Atomic Layer Deposition of Zirconia on Cobalt–Chromium Alloys for Dental Prosthetics: Surface Functionalization Under MDR 2017/745
Abstract
1. Introduction
2. Materials and Methods
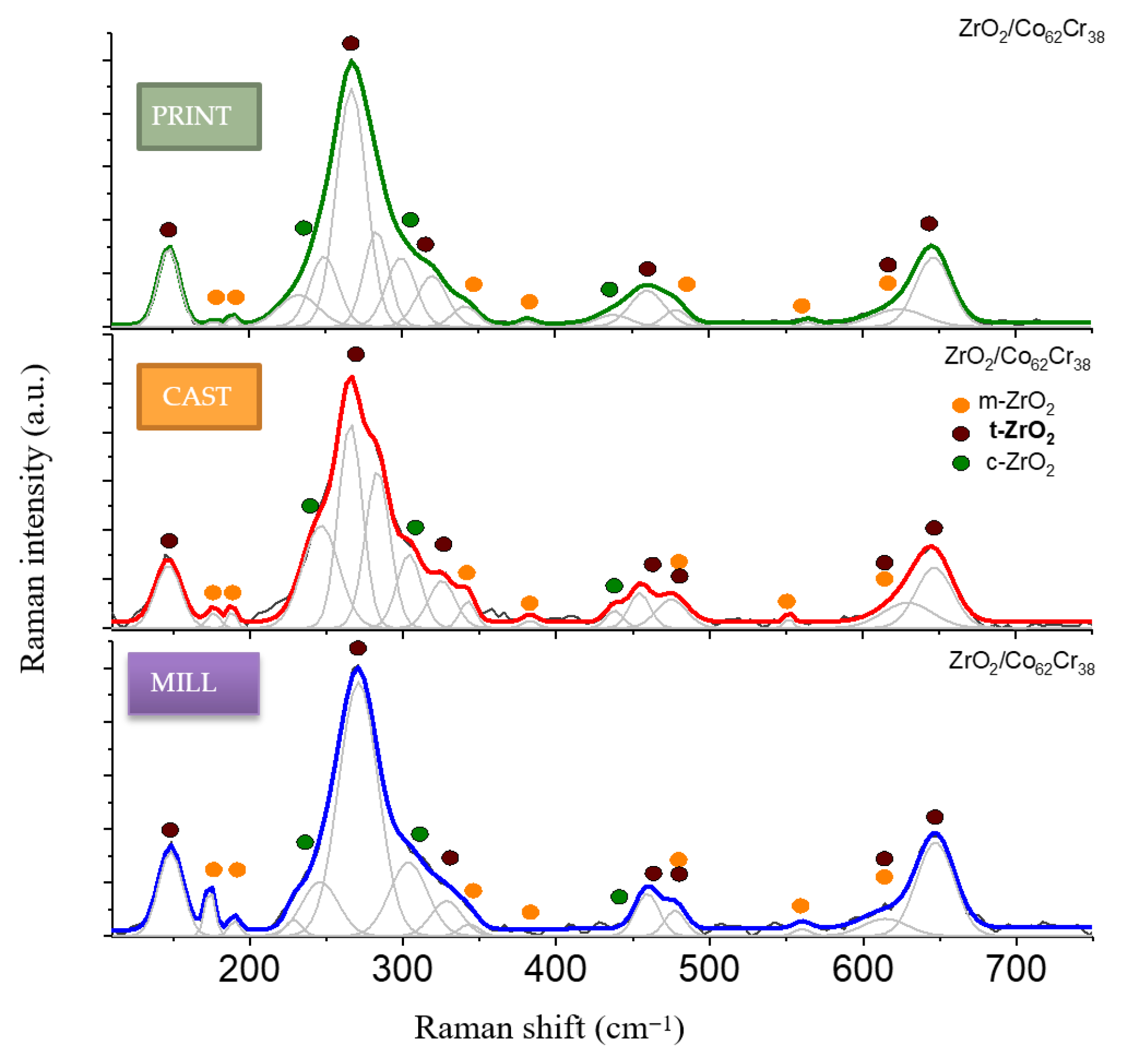
2.1. Phase Composition of the Surface Layer
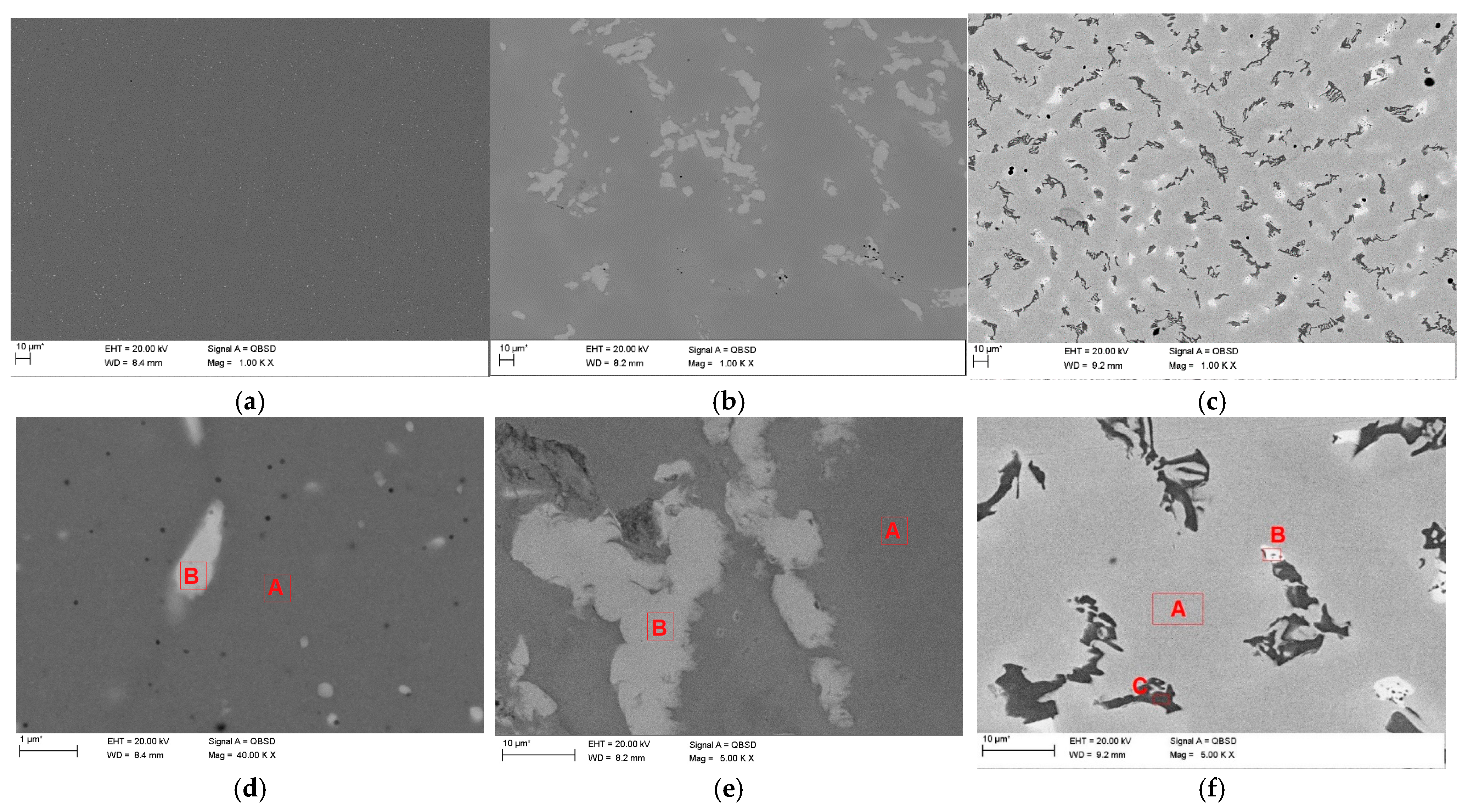
