Formation of Black Coatings on AA7075 and AA6061 by Low-Voltage Plasma Electrolytic Oxidation for Use as Flat Solar Absorbers in the Aerospace
Abstract
1. Introduction
2. Materials and Methods
2.1. PEO Experimental Condition
2.2. PEO Coatings Morphology, Phase, and Chemical Characterisation
2.3. Evaluation of Solar Absorptance ()
2.4. Thermal Shock Evaluation
2.5. Corrosion Resistance Evaluation
3. Results and Discussion
3.1. PEO Process—Cell Voltage
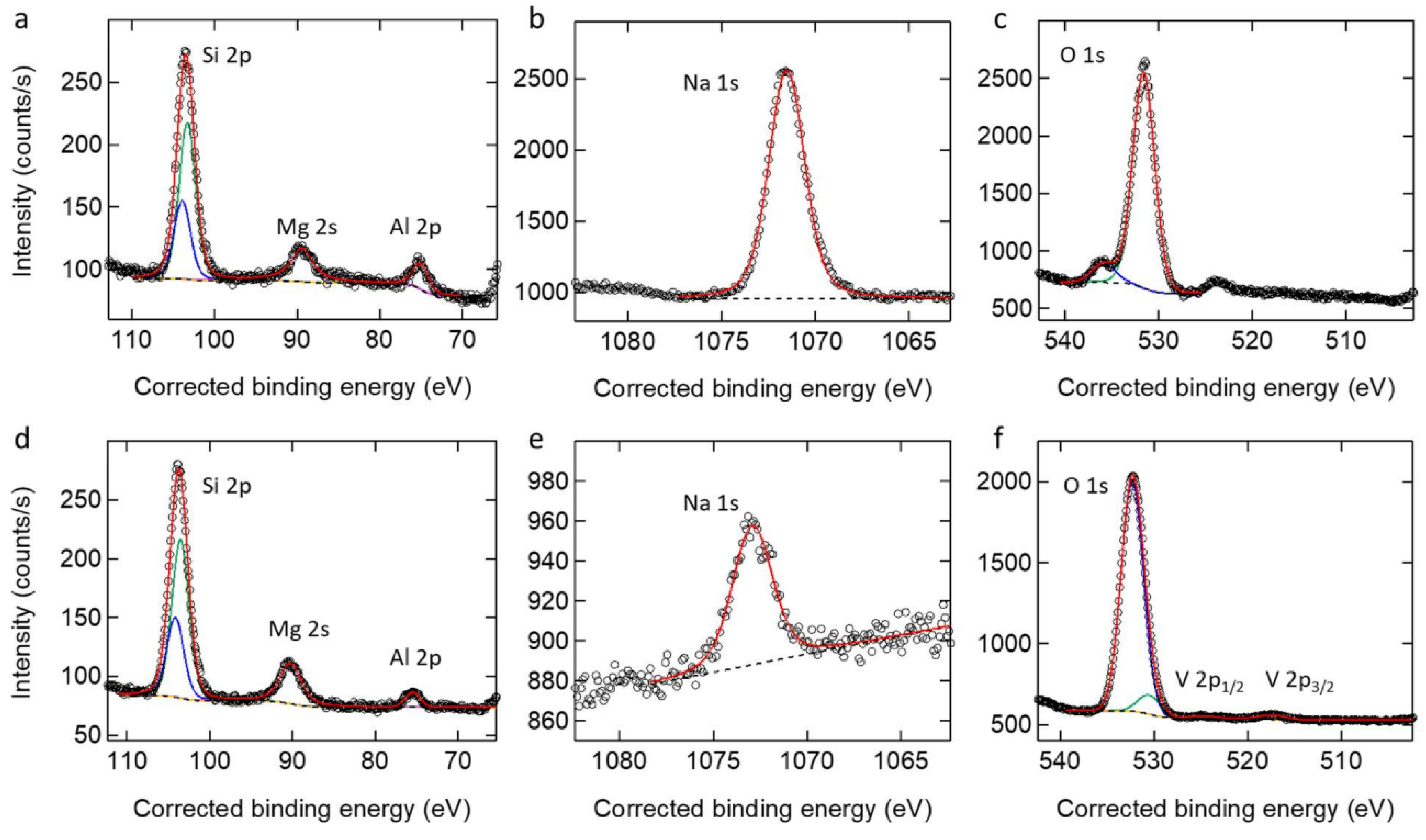
3.2. PEO Coatings Morphology, Chemical, and Phase Evaluation
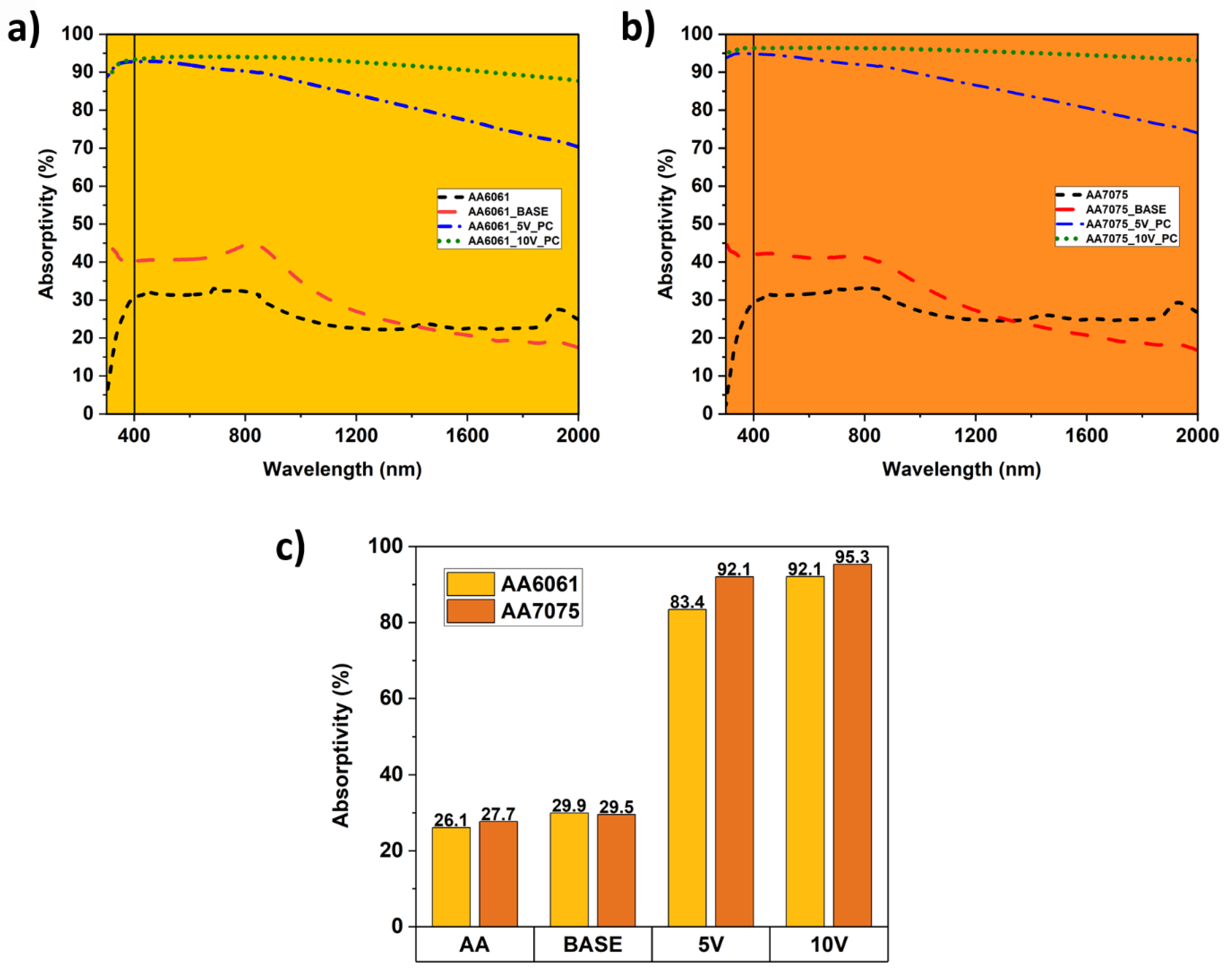
3.3. Optical Properties of the Coatings
