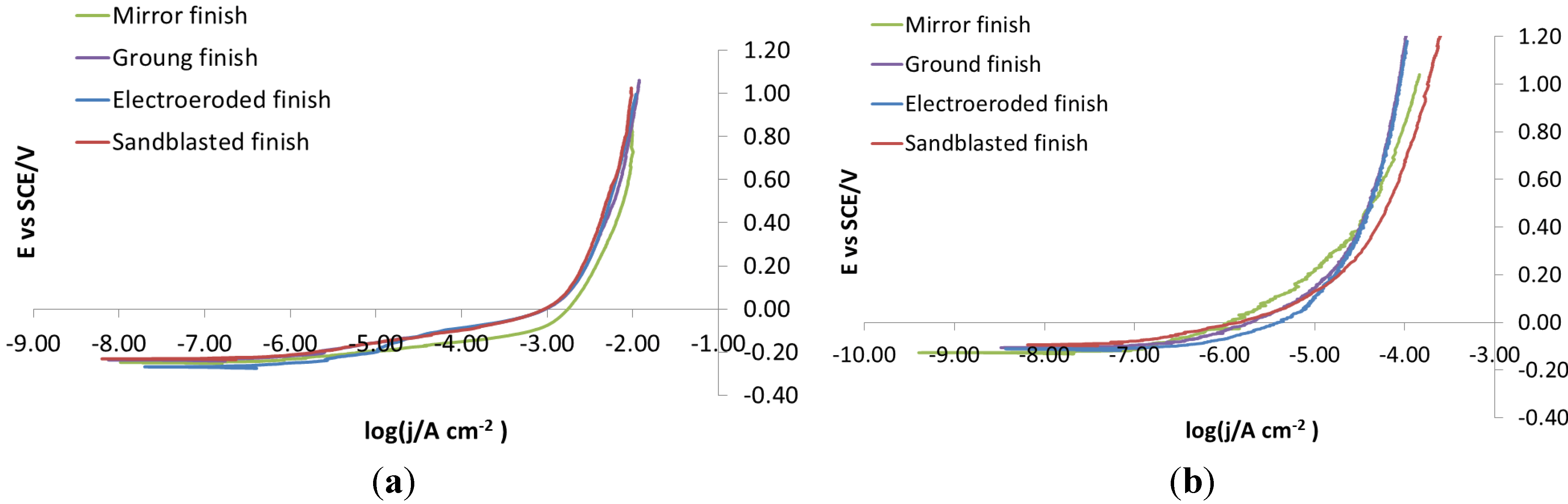
Figure 5a, potentiodynamic polarizations recorded on bare steels on each surface finish in NaCl 0.1 M do not show any difference in the corrosion behavior between the four different surface finishes. This is because the concentration of NaCl was too high, so much that the corrosive attack takes place immediately and extensively. To avoid these problems, potentiodynamic polarization was re-recorded in NaCl 0.001 M,
Figure 5b. In this case, the curves show slightly, but definitely different, trends, depending on which surface they were recorded. For this reason, 0.001 M was chosen as the NaCl concentration for carrying out potentiodynamic polarizations.
Figure 5.
X105CrCoMo18-2 bare steel: polarization curve on different finishes. (a) NaCl 0.1 M; (b) NaCl 0.001 M.
Figure 5.
X105CrCoMo18-2 bare steel: polarization curve on different finishes. (a) NaCl 0.1 M; (b) NaCl 0.001 M.
In
Figure 6, recorded on mirror finish surfaces, the same corrosion behavior for the four bare steels can be noticed. Actually, all steels show active dissolution. The same active behavior is maintained for all steels after the introduction of monolayer coatings. Even if the applied potential is the same, the curves of X37CrMoV5-1 and X2NiCoMo18-8-5 steels move towards lower currents than the curves of X105CrCoMo18-2 and X40CrMo15 steels. The corrosion behavior improves greatly for X37CrMoV5-1, X2NiCoMo18-8-5 and X105CrCoMo18-2 steels with Multilayer 4 coatings, while for X40CrMo15 steel, it appears that corrosive attack takes place after coating breakdown. Finally, the corrosion behavior improves greatly for all steels if Multilayer 8 coatings are applied. The shape of the curves is indicative of a barrier effect that is created against the external environment, which slows down the kinetics of corrosion, putting it under diffusion control.
Figure 6.
Mirror finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
Figure 6.
Mirror finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
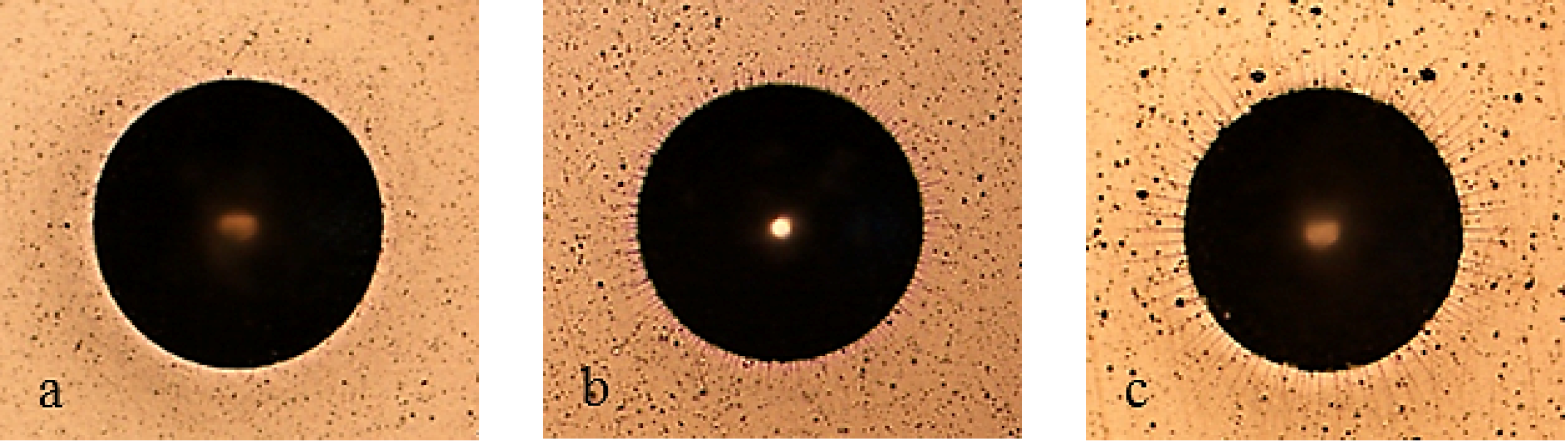
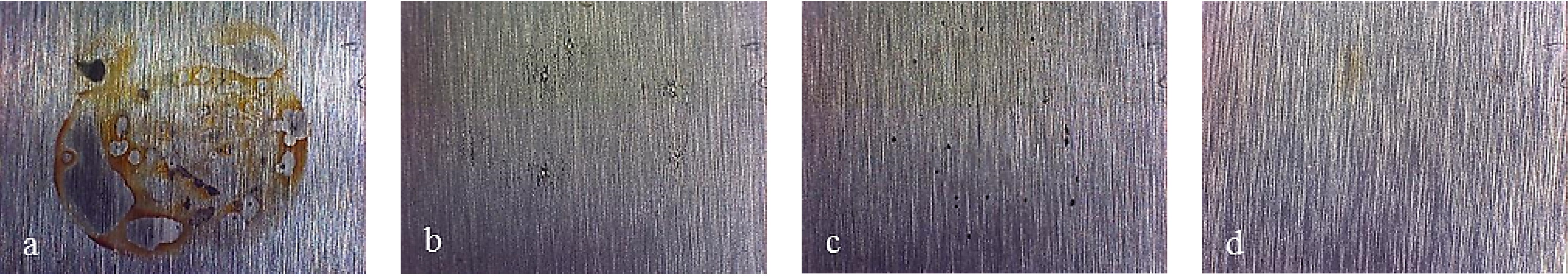