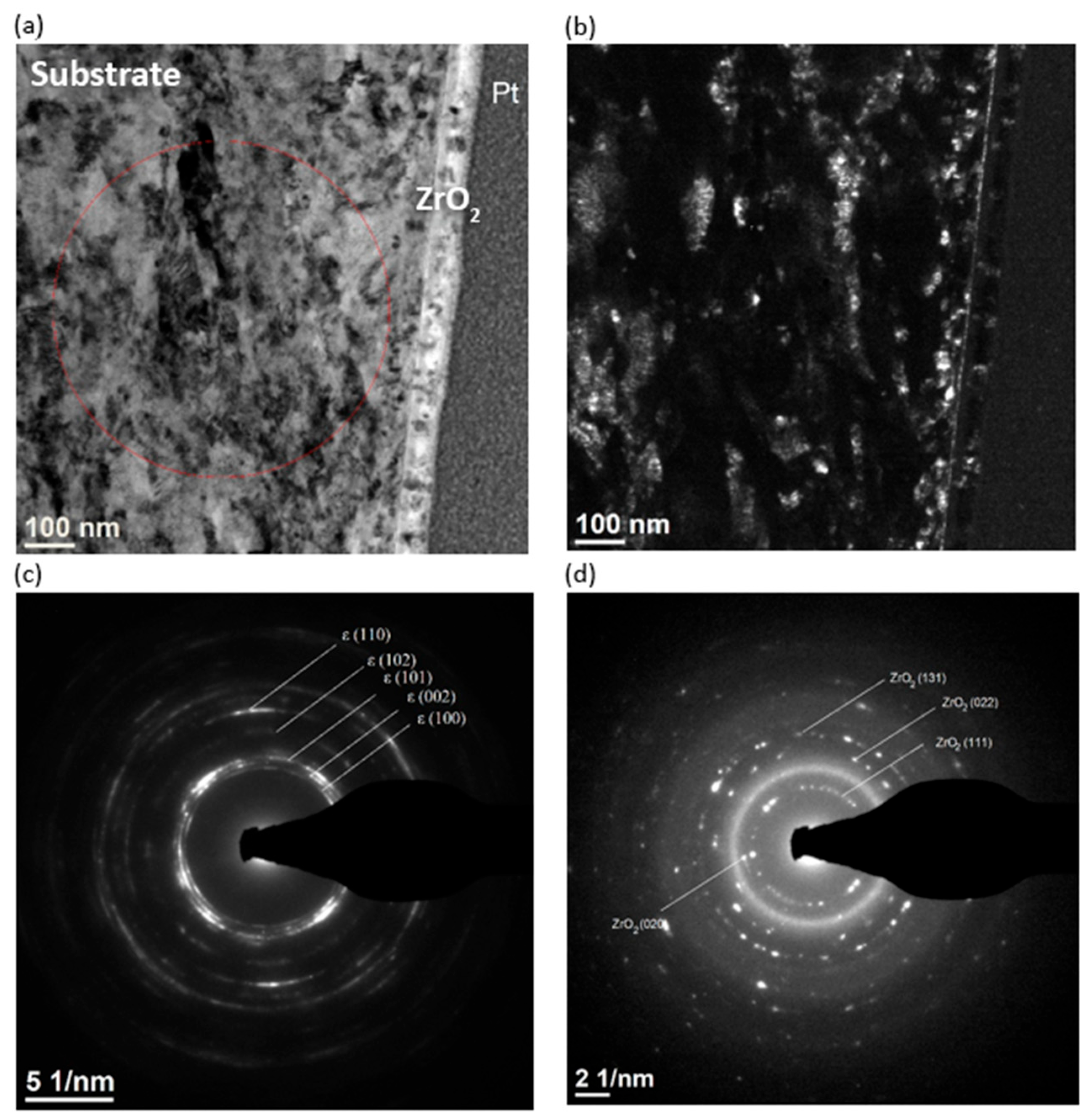
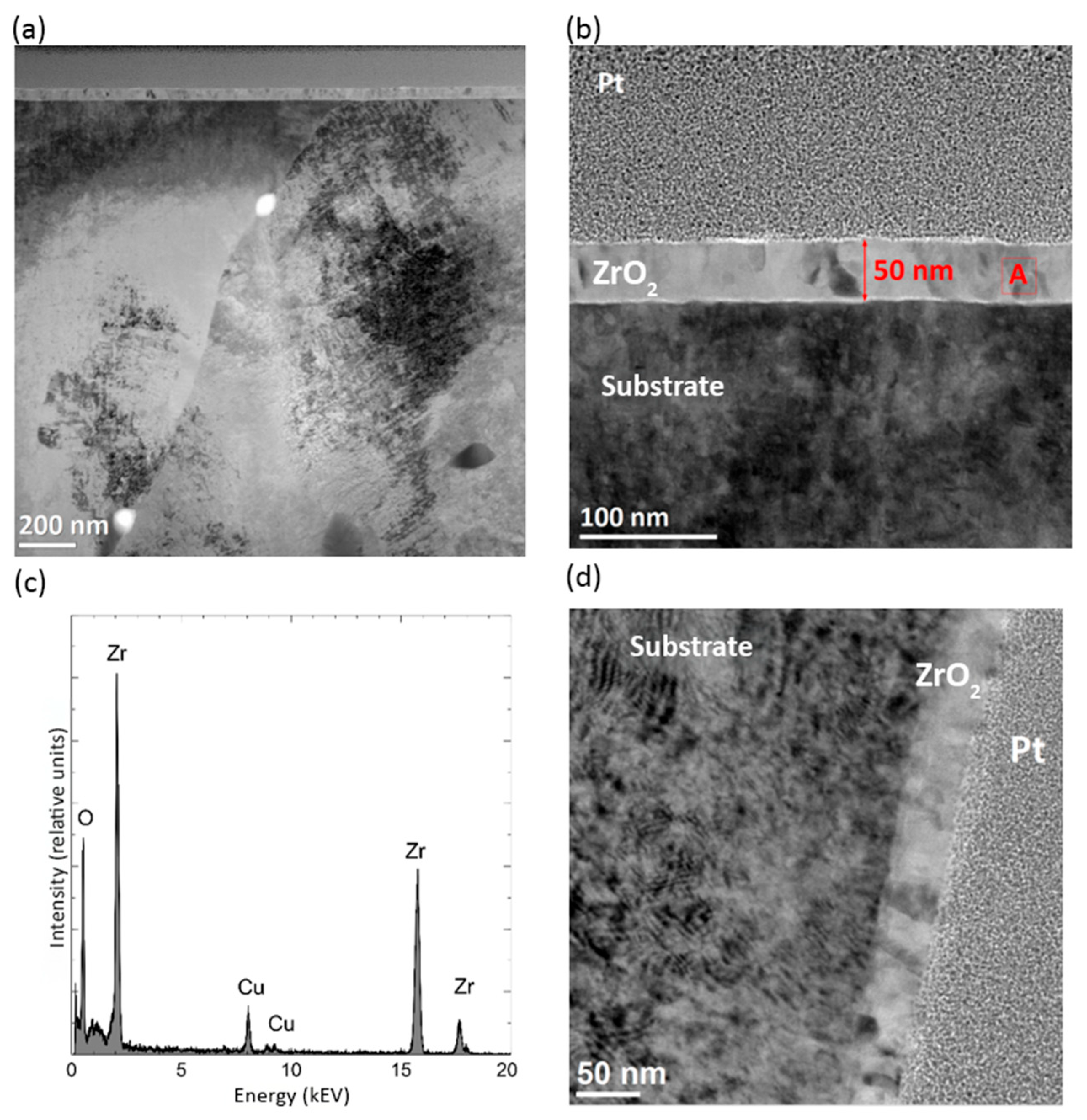
2.2. Microstructure of the Surface Layer
2.3. Wettability and Surface Energy
3. Results
3.1. Phase Composition of the Surface Layer
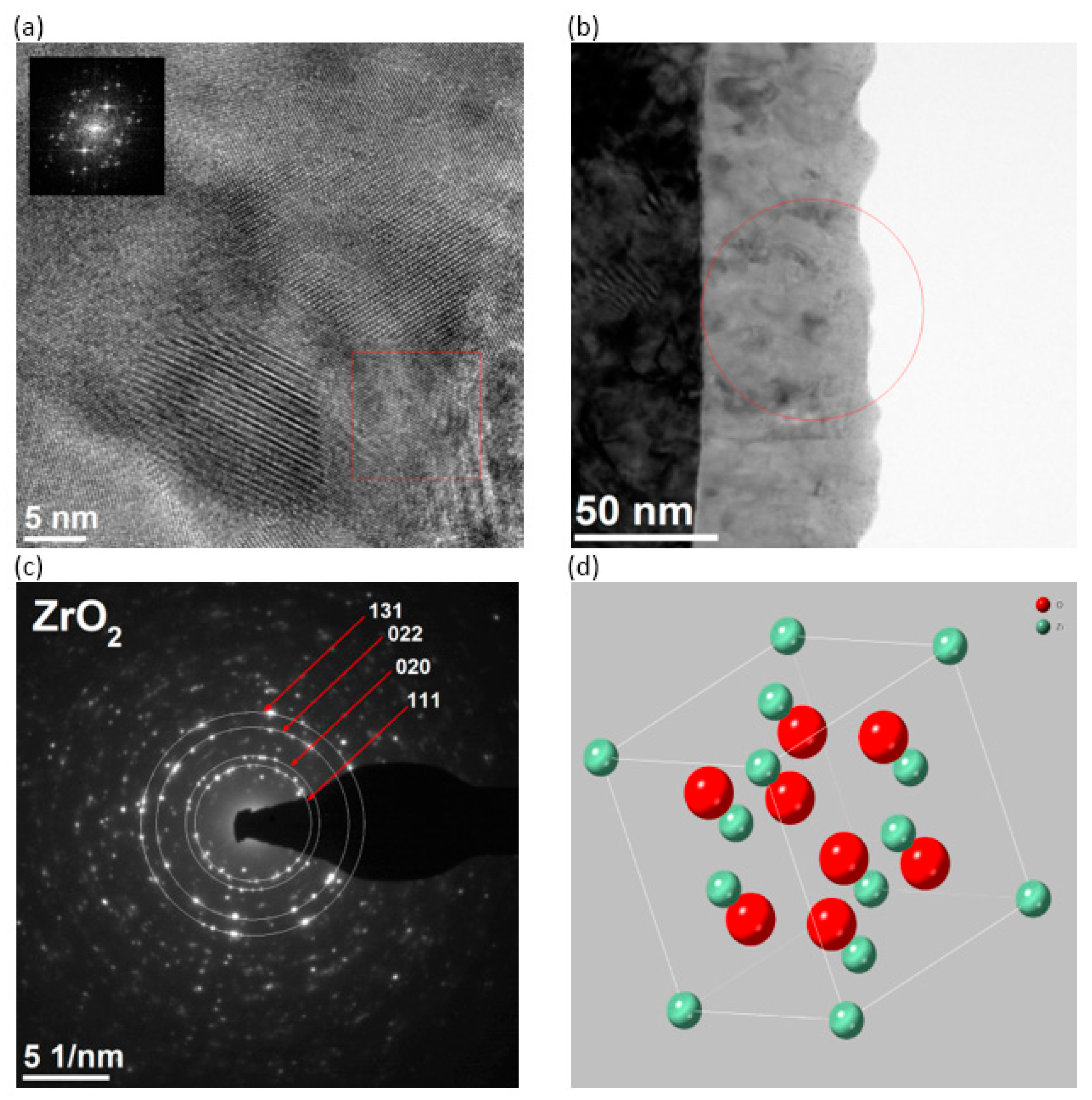
3.2. Microstructure of the Surface Layer
3.3. Wettability and Surface Energy
4. Discussion
- ➢
- Annex I, Chapter II—Risk Management: Reduced microbial adhesion lowers infection risk [16].;
- ➢
- Annex I, Chapter III—Performance Requirements: Coatings maintain structural integrity and do not interfere with the primary function;
- ➢
- Annex I, Chapter VI—Information Supplied: The applied coating process must be traceable and documented for conformity assessment;
- ➢
- Annex XIV, Clinical Evaluation—in that context (future studies required): In vitro test results support clinical data when coatings are used on proven materials (Co–Cr). Furthermore, according to MDCG 2020-6 [4], surface modifications (nano/coating) are considered "significant changes" and require separate technical documentation. This highlights the need for reproducible coating processes in accordance with ISO 13485 [36].