3.4. Thermal Shocks Evaluation
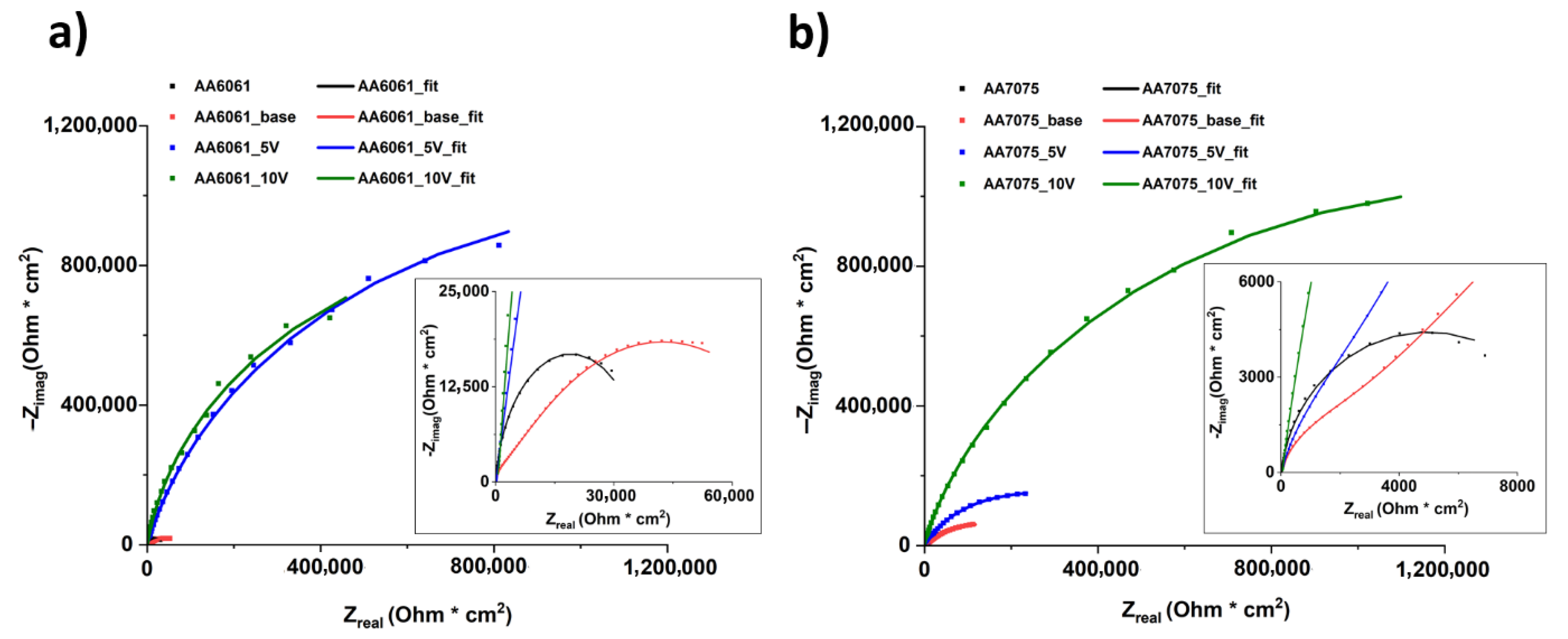
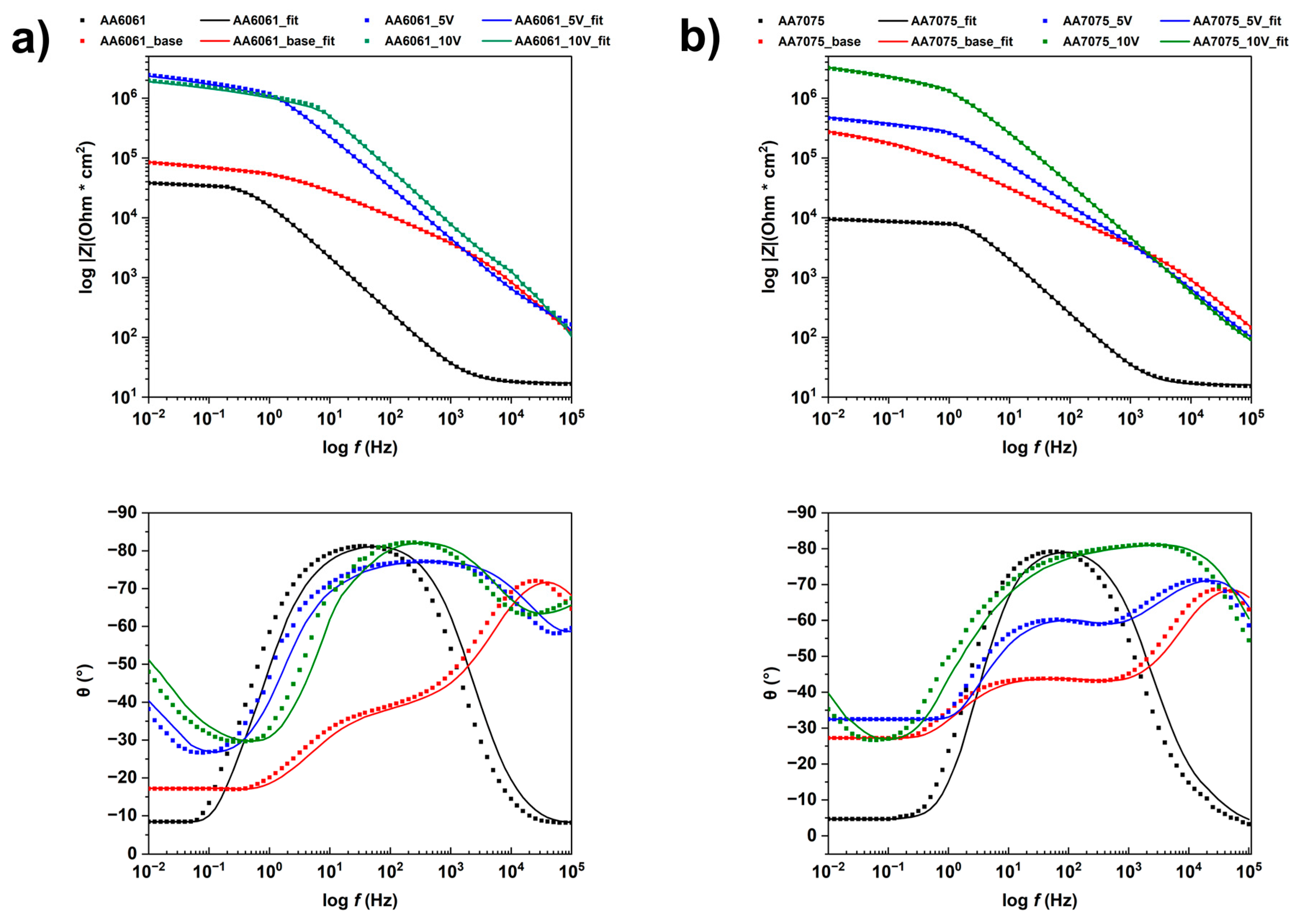
3.5. Corrosion Resistance Evaluation
4. Conclusions
- Vanadium was confirmed in all coatings where it was used as an additive in the PEO electrolyte by XPS, EDS, and XRD analyses.