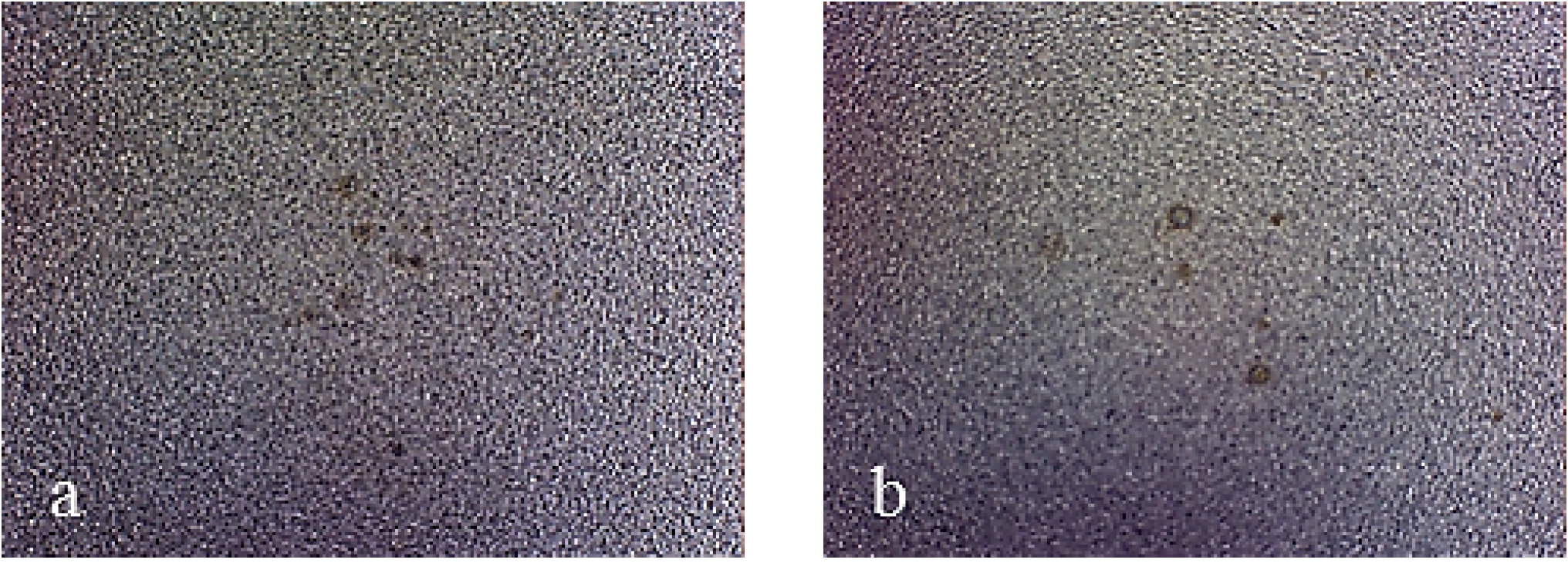
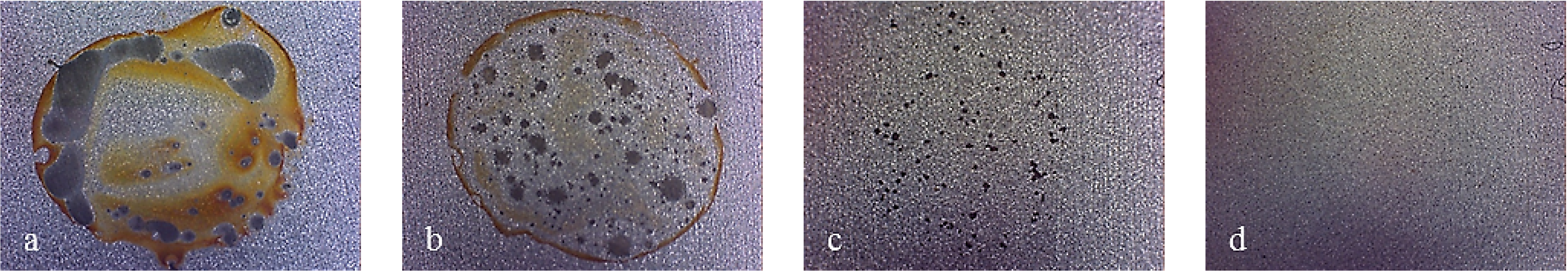
Figure 7 shows pictures of the working area after polarization of mirror finished X2NiCoMo18-8-5 steel. On this picture, uniform corrosion can be noticed on bare steel, while for monolayer-coated steel, corrosion becomes localized. As Multilayer 4 is applied, the result of corrosive attack is the loss of brilliance of the surface, due to the increase in porosity of the coating. Finally, the surface protected with Multilayer 8 does not appear damaged, except for a light loss of brilliance.
Figure 8 shows that other bare steels exhibited different corrosion attack, but the coated ones showed the same improvement of surface conditions as for X2NiCoMo18-8-5 coated steel.
Figure 7.
Working area after polarization on X2NiCoMO18-8-5 steel, mirror finished. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 7.
Working area after polarization on X2NiCoMO18-8-5 steel, mirror finished. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 8.
Working area after polarization on bare mirror finish: (a) X37CrMoV5-1; (b) X105CrCoMo18-2; (c) X40CrMo15.
Figure 8.
Working area after polarization on bare mirror finish: (a) X37CrMoV5-1; (b) X105CrCoMo18-2; (c) X40CrMo15.
In
Figure 9, all ground finish steels show the same corrosion behavior. Only the Multilayer 8 coating protects from corrosion. The monolayer and Multilayer 4 coatings, due to the small thickness, are unable to fit the basic finish, thus resulting in being unsuccessful in providing protection.
Figure 9.
Ground finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
Figure 9.
Ground finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
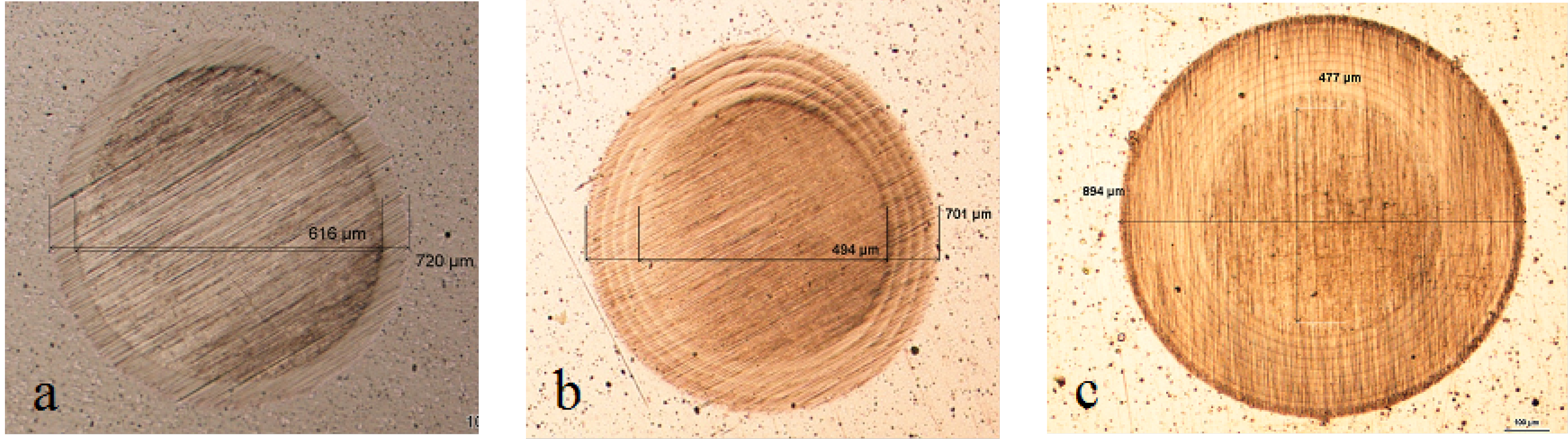
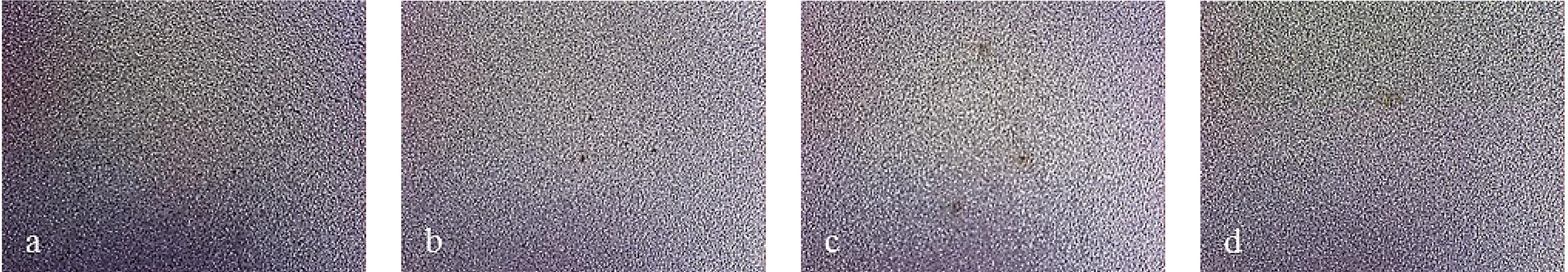
Figure 10 displays the characteristic behavior observed for all ground finish coated samples. In particular, as the number of layers grow, the size of localized attack decreases. As Multilayer 8 is applied, spots of breakdown are no longer visible. Bare steels showed different corrosion attack also in this case (
Figure 11).