5. Conclusions
- For the selected cobalt–chromium alloys, slight differences in their chemical composition and substrate structure, additionally modified by surface treatment, do not significantly affect the final thickness and quality of the ZrO2 coating;
- The universal low-temperature process method used allows for obtaining a coating with comparable performance characteristics and biocompatibility and can be promoted for clinical applications in the production of new-generation removable prosthetic restorations in relation to the scenario assuming the implementation of high quality and safety standards for removable partial dentures, in the context of the use of CMR substances in the dental alloy and the applicable package of regulations (MDR, REACH, CLP) [2,5];
- ZrO2 coatings applied by the ALD method could be a potential method of surface modification for cobalt–chromium alloys, improving corrosion protection and creating an antifungal and bacterial barrier for removable partial dentures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:en:PDF (accessed on 12 August 2025).
- Regulation (EU) 2017/745 of The European Parliament and of The Council of 5 April 2017. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj/eng (accessed on 12 August 2025).
- Ziębowicz, A.; Woźniak, A.; Ziębowicz, B. The Influence of Technology on the Physicochemical and Electrochemical Properties of the Prosthetic Materials. In Proceedings of the Innovations in Biomedical Engineering IBE, Zabrze, Poland, 19–20 October 2017; Gzik, M., Tkacz, E., Paszenda, Z., Piętka, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 349–357. [Google Scholar]
- MDCG 2020-6 Medical Device Coordination Group Document. Regulation (EU) 2017/745: Clinical Evidence Needed for Medical Devices Previously CE Marked Under Directives 93/42/EEC or 90/385/EEC. Available online: https://health.ec.europa.eu/system/files/2020-09/md_mdcg_2020_6_guidance_sufficient_clinical_evidence_en_0.pdf (accessed on 12 August 2025).
- Available online: https://echa.europa.eu/en/home (accessed on 12 August 2025).
- Lucchetti, M.C.; Fratto, G.; Valeriani, F.; De Vittori, E.; Giampaoli, S.; Papetti, P.; Spica, V.R.; Manzon, L. Cobalt-chromium alloys in dentistry: An evaluation of metal ion release. J. Prosthet. Dent. 2015, 114, 602–608. [Google Scholar] [CrossRef]
- Shapiro, J.A.; Eskildsen, S.M.; Del Gaizo, D.J. Systemic cobaltism manifesting as oral mucosal discoloration and metallic gustation after metal-on-metal hip resurfacing. Arthroplast. Today 2018, 16, 436–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woźniak, A.; Adamiak, M. The Electrochemical Impedance Spectroscopy and wettability analysis of Ti6Al4V alloy modified by Atomic Layer Deposition method. Acta Phys. Pol. A 2022, 142, 88–91. [Google Scholar] [CrossRef]
- Walke, W. Surface Phenomena on Titanium Substrates with SiO2 Coating in the Circulatory System; Silesian University of Technology Publishing House: Gliwice, Poland, 2017. (In Polish) [Google Scholar]
- Liu, Z.; Zhu, Y.; Liu, X.; Yeung, K.W.K.; Wu, S. Construction of poly (vinyl alcohol)/poly (lactide-glycolide acid)/vancomycin nanoparticles on titanium for enhancing the surface self-antibacterial activity and cytocompatibility. Colloids Surf. B Biointerfaces 2017, 151, 165–177. [Google Scholar] [CrossRef]
- Huang, L.; Jing, S.; Zhuo, O.; Meng, X.; Wang, X. Surface hydrophilicity and antifungal properties of TiO2 films coated on a Co-Cr substrate. BioMed Res. Int. 2017, 2054723. [Google Scholar] [CrossRef]
- Mohseni, P.; Sou, A.; Ramos Chrcanovic, B. Clinical outcomes of zirconia implants: A systematic review and meta-analysis. Clin. Oral Investig. 2024, 28, 15. [Google Scholar] [CrossRef]
- Kui, A.; Manziuc, M.; Petruțiu, A.; Buduru, S.; Labuneț, A.; Negucioiu, M.; Chisnoiu, A. Translucent zirconia in fixed prosthodontics—An integrative overview. Biomedicines 2023, 11, 3116. [Google Scholar] [CrossRef]
- Uggowitzer, P.J.; Magdowski, R.; Speidel, M.O. Nickel free high nitrogen austenitic steels. ISIJ Int. 1996, 36, 901–908. [Google Scholar] [CrossRef]
- EOS. Instruction for Use & Technical Data EOS CobaltChrome RPD; EOS: Seattle, WA, USA, 2018. [Google Scholar]
- Ziębowicz, A.; Matus, K.; Pakieła, W.; Matula, G.; Pawlyta, M. Comparison of the crystal structure and wear resistance of Co-Based alloys with low carbon content manufactured by Selective Laser Sintering and Powder Injection Molding. Crystals 2020, 10, 197. [Google Scholar] [CrossRef]
- ISO 22674; Dentistry—Metallic Materials for Fixed and Removable Restorations and Appliances. International Organization for Standardization: Geneva, Switzerland, 2016.