- Produced black PEO coatings show excellent optical properties with a solar absorbance, , exceeding 83% for samples with vanadium additive, where the maximum of 95.3% is detected for the sample with 10 g/L of vanadium additive on AA7075 substrate.
- All PEO coatings, with and without an additive, have a characteristic porous PEO structure, with a larger pore size, as well as a higher thickness of the “base” coatings obtained by DC PEO mode.
- The coatings are mainly composed of aluminium and silicate oxides in amorphous or crystalline form, depending on the sample. Also, a higher degree of oxidation was confirmed by XPS and XRD analysis for samples on AA7075.
- After cycling at extreme temperature exposure in the thermal shock test, there were no visible signs of delamination, creaks, or any damage.
- All PEO coatings improve the corrosion resistance of the tested aluminium alloys by two or three orders of magnitude, with inner layer resistance, , in the MΩ· range.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Aluminium Alloy |
| PEO | Plasma Electrolytic Oxidation |
| SEM-EDS | Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction Spectroscopy |
| UV-VIS-NIR | Ultraviolet–Visible–Near-Infrared Spectroscopy |
| EIS | Electrochemical Impedance Spectroscopy |
| Solar Absorptance | |
| Thermal Emittance | |
| ISO/FEPA | International Organisation for Standardization/Federation of European Producers of Abrasives |
| DC | Direct Current |
| wt. % | Weight Percent |
| BE | Binding Energy |
| at. % | Atomic Percentages |
| Solar Spectrum | |
| SCE | Saturated Calomel Electrode |
| OCP | Open Circuit Potential |
| EEC | Equivalent Electrical Circuits |
| CPE | Constant Phase Element |
References
- Snizhko, L.O.; Yerokhin, A.L.; Pilkington, A.; Gurevina, N.L.; Misnyankin, D.O.; Leyland, A.; Matthews, A. Anodic Processes in Plasma Electrolytic Oxidation of Aluminium in Alkaline Solutions. Electrochim. Acta 2004, 49, 2085–2095. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A Review of Recent Work on Discharge Characteristics during Plasma Electrolytic Oxidation of Various Metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Mohedano, M.; Lopez, E.; Mingo, B.; Moon, S.; Matykina, E.; Arrabal, R. Energy Consumption, Wear and Corrosion of PEO Coatings on Preanodized Al Alloy: The Influence of Current and Frequency. J. Mater. Res. Technol. 2022, 21, 2061–2075. [Google Scholar] [CrossRef]
- Premchand, C.; Manojkumar, P.; Lokeshkumar, E.; Rama Krishna, L.; Ravisankar, B.; Rameshbabu, N. Surface Characteristics of AC PEO Coatings Fabricated on Commercial Al Alloys. Surf. Coat. Technol. 2022, 449, 128975. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Cao, G.; Chen, C.; Zhu, Y.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Zou, Y.; Ouyang, J.; et al. High Temperature Oxidation and Hot Corrosion Behaviors of PEO and PEO/Polysilazane Preceramic-Based Dual-Layer Coatings on Ti6Al4V Alloy. Corros. Sci. 2023, 216, 111076. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma Electrolytic Oxidation Coatings with Particle Additions—A Review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Ignjatović, S.; Blawert, C.; Serdechnova, M.; Karpushenkov, S.; Damjanović, M.; Karlova, P.; Wieland, D.C.F.; Starykevich, M.; Stojanović, S.; Damjanović-Vasilić, L.; et al. Formation of Multi-Functional TiO2 Surfaces on AA2024 Alloy Using Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2021, 544, 148875. [Google Scholar] [CrossRef]
- Magniez, L.; Tousch, C.D.S.; Fontana, S.; Cahen, S.; Martin, J.; Hérold, C.; Henrion, G. Plasma Electrolytic Oxidation of Aluminium in Electrolytes Containing Various Concentrations of Carbon Black Nanoparticles. Surf. Coat. Technol. 2023, 473, 129990. [Google Scholar] [CrossRef]