Figure 10.
Working area after polarization on X2NiCoMO18-8-5 steel, ground finished. (a) Bare; (b) monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 10.
Working area after polarization on X2NiCoMO18-8-5 steel, ground finished. (a) Bare; (b) monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 11.
Working area after polarization on the bare ground finish: (a) X37CrMoV5-1; (b) X105CrCoMo18-2; (c) X40CrMo15.
Figure 11.
Working area after polarization on the bare ground finish: (a) X37CrMoV5-1; (b) X105CrCoMo18-2; (c) X40CrMo15.
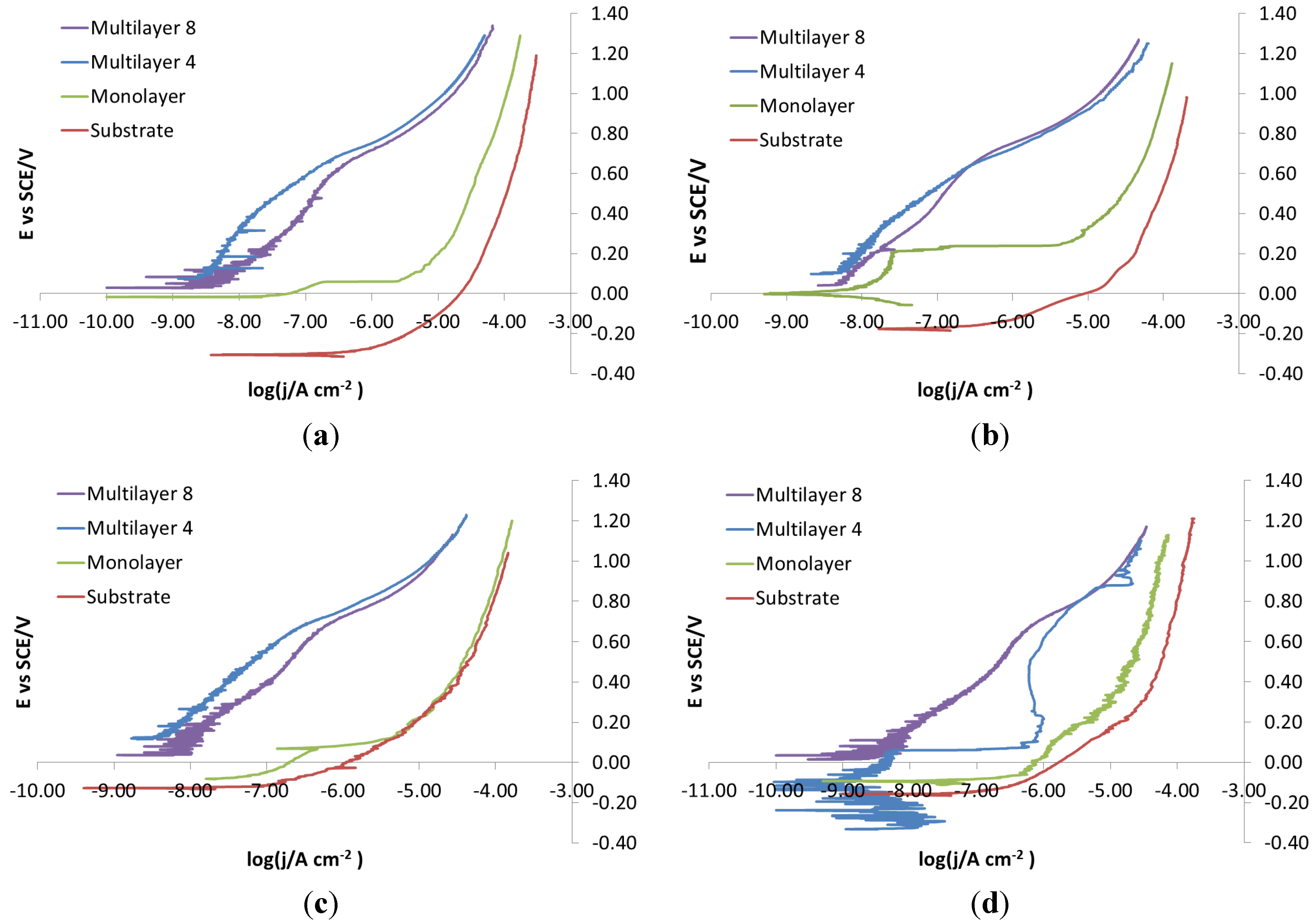
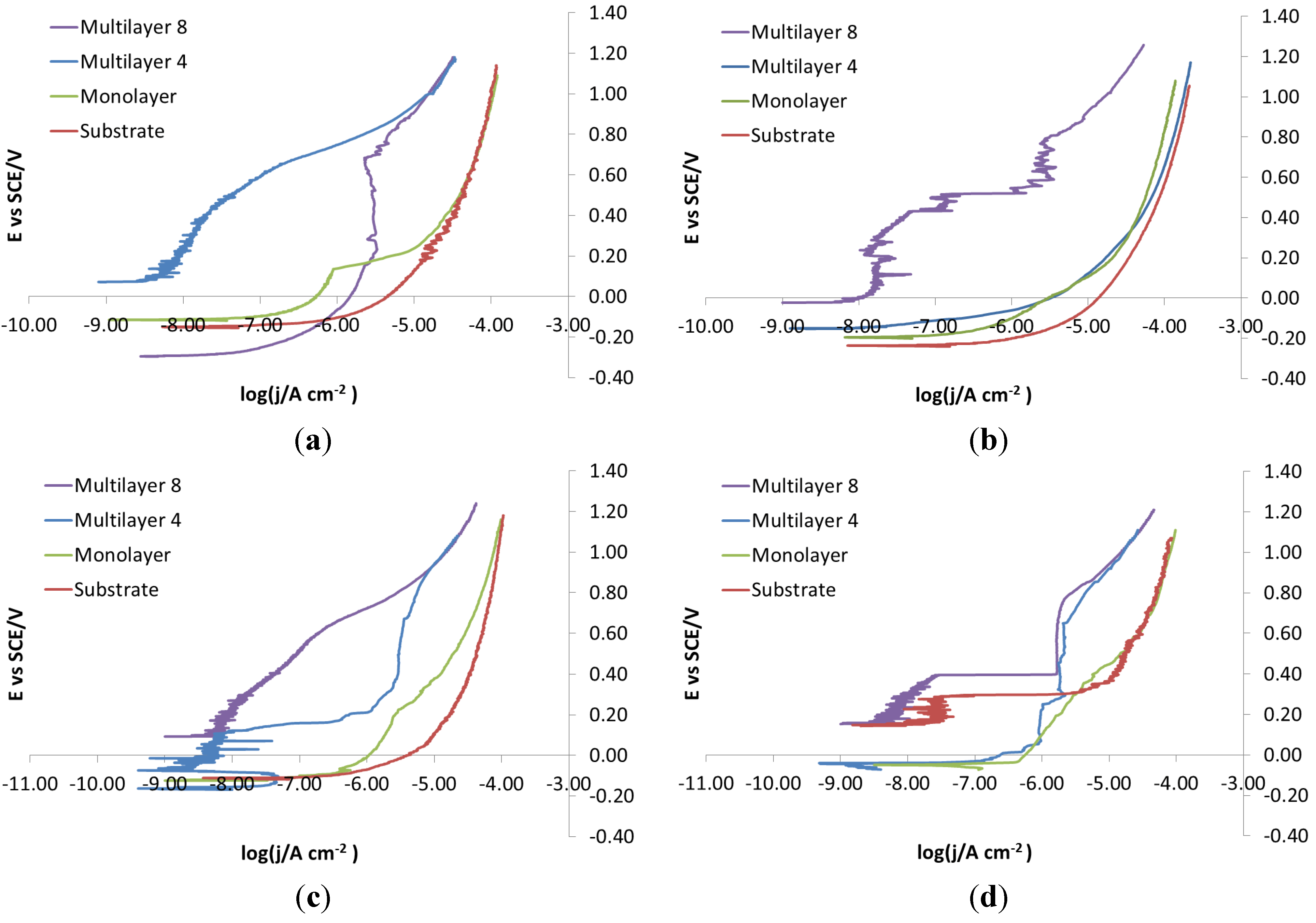
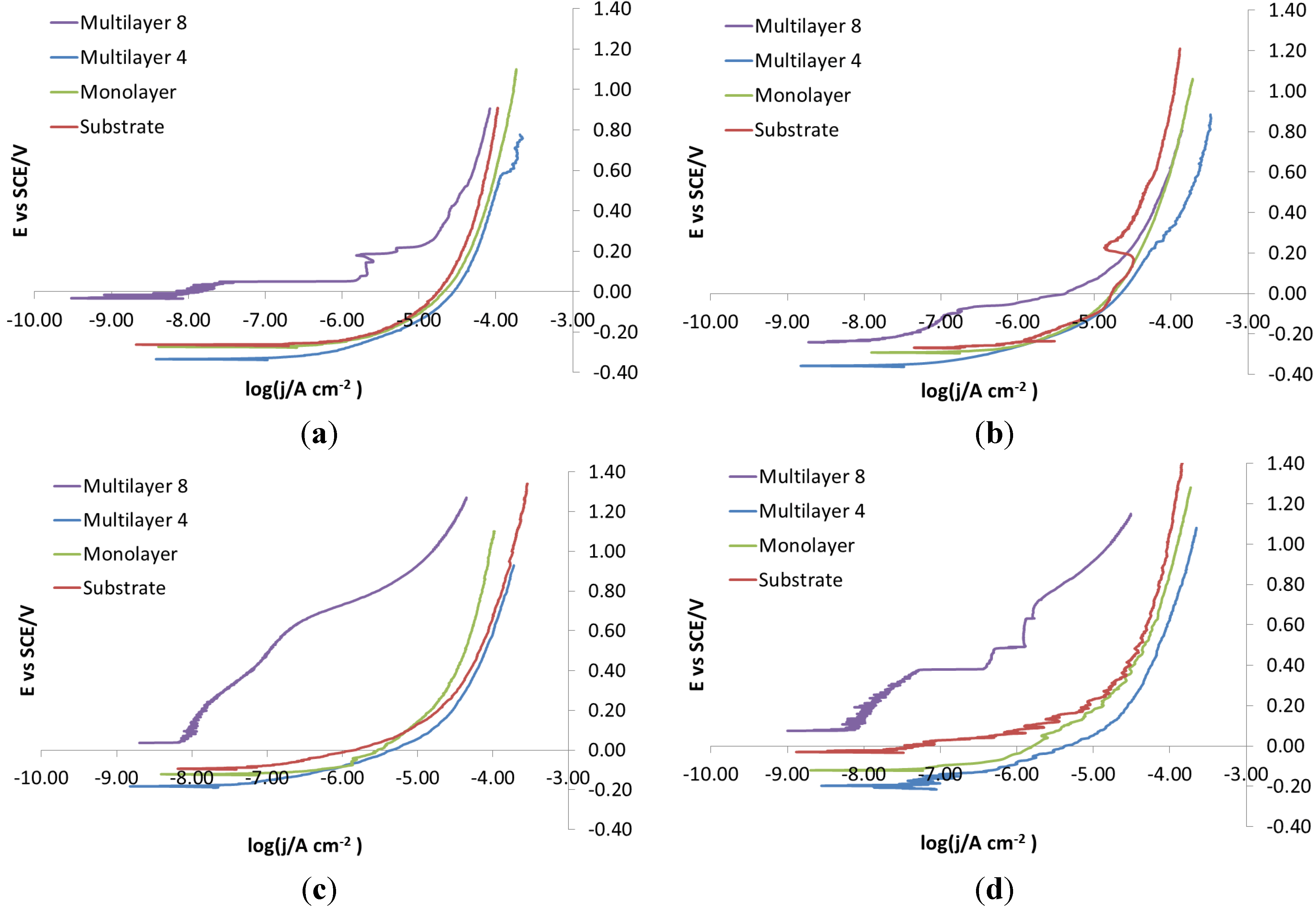
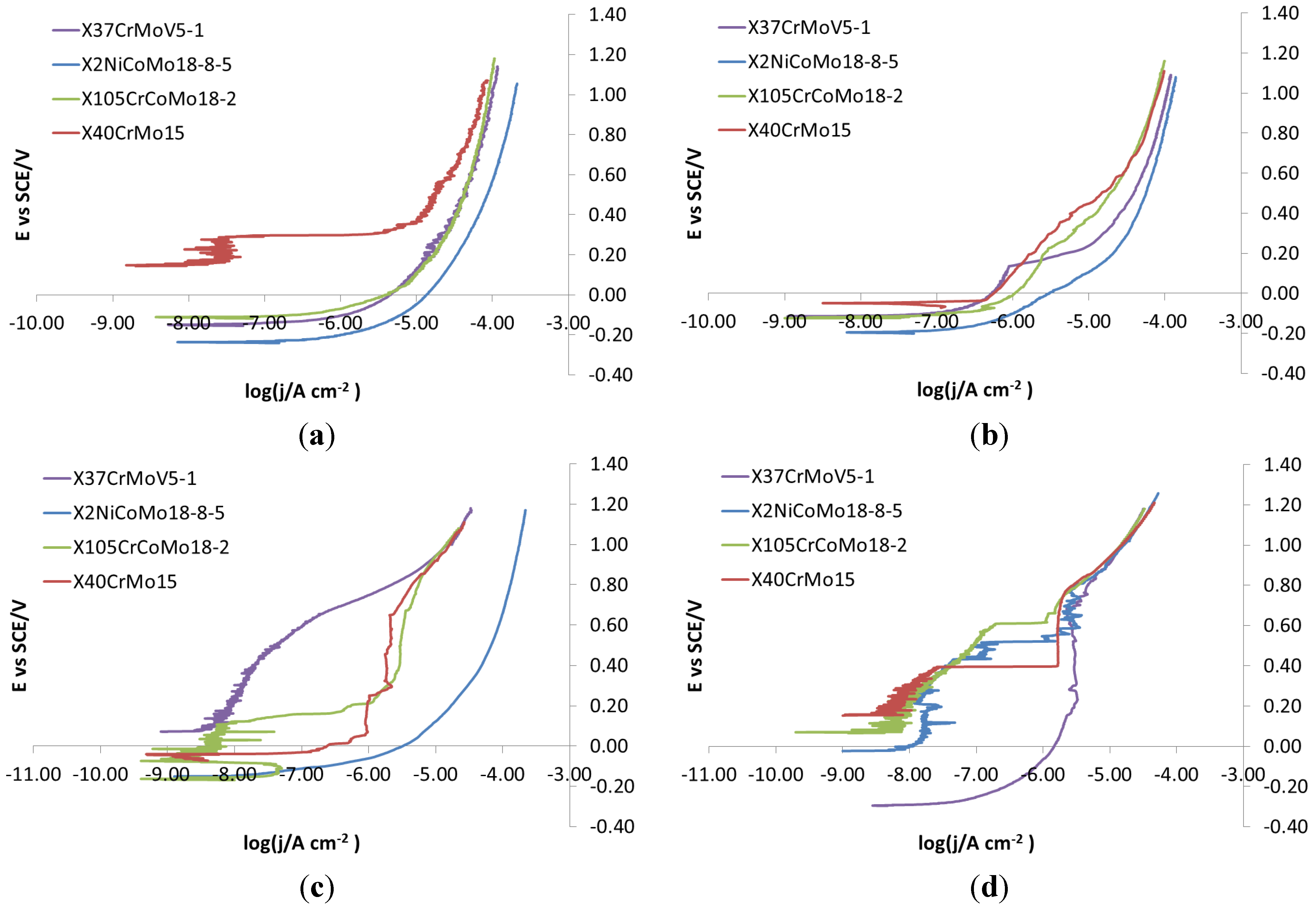
As for the electro-eroded finish (
Figure 12), both bare and monolayer-coated steels show active dissolution. On the contrary, for others with coating in the same figure, a difference in the corrosion behavior of X37CrMoV5-1 and X2NiCoMo18-8-5 can be noticed. For X37CrMoV5-1, the corrosion behavior