- Ziębowicz, A.; Sambok-Kiełbowicz, A.; Walke, W.; Mzyk, A.; Kosiel, K.; Kubacki, J.; Bączkowski, B.; Pawlyta, M.; Ziębowicz, B. Evaluation of bacterial adhesion to the ZrO2 atomic layer deposited on the surface of cobalt-chromium dental alloy produced by DMLS method. Materials 2021, 14, 1079. [Google Scholar] [CrossRef]
- Xin, Q. Phase Transformation in the Surface Region of Zirconia Detected by UV Raman Spectroscopy. J. Phys. Chem. B 2001, 105, 8107–8111. [Google Scholar] [CrossRef]
- Shi, L.; Tin, K.; Wong, N. Tetragonal zirconia (t-ZrO2) begins to change into monoclinic. J. Mater. Sci. 1999, 34, 3367. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Pezzotti, G.; Porporati, A.A. Raman spectroscopic analysis of phase-transformation and stress patterns in zirconia hip joints. J.Biomed. Opt. 2004, 9, 372–384. [Google Scholar] [CrossRef]
- Clarke, D.R.; Adar, F. Measurement of the crystallographically transformed zone produced by fracture in ceramics containing tetragonal zirconia. J. Am. Ceram. Soc. 1982, 65, 284–288. [Google Scholar] [CrossRef]
- Gazzoli, D.; Mattei, G.; Valigi, M. Raman and X-ray investigations of the incorporation of Ca2+ and Cd2+ in the ZrO2 structure. J.Raman Spectrosc. 2007, 38, 824–831. [Google Scholar] [CrossRef]
- Chervin, C.N.; Clapsaddle, B.J.; Chiu, H.W.; Gash, A.E.; Satcher, J.H.; Kauzlarich, S.M. Aerogel synthesis of yttria-stabilized zirconia by a non-alkoxide sol-gel route. Chem. Mater. 2005, 17, 3345–3351. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Orkoula, M. Quantitative determination of the cubic, tetragonal and monoclinic phases in partially stabilized zirconias by Raman spectroscopy. J. Mater. Sci. 1994, 29, 5316–5320. [Google Scholar] [CrossRef]
- Pêcheur, D.; Lefebvre, F.; Motta, A.T.; Lemaignan, C.J.; Wadier, F. Precipitate evolution in the Zircaloy-4 oxide layer. J. Nucl. Mater. 1992, 189, 318–332. [Google Scholar] [CrossRef]
- Beltrán, J.; Hafner, J.; Muñoz, M.C. Structural, electronic and magnetic properties of the surfaces of tetragonal and cubic HfO2. New J. Phys. 2008, 10, 2–20. [Google Scholar] [CrossRef]
- Katz, G. Crystal Structure of ZrO2. J. Am. Ceram. Soc. 1971, 54, 531. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef]
- Pasich, E.; Walczewska, M.; Pasich, A.; Marcinkiewicz, J. Mechanism and risk factors of oral bacterial biofilm formation. Adv. Hyg. Exp. Med. 2013, 67, 736–741. (In Polish) [Google Scholar]
- Kozmos, M.; Virant, P.; Rojko, F.; Abram, A.; Rudolf, R.; Raspor, P.; Bohinc, A.; Klemen, Z. Bacterial adhesion of Streptococcus mutans to dental material surfaces. Molecules 2021, 26, 1152. [Google Scholar] [CrossRef] [PubMed]
- Hjerppe, J.; Rodas, S.; Korvala, J.; Pesonen, P.; Kaisanlahti, A.; Özcan, M.; Suojanen, J.; Reunanen, J. Surface roughness and Streptococcus mutans adhesion on metallic and ceramic fixed prosthodontic materials after scaling. Materials 2021, 14, 1027. [Google Scholar] [CrossRef]
- Boryło, P.; Łukaszkowicz, K. ZnO thin films prepared by Atomic Layer Deposition. Mater. Eng. Mater. Inźinierstvo 2017, 24, 21–25. [Google Scholar]
- Basiaga, M.; Walke, W.; Lisoń, J.; Taratuta, A.; Szewczenko, J.; Ziębowicz, B.; Szindler, M.; Dyner, A. Tin dioxide in terms of physical properties on steel AISI 316 LVM. Mater. Werkst. 2022, 54, 517–525. [Google Scholar] [CrossRef]
- ISO 13485; Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. International Organization for Standardization: Geneva, Switzerland, 2016.