- Torskaya, E.; Shkalei, I.; Morozov, A.; Stepanov, F.; Malyshev, V.; Svistkov, A. Structure, Friction and Wear of AlZn5.5MgCu Based PEO Coatings Modified by Diamond Nanoparticles and Silver Micropowder. Tribol. Int. 2025, 203, 110417. [Google Scholar] [CrossRef]
- Shahri, Z.; Allahkaram, S.R.; Soltani, R.; Jafari, H. Modeling of PEO Coatings by Coupling an Artificial Neural Network and Taguchi Design of Experiment. J. Mater. Eng. Perform. 2024, 33, 7111–7122. [Google Scholar] [CrossRef]
- Tu, W.; Cheng, Y.; Wang, X.; Zhan, T.; Han, J.; Cheng, Y. Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy in Aluminate-Tungstate Electrolytes and the Coating Formation Mechanism. J. Alloys Compd. 2017, 725, 199–216. [Google Scholar] [CrossRef]
- Dorneles, M.T.; de Castro, V.V.; dos Santos, A.G.; Aguzzoli, C.; de Andrade, A.M.H.; Schroeder, R.M.; de Fraga Malfatti, C. Tribological and Corrosion Behavior of PEO Coatings on AA 2024-T3 Aluminum Alloy Obtained with Optimized Electrical Parameters under Low Current Densities. J. Braz. Soc. Mech. Sci. Eng. 2024, 46, 479. [Google Scholar] [CrossRef]
- Yeshmanova, G.; Blawert, C.; Serdechnova, M.; Wieland, D.C.F.; Starykevich, M.; Gazenbiller, E.; Höche, D.; Smagulov, D.; Zheludkevich, M.L. Effect of Electrolyte Composition on the Formation of PEO Coatings on AA2024 Aluminium Alloy. Surf. Interfaces 2024, 44, 103797. [Google Scholar] [CrossRef]
- del Olmo, R.; López, E.; Matykina, E.; Tiringer, U.; Mol, J.M.C.; Mohedano, M.; Arrabal, R. Hybrid PEO/Sol-Gel Coatings Loaded with Ce for Corrosion Protection of AA2024-T3. Prog. Org. Coat. 2023, 182, 107667. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Asghar, H.; Maurino, V.; Matykina, E.; Arrabal, R.; Mohedano, M. Evaluating the Energy Consumption, Structural, and Corrosion Resistance Properties of Photocatalytic TiO2-Based PEO Coatings on Pre-Anodized AA2024-Al. Surf. Interfaces 2024, 44, 103659. [Google Scholar] [CrossRef]
- Mohedano, M.; Mingo, B.; Mora-Sánchez, H.; Matykina, E.; Arrabal, R. Effects of Pre-Anodizing and Phosphates on Energy Consumption and Corrosion Performance of PEO Coatings on AA6082. Surf. Coat. Technol. 2021, 409, 126892. [Google Scholar] [CrossRef]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K.; Bera, P.; Poornima, S.; Sampath, S. Development of Vanadium Impregnated Flat Absorber Composite PEO Coating on AA6061 Alloy. Surf. Coat. Technol. 2021, 410, 126891. [Google Scholar] [CrossRef]
- Yao, R.; Yao, Z.; Li, Y.; Zhang, P.; Lu, S.; Wu, X. Preparation of Black Thermal Control PEO Coatings on TC4 with Enhanced Electrical Conductivity by Gas Thermal Penetration Post-Treatment. Surf. Coat. Technol. 2023, 454, 129152. [Google Scholar] [CrossRef]
- Goueffon, Y.; Arurault, L.; Mabru, C.; Tonon, C.; Guigue, P. Black Anodic Coatings for Space Applications: Study of the Process Parameters, Characteristics and Mechanical Properties. J. Mater. Process Technol. 2009, 209, 5145–5151. [Google Scholar] [CrossRef]
- Sharma, A.K. Surface Engineering for Thermal Control of Spacecraft. Surf. Eng. 2005, 21, 249–253. [Google Scholar] [CrossRef]
- Madhuri, D.; Ghosh, R.; Hasan, M.A.; Dey, A.; Pillai, A.M.; Angamuthu, M.; Anantharaju, K.S.; Rajendra, A. Flat Absorber Black PEO Coatings on Ti6Al4V for Spacecraft Thermal Control Application. Ceram. Int. 2022, 48, 35906–35914. [Google Scholar] [CrossRef]
- Liang, J.; Peng, Z.; Li, R.; Wang, B. Preparation of White ZrO2 Coating with Low Solar Absorptance on Aluminum Alloy by Plasma Electrolytic Oxidation. Ceram. Int. 2023, 49, 29133–29140. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, Z.; Zhuang, X.; Cheng, Y.; Skeldon, P. Effect of Frequency on Black Coating Formation on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation in Aluminate-Tungstate Electrolyte. Surf. Coat. Technol. 2019, 372, 34–44. [Google Scholar] [CrossRef]