improves greatly with Multilayer 4. As for this coating, thanks to a barrier effect, the shift of the curves towards lower currents, at the same applied potential, can be seen. Multilayer 8 coating shows a passive behavior, characteristic of a superficial chromium layer. The disappearance of the barrier effect with the Multilayer 8 coating can be explained as follows. The increase in thickness causes greater brittleness, because of rising of tensions inside the coating, plus tensions accumulated in the steel during electro-erosion. On the contrary, for X2NiCoMo18-8-5, Multilayer 4 does not provide protection against corrosion, which is effectively provided by the Multilayer 8 coating. The absence of brittleness in the coating with the maximum thickness is due to the capacity of X2NiCoMo18-8-5 to relax tensions accumulated during electro-erosion.
In
Figure 13, it can be noticed that as Multilayer 8 is applied on X37CrMoV5-1 steel, there is a worsening of corrosive attack with respect to Multilayer 4.
Figure 14 shows that the morphology of the corrosive attack of X40CrMo15 is related to the electrochemical behavior reported in
Figure 12, since there is a worsening of the corrosive attack from bare steels to Multilayer 4. Only with Multilayer 8 are the spots of breakdown no longer visible.
In
Figure 15, it is seen that for X2NiCoMo18-8-5 steel with a growing number of layers, the corrosive attack decreases. The same corrosive attack was observed for X105CrCoMo18-2 coated steel.
Figure 12.
Electro-eroded finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
Figure 12.
Electro-eroded finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
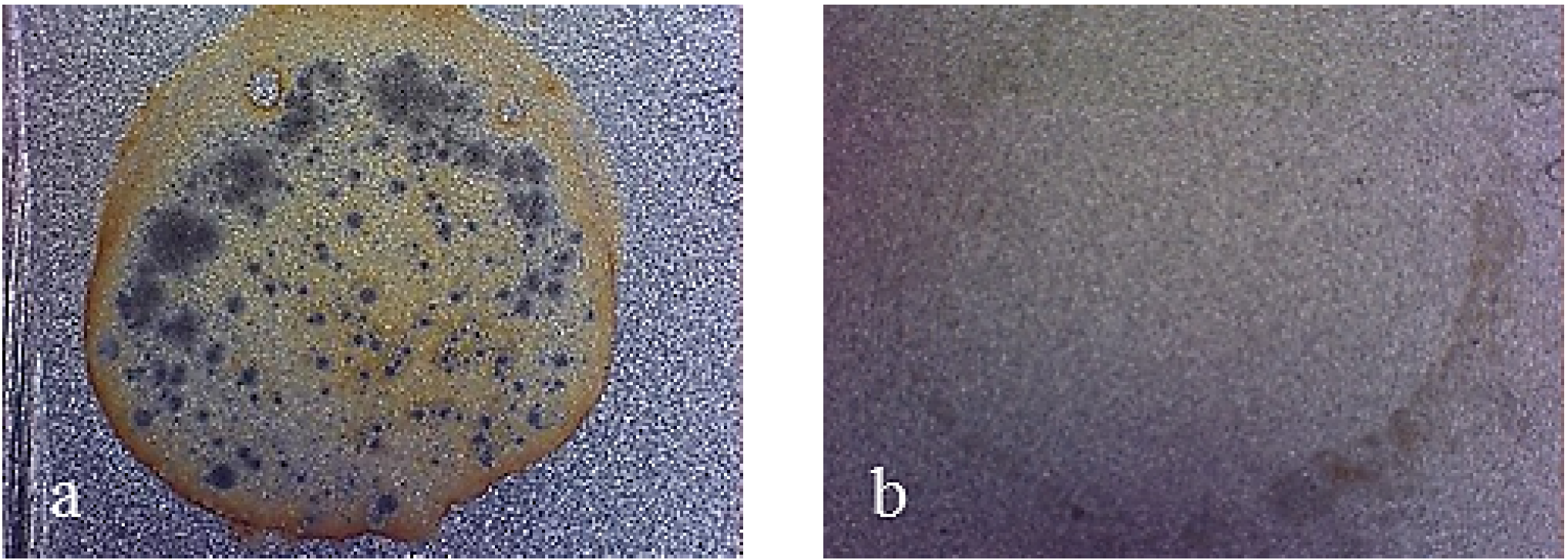
Figure 13.
Working area after polarization on X37CrMoV5-1 steel, electro-eroded finish. (a) Multilayer 4; (b) Multilayer 8.
Figure 13.
Working area after polarization on X37CrMoV5-1 steel, electro-eroded finish. (a) Multilayer 4; (b) Multilayer 8.
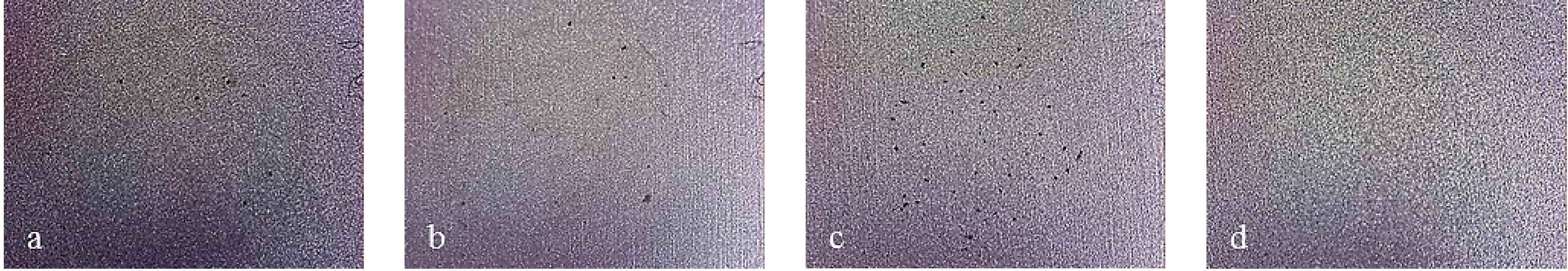
Figure 14.