| Type of Material | Co | Cr | Mo | W | Si |
|---|---|---|---|---|---|
| CAST substrate | 66 | 28 | 5 | - | 1 |
| CAST bright precipitates | 50 | 25 | 24 | - | 1 |
| CAST dark precipitates | 19 | 71 | 10 | - | - |
| MILL substrate | 58 | 27 | 5 | 11 | - |
| MILL bright precipitates | 44 | 26 | 12 | 18 | - |
| PRINT substrate | 62 | 26 | 5 | 7 | - |
| PRINT bright precipitates | 39 | 16 | 20 | 25 | - |
| Average Contact Angle θ, [o] | Surface Free Energy and Its Components, [mJ/m2] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distilled Water θw¬av | Diiodomethane θdav | γs | Polar γsp | Dispersive γsd | ||||||
| CAST | 71.21 | ±1.24 | 51.76 | ±0.24 | 36.28 | ±0.20 | 13.27 | ±0.33 | 23.01 | ±0.21 |
| CAST + ZrO2 | 100.31 | ±1.81 | 59.38 | ±0.22 | 31.43 | ±0.18 | 0.53 | ±0.04 | 30.91 | ±0.26 |
| MILL | 83.19 | ±1.82 | 58.65 | ±0.29 | 30.99 | ±0.17 | 7.49 | ±0.10 | 23.51 | ±0.22 |
| MILL + ZrO2 | 99.59 | ±1.45 | 57.45 | ±0.25 | 32.82 | ±0.16 | 0.57 | ±0.04 | 32.24 | ±0.31 |
| 62.38 | ±1.59 | 51.71 | ±0.27 | 34.08 | ±0.26 | 9.21 | ±0.17 | 25.36 | ±0.28 | |
| PRINT + ZrO2 | 94.75 | ±1.41 | 60.34 | ±0.22 | 29.23 | ±0.16 | 1.88 | ±0.12 | 27.36 | ±0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziębowicz, A.; Pawlyta, M. Atomic Layer Deposition of Zirconia on Cobalt–Chromium Alloys for Dental Prosthetics: Surface Functionalization Under MDR 2017/745. Coatings 2025, 15, 994. https://doi.org/10.3390/coatings15090994
Ziębowicz A, Pawlyta M. Atomic Layer Deposition of Zirconia on Cobalt–Chromium Alloys for Dental Prosthetics: Surface Functionalization Under MDR 2017/745. Coatings. 2025; 15(9):994. https://doi.org/10.3390/coatings15090994
Chicago/Turabian StyleZiębowicz, Anna, and Mirosława Pawlyta. 2025. "Atomic Layer Deposition of Zirconia on Cobalt–Chromium Alloys for Dental Prosthetics: Surface Functionalization Under MDR 2017/745" Coatings 15, no. 9: 994. https://doi.org/10.3390/coatings15090994
APA StyleZiębowicz, A., & Pawlyta, M. (2025). Atomic Layer Deposition of Zirconia on Cobalt–Chromium Alloys for Dental Prosthetics: Surface Functionalization Under MDR 2017/745. Coatings, 15(9), 994. https://doi.org/10.3390/coatings15090994


.jpg)