- Nagumothu, R.B.; Thangavelu, A.; Nair, A.M.; Sukumaran, A.; Anjilivelil, T. Development of Black Corrosion-Resistant Ceramic Oxide Coatings on AA7075 by Plasma Electrolytic Oxidation. Trans. Indian Inst. Met. 2019, 72, 47–53. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Rama Krishna, L.; Anoop, S.; Uma Rani, R.; Rameshbabu, N. Fabrication of Multifunctional Black PEO Coatings on AA7075 for Spacecraft Applications. Surf. Coat. Technol. 2016, 307, 735–746. [Google Scholar] [CrossRef]
- Pan, J.; Wen, Y.; Wang, L.; Wu, Z.; Dong, H.; Ye, Z. Doping and Defects: The Coloring Mechanism of Black Plasma Electrolytic Oxidation (PEO) Films on Aluminum Alloys. Surf. Coat. Technol. 2022, 431, 128035. [Google Scholar] [CrossRef]
- Liang, J.; Peng, Z.; Cui, X.; Li, R.; Wang, B. Characterization of V-Containing Black Plasma Electrolytic Oxide Coatings on Aluminium Alloy: Impact of Base Electrolytes. J. Mater. Res. Technol. 2024, 30, 110–119. [Google Scholar] [CrossRef]
- Yao, Z.; Li, X.; Wei, H.; Xia, Q.; Wang, Y.; Li, D.; Jiang, Z. Black Ceramic Coatings on Ti Alloy with Enhanced High Absorptivity and High Emissivity by Plasma Electrolytic Oxidation. Int. J. Appl. Ceram. Technol. 2019, 16, 994–1003. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Van Tuan, T.; Khabibullina, Z.V.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Zheludkevich, M.L.; Gladkova, A.A. Role of Cobalt Additive on Formation and Anticorrosion Properties of PEO Coatings on AA2024 Alloy in Alkali-Silicate Electrolyte. Surf. Coat. Technol. 2022, 433, 128075. [Google Scholar] [CrossRef]
- Aliramezani, R.; Raeissi, K.; Santamaria, M.; Hakimizad, A. Characterization and Properties of PEO Coatings on 7075 Al Alloy Grown in Alkaline Silicate Electrolyte Containing KMnO4 Additive. Surf. Coat. Technol. 2017, 329, 250–261. [Google Scholar] [CrossRef]
- Husak, Y.; Olszaniecki, J.; Pykacz, J.; Ossowska, A.; Blacha-Grzechnik, A.; Waloszczyk, N.; Babilas, D.; Korniienko, V.; Varava, Y.; Diedkova, K.; et al. Influence of Silver Nanoparticles Addition on Antibacterial Properties of PEO Coatings Formed on Magnesium. Appl. Surf. Sci. 2024, 654, 159387. [Google Scholar] [CrossRef]
- Pillai, A.M.; Ghosh, R.; Dey, A.; Prajwal, K.; Rajendra, A.; Sharma, A.K.; Sampath, S. Crystalline and Amorphous PEO Based Ceramic Coatings on AA6061: Nanoindentation and Corrosion Studies. Ceram. Int. 2021, 47, 14707–14716. [Google Scholar] [CrossRef]
- Hao, Y.; Ye, Z.; Ye, M.; Dong, H.; Wang, L.; Du, Y. Construction and Growth of Black PEO Coatings on Aluminum Alloys for Enhanced Wear and Impact Resistance. Ceram. Int. 2023, 49, 30782–30793. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Liu, Z.; Xu, D.; Dong, K.; Han, E.H. Optimization of Thermal Control and Corrosion Resistance of PEO Coatings on 7075 Aluminum Alloy by Frequency Alteration. Surf. Coat. Technol. 2022, 446, 128797. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Wang, Q.; Lu, S. Optimal of Black Anti-Static AZO/Al2O3-SiO2-VxOy Coatings for Spacecraft Stray Light on High Silicon Aluminum Alloys. Curr. Appl. Phys. 2023, 55, 44–52. [Google Scholar] [CrossRef]
- Kostelac, L.; Pezzato, L.; Colusso, E.; Natile, M.M.; Brunelli, K.; Dabalà, M. Black PEO Coatings on Titanium and Titanium Alloys Produced at Low Current Densities. Appl. Sci. 2023, 13, 12280. [Google Scholar] [CrossRef]
- Yao, R.; Li, Y.; Yao, Z.; Zhang, P.; Lu, S.; Wu, X. Black PEO Coating with Enhanced Thermal Stability on Titanium Alloy and Its Thermal Control Properties. Surf. Coat. Technol. 2022, 429, 127934. [Google Scholar] [CrossRef]
- Dilimon, V.S.; Shibli, S.M.A. A Review on the Application-Focused Assessment of Plasma Electrolytic Oxidation (PEO) Coatings Using Electrochemical Impedance Spectroscopy. Adv. Eng. Mater. 2023, 25, 2201796. [Google Scholar] [CrossRef]