Working area after polarization on X40CrMo15 steel, sandblasted finish. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 14.
Working area after polarization on X40CrMo15 steel, sandblasted finish. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 15.
Working area after polarization on X2NiCoMo18-8-5 steel, sandblasted finish. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 15.
Working area after polarization on X2NiCoMo18-8-5 steel, sandblasted finish. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
In
Figure 16, on a sandblasted finish, monolayer and Multilayer 4 coatings are seen not to provide any protection against corrosion for all steels. In some cases, as these coatings are applied, worse performance than for bare steels is even observed. For X37CrMoV5-1, X2NiCoMo18-8-5 and X40CrMo15 steels, the corrosion behavior does not improve significantly, even as the Multilayer 8 coating is applied. On the contrary, X105CrCoMo18-2 steel coated with Multilayer 8 shows great improvements in the corrosion behavior.
Figure 16.
Sandblasted finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
Figure 16.
Sandblasted finish: polarization curves on bare and coated steels. (a) X37CrMoV5-1; (b) X2NiCoMo18-8-5; (c) X105CrCoMo18-2; (d) X40CrMo15.
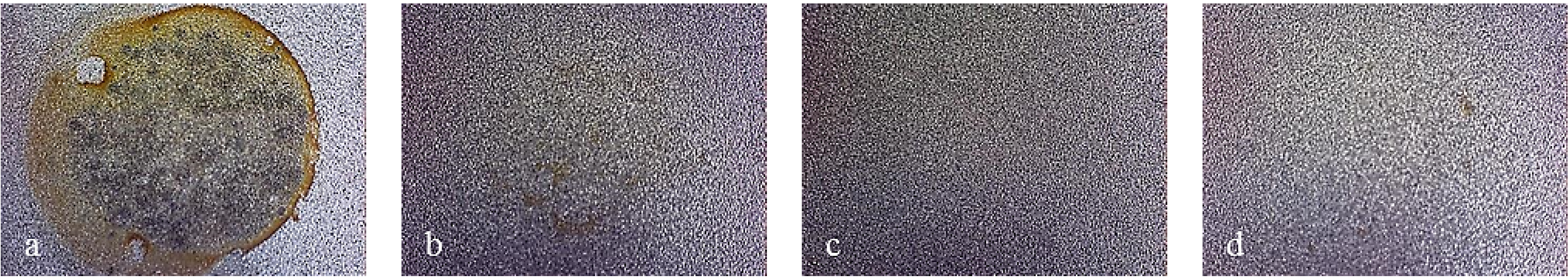
Figure 17 displays the behavior observed with X37CrMoV5-1 sandblasted finish samples. In particular, it is noticed that the bare steel shows uniform corrosion that becomes localized once the monolayer is applied. Furthermore, as the number of layers grows, the extent of localized attack decreases. As Multilayer 8 is applied, spots of breakdown were no longer visible. X2NiCoMo18-8-5 and X105CrCoMo18-2 bare steels showed different corrosion attack, but the coated ones showed the same improvement of the surface condition as that seen for X37CrMoV5-1 coated steel (
Figure 18).
Figure 17.
Working area after polarization on X37CrMoV5-1 steel, sandblasted finished. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 17.
Working area after polarization on X37CrMoV5-1 steel, sandblasted finished. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 18.
Working area after polarization on bare sandblasted finish: (a) X2NiCoMo18-8-5; (b) X105CrCoMo18-2.
Figure 18.
Working area after polarization on bare sandblasted finish: (a) X2NiCoMo18-8-5; (b) X105CrCoMo18-2.
Figure 19 shows that the morphology of corrosive attack of X40CrMo15 corresponds to the electrochemical behavior reported in
Figure 16. Actually, worsening of corrosive attack is observed from bare steels to Multilayer 4. Only as Multilayer 8 is applied do spots of breakdown become no longer visible.
Figure 19.
Working area after polarization on X40CrMo15 steel, sandblasted finish. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 19.
Working area after polarization on X40CrMo15 steel, sandblasted finish. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
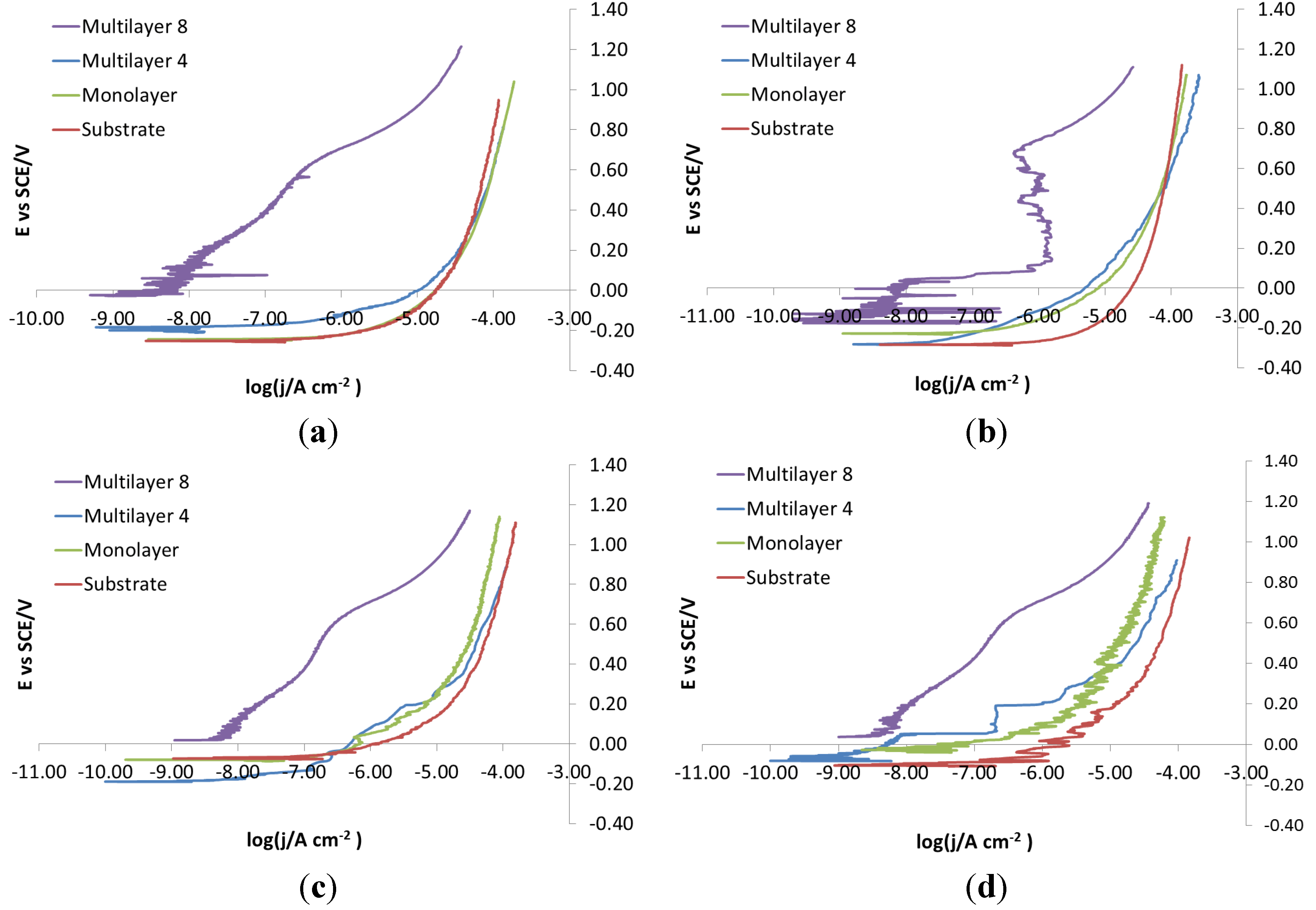
Figure 20 displays curves showing the effect of substrate steel with an electro-eroded finish. The corrosion behavior of X105CrCoMo18-2 and X2NiCoMo18-8-5 steels can be readily explained by the fact that only coatings as thick as Multilayer 8 can provide a homogeneous surface without cracks and pinholes that acts as a barrier. On the contrary, X37CrMoV5-1 steel protected with coatings at intermediate thickness, such as Multilayer 4, shows better corrosion behavior than with Multilayer 8. Finally, the behavior of X40CrMo15 is very particular. As shown in
Figure 20, the curves recorded for bare steel show a tentative passivation and a shift to higher potential than curves for steel coated with a monolayer and Multilayer 4. With Multilayer 8, finally, the barrier effect of the coating can be observed.