- Dehnavi, V.; Shoesmith, D.W.; Luan, B.L.; Yari, M.; Liu, X.Y.; Rohani, S. Corrosion Properties of Plasma Electrolytic Oxidation Coatings on an Aluminium Alloy—The Effect of the PEO Process Stage. Mater. Chem. Phys. 2015, 161, 49–58. [Google Scholar] [CrossRef]
- Settimi, A.G.; Pezzato, L.; Longato, A.; Brunelli, K.; Martucci, A.; Gross, S.; Dabalà, M. Photoluminescent Plasma Electrolytic Oxidation Coatings Containing YAG: Ce Produced on 1050 Aluminum Alloy. J. Mater. Eng. Perform. 2023, 32, 5967–5979. [Google Scholar] [CrossRef]
- Mojsilović, K.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Stojadinović, S.; Vasilić, R. In-Situ Incorporation of LDH Particles during PEO Processing of Aluminium Alloy AA2024. Appl. Surf. Sci. 2024, 654, 159450. [Google Scholar] [CrossRef]
- Moga, S.G.; Negrea, D.A.; Ducu, C.M.; Malinovschi, V.; Schiopu, A.G.; Coaca, E.; Patrascu, I. The Influence of Processing Time on Morphology, Structure and Functional Properties of PEO Coatings on AZ63 Magnesium Alloy. Appl. Sci. 2022, 12, 12848. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, W.; Liu, H.; Liu, L.; Wang, F.; Qiao, Z. Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges. Materials 2022, 15, 4635. [Google Scholar] [CrossRef]
- Barr, T.L. An XPS study of Si as it occurs in adsorbents, catalysts, and thin films. Appl. Surf. Sci. 1983, 15, 1–35. [Google Scholar] [CrossRef]
- Hartney, M.A.; Chiang, J.N.; Hess, D.W.; Soane, D.S. Oxide Formation during Plasma Etching of Silicon-Containing Resists. Appl. Phys. Lett. 1989, 54, 1510–1512. [Google Scholar] [CrossRef]
- Derek Haycock, B.E.; Nicholls, C.J.; Urch, D.S.; Webber, M.J.; Wiech, G. The electronic structure of magnesium dialuminium tetraoxide (spinel) using X-ray emission and X-ray photoelectron spectroscopies. J. Chem. Soc. 1978, 12, 1785–1790. [Google Scholar] [CrossRef]
- Hughes, A.E.; Hedges, M.M.; Sexton, B.A. Reactions at the Al/SiO2/SiC layered interface. J. Mater. Sci. 1990, 25, 4856–4865. [Google Scholar] [CrossRef]
- Wagner, C.D. Chemical shifts of Auger lines, and the Auger parameter. Faraday Discuss. 1975, 60, 291–300. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M. Bonding-state characterization of the constituent elements of silicate minerals by X-ray photoelectron spectroscopy. J. Chem. Soc. Faraday Trans. 1 1985, 81, 485–495. [Google Scholar] [CrossRef]
- Kovacich, J.A.; Lichtman, D. A qualitative and quantitative study of the oxides of aluminium and silicon using AES and XPS. J. Electron Spectrosc. Relat. Phenom. 1985, 35, 7–18. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Firsov, M.N.; Shaplygin, I.S. Electronic structures of MRhO2, MRh2O4, RhMO4 and Rh2MO6 on the basis of X-ray spectroscopy and ESCA data. J. Electron Spectrosc. Relat. Phenom. 1982, 26, 65–78. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Arrua, L.A.; Nieto, J.M.L.; Kremenic, G. Surface properties of Coprecipitated VTiO catalysts and their relation to the selectiveoxidation of isobutene. Appl. Catal. 1988, 37, 323–338. [Google Scholar] [CrossRef]
- Bond, G.C.; Flamerz, S. Structure and reactivity of titania-supported oxides: IV. Characterisation of dried vanadia/titania catalyst precursors. Appl. Catal. 1989, 46, 89–102. [Google Scholar] [CrossRef]
- Lee, A.Y.; Powell, C.J.; Gorham, J.M.; Morey, A.; Scott, J.H.J.; Hanisch, R.J. Development of the NIST X-Ray Photoelectron Spectroscopy (XPS) Database, Version 5. Data Sci. J. 2024, 23, 45. [Google Scholar] [CrossRef]
- Jaleh, B.; Nasri, A.; Chaharmahali, R.; Kaseem, M.; Fattah-alhosseini, A. Exploring Wear, Corrosion, and Microstructure in PEO Coatings via Laser Surface Treatments on Aluminum Substrates. Opt. Laser Technol. 2025, 181, 111958. [Google Scholar] [CrossRef]