Figure 20.
Electro-eroded finish: polarization curves on different steels. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
Figure 20.
Electro-eroded finish: polarization curves on different steels. (a) Bare; (b) Monolayer; (c) Multilayer 4; (d) Multilayer 8.
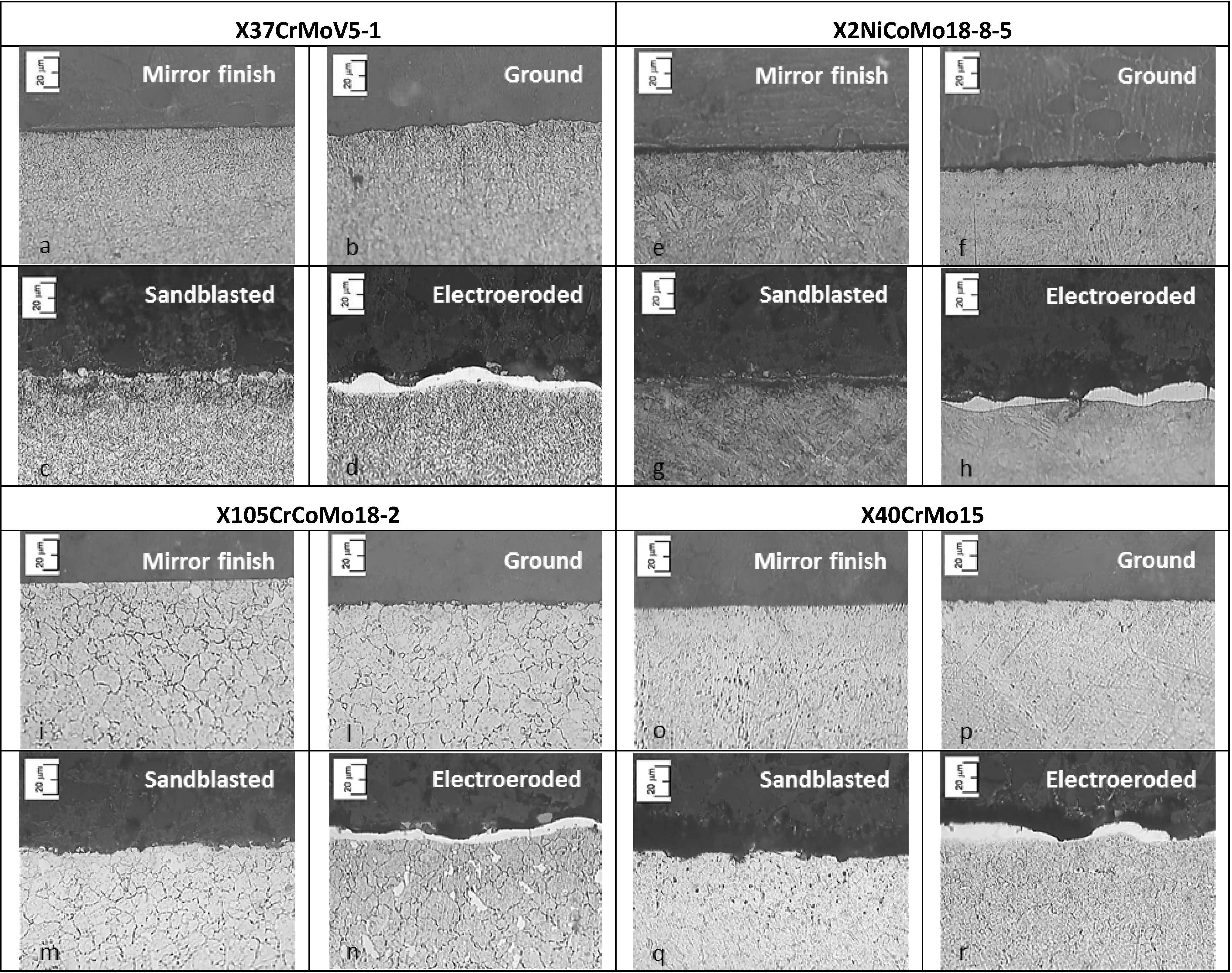
3.7. SEM Analysis
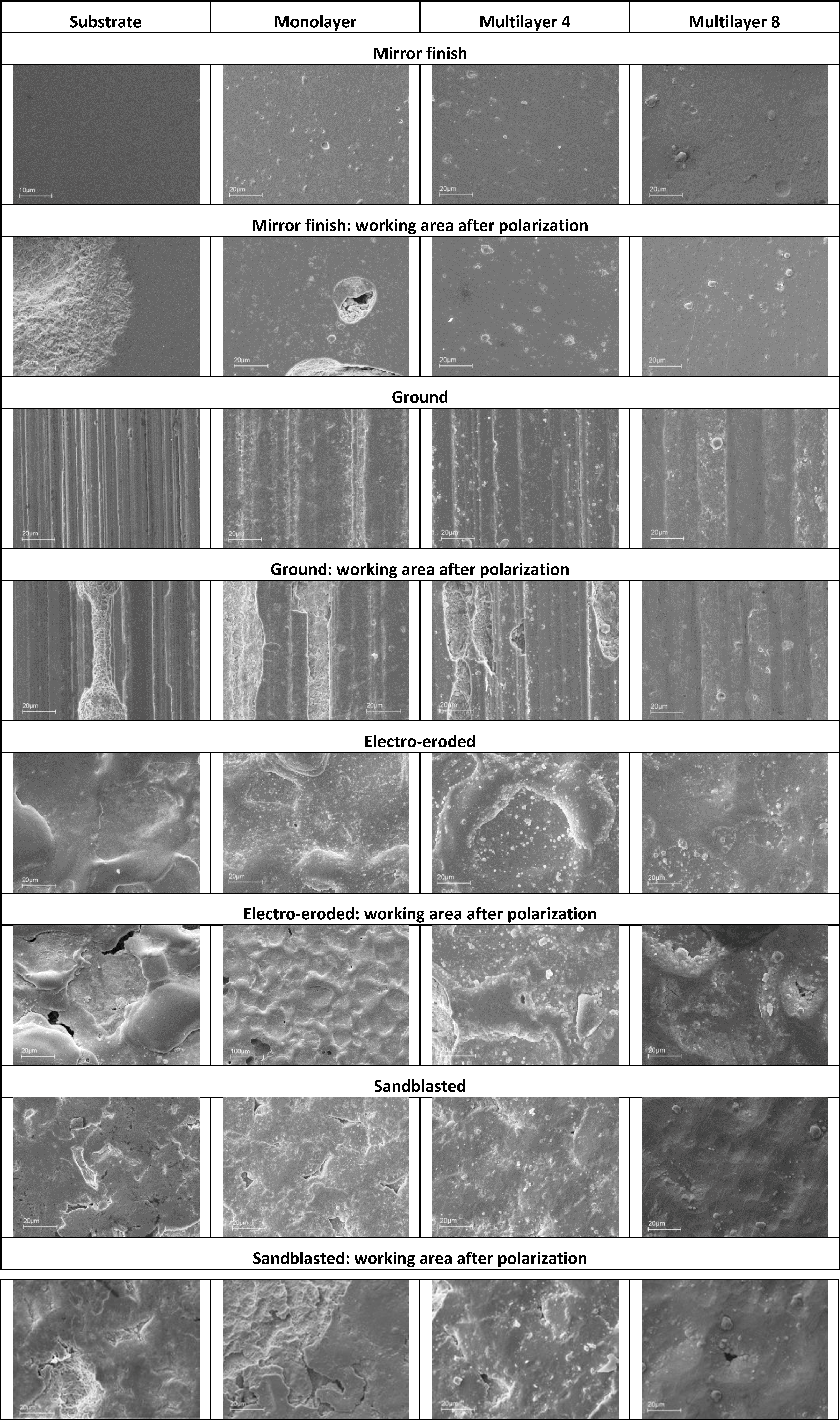
SEM pictures of X37CrMoV5-1 steel are presented in
Figure 21. The coatings applied on the mirror finish show a homogeneous surface and less defects, with the exception of the presence of droplets. From the working areas of the monolayer, it can be noticed that corrosive attack and breakdown of the coating take place next to the droplets. The working areas of Multilayers 4 and 8 are practically intact, but there is an increase of porosity in the regions next to the droplets.
Figure 21.
SEM images of X37CrMoV5-1 sample surfaces of different finished and coatings before and after polarization.
Figure 21.
SEM images of X37CrMoV5-1 sample surfaces of different finished and coatings before and after polarization.
As for the ground finish substrate, the corrosive attack follows the grinding crests and spreads as generalized corrosion. As for coatings on such a finish, it is noticed that the droplets are located on the grinding crests. The same corrosion behavior of the substrate is observed for the monolayer and Multilayer 4. Multilayer 8 shows localized corrosion, which does not depend on the morphology of the ground lines. For coated samples of electro-eroded finish surfaces, the concentration of droplets is greater in the areas between crests and depressions, while it is lower on the crest top. The working surface of the substrate shows localized corrosion in the regions between crests and depressions, where the white layer is smaller, as seen after the metallographic analysis. As for the monolayer and Multilayer 4, corrosion attack and the resulting coating breakdown are located in the same critical areas of the substrate. Multilayer 8 shows a surface characterized by the presence of cracks. The surface of the sandblasted finish substrate shows a great number of sharp holes and crests, which cannot be covered by the monolayer and Multilayer 4. The substrate working surface shows that localized corrosion tends to be widespread. This takes place next to the holes of the surface. As for the monolayer and Multilayer 4, corrosion attack and the resulting coating breakdown are localized in the same critical areas already observed in the substrate. Multilayer 8 shows localized corrosion attack as lighter compared to the previous coatings applied on this finish [
8].
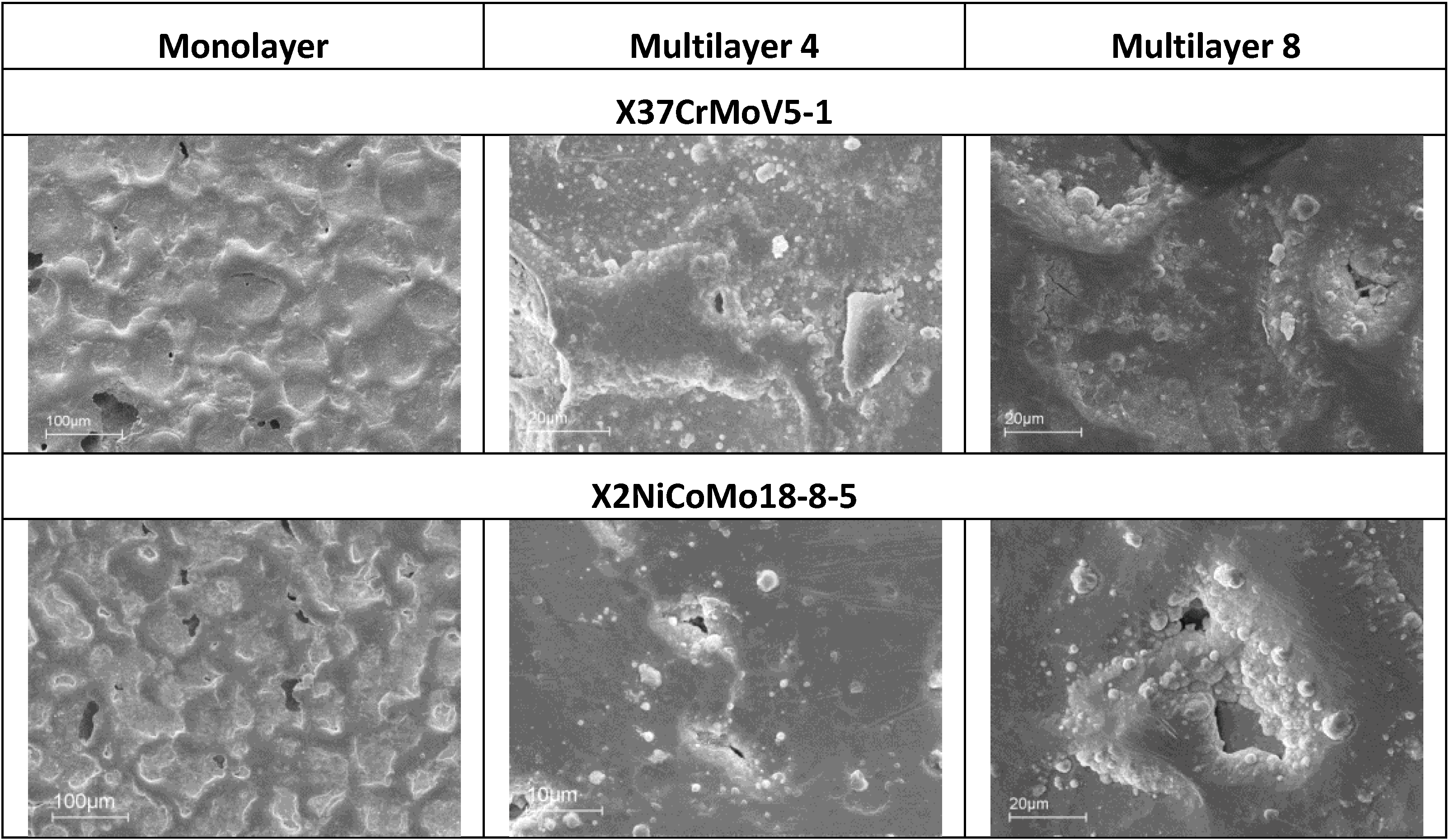
SEM analysis can help to understand differences in the corrosion observed during electrochemical characterization. For example, if electro-eroded surfaces are compared after polarization of X37CrMoV5-1 and X2NiCoMo18-8-5 steels, it is noticed that the surface of the Multilayer 8 coating on X37CrMoV5-1 steel is richer in cracks than the same coating on X2NiCoMo18-8-5 steel. That can explain why the polarization curves of X2NiCoMo18-8-5 coated with Multilayer 8 show a barrier effect, while the same polarization recorded on X37CrMoV5-1 coated with the same Multilayer 8 gives curves shifted to higher currents without the barrier effect (
Figure 12). The situation is different for these two steels with the Multilayer 4 coating, since with this coating, X37CrMoV5-1 steel shows a surface with a few spots of breakdown, while for X2NiVoMo18-8-5 steel, the number and extent of breakdown is higher. It is this difference that can explain why curves recorded on Multilayer 4-coated X37CrMoV5-1 steel shows a barrier effect, whereas the same polarization on X2NiCoMo18-8-5 coated with the same Multilayer 4 gives curves indicating the absence of protective actions (
Figure 12). Finally,
Figure 22 shows that both surfaces of X37CrMoV5-1 and X2NiCoMo18-8-5 steels coated with monolayers are extremely damaged. Accordingly, the respective polarization curves (
Figure 12) show the same active behavior typically of the absence of protection.
Figure 22.
SEM images of X37CrMoV5-1 and X2NiCoMo18-8-5 electro-eroded surfaces after polarization.
Figure 22.
SEM images of X37CrMoV5-1 and X2NiCoMo18-8-5 electro-eroded surfaces after polarization.