- Yang, D.; Gerelt Od, D.; Ramu, A.G.; Choi, D. Fabrication of Enhanced Corrosion Protection of PEO/PFOTES Nanocomposite Film Coatings on Aluminum Alloy Deposited by Plasma Electrolytic Oxidation. Mater. Lett. 2022, 315, 131898. [Google Scholar] [CrossRef]
- Nadimi, M.; Dehghanian, C.; Etemadmoghadam, A. Influence of SiO2 Nanoparticles Incorporating into Ceramic Coatings Generated by PEO on Aluminium Alloy: Morphology, Adhesion, Corrosion, and Wear Resistance. Mater. Today Commun. 2022, 31, 103587. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Raeissi, K.; Ashrafizadeh, F.; Hakimizad, A.; Santamaria, M.; Lampke, T. Silicate and Hydroxide Concentration Influencing the Properties of Composite Al2O3-TiO2 PEO Coatings on AA7075 Alloy. Coatings 2022, 12, 33. [Google Scholar] [CrossRef]
- Pezzato, L.; Kostelac, L.; Tonelli, L.; Elsayed, H.; Kajánek, D.; Bernardo, E.; Martini, C.; Dabalà, M.; Brunelli, K. Effect of Different Types of Glass Powders on the Corrosion and Wear Resistance of Peo Coatings Produced on 6061 Aluminum Alloy. Met. Mater. Int. 2024, 31, 636–653. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, J.; Gong, Y.; Zhao, D.; Zhang, H.; Chen, D.; Wang, M.; Wang, H. Insight into Continuous Growth Mechanisms of ZrO2-Al2O3 Composite PEO Coatings with Superior Cavitation Erosion Resistance. Surf. Coat. Technol. 2024, 477, 130355. [Google Scholar] [CrossRef]







| Substrate | Cell Voltage (V) | Electrolyte (Base) | Electrolyte (Additive) | Solar Absorptance | Thermal Emittance | Ref. |
|---|---|---|---|---|---|---|
| AA6061 | >500 |
|
| 0.92 | 0.88 | [17] |
| AA6061 | >450 |
|
| 0.93 | - | [33] |
| AA6061 | >450 |
|
| 0.895 0.915 | 0.81 0.83 | [27] |
| AA7075 | >500 |
|
| 0.9 | 0.79 | [25] |
| AA7075 | >450 |
|
| - | - | [30] |
| AA7075 | >450 |
|
| - | 0.85 | [34] |
| Element (wt. %) | Al | Mg | Si | Cu | Mn | Fe | Cr | Zn |
|---|---|---|---|---|---|---|---|---|
| AA6061 | Bal. | 0.9 | 0.7 | - | 0.05 | 0.6 | 0.25 | 0.20 |
| AA7075 | Bal. | 2.5 | 0.08 | 1.5 | 0.04 | 0.3 | 0.18 | 5.6 |
| Substrate | Electrolyte (Base) | Electrolyte (Additive) | Electrical Mode | Current Density (A/cm2) | Frequency (Hz) | Duty Cycle (%) | Time (min) |
|---|---|---|---|---|---|---|---|
| AA6061 base | 2 g/L ·9 2 g/L NaOH | - | DC | 0.2 | - | - | 10 |
| AA6061 5V ** | 2 g/L ·9 2 g/L NaOH | 5 g/L | DC | 0.2 | - | - | 10 |
| AA6061 base * | 2 g/L ·9 2 g/L NaOH | - | pulsed | 0.8 | 500 | 50 | 8 |
| AA6061 5V | 2 g/L ·9 2 g/L NaOH | 5 g/L | pulsed | 0.8 | 500 | 50 | 8 |
| AA6061 10V | 2 g/L ·9 2 g/L NaOH | 10 g/L | pulsed | 0.8 | 500 | 50 | 8 |
| AA7075 base | 2 g/L ·9 2 g/L NaOH | - | DC | 0.2 | - | - | 10 |
| AA7075 5V ** | 2 g/L ·9 2 g/L NaOH | 5 g/L | DC | 0.2 | - | - | 10 |
| AA7075 base * | 2 g/L ·9 2 g/L NaOH | - | pulsed | 0.8 | 500 | 50 | 8 |
| AA7075 5V | 2 g/L ·9 2 g/L NaOH | 5 g/L | pulsed | 0.8 | 500 | 50 | 8 |
| AA7075 10V | 2 g/L ·9 2 g/L NaOH | 10 g/L | pulsed | 0.8 | 500 | 50 | 8 |
| AA6061 Base | AA6061 5V | AA6061 10V | AA7075 Base | AA7075 5V | AA7075 10V | |
|---|---|---|---|---|---|---|
| Thickness (µm) | 36.1 ± 2.1 | 15.9 ± 1.0 | 12.9 ± 0.7 | 81.6 ± 1.7 | 8.2 ± 0.5 | 16.3 ± 1.0 |
| At. % | AA6061 Base | AA6061 5V | AA6061 10V | AA7075 Base | AA7075 5V | AA7075 10V |
|---|---|---|---|---|---|---|
| O | 64.13 | 58.58 | 57.56 | 64.92 | 56.03 | 58.26 |
| Na | 1.91 | 0.27 | 0.13 | 2.11 | 1.07 | 0.53 |
| Si | 23.08 | 19.47 | 18.26 | 21.37 | 21.35 | 18.77 |
| Al | 10.51 | 19.25 | 18.92 | 11.89 | 18.68 | 17.49 |
| Mg | 0.37 | 0.26 | 0.12 | 0.60 | 0.31 | 0.30 |
| V | - | 2.17 | 5.01 | - | 2.55 | 4.65 |
| At. % | AA6061 Base | AA6061 10V | AA7075 Base | AA7075 10V |
|---|---|---|---|---|
| O | 63.97 | 71.63 | 55.42 | 69.46 |
| Na | 10.18 | 2.56 | 27.07 | 2.08 |
| Si | 16.82 | 18.09 | 10.91 | 18.43 |
| Al | 3.52 | 1.83 | 1.60 | 1.30 |
| Mg | 5.52 | 4.58 | 5.01 | 8.14 |
| V | - | 1.31 | - | 0.60 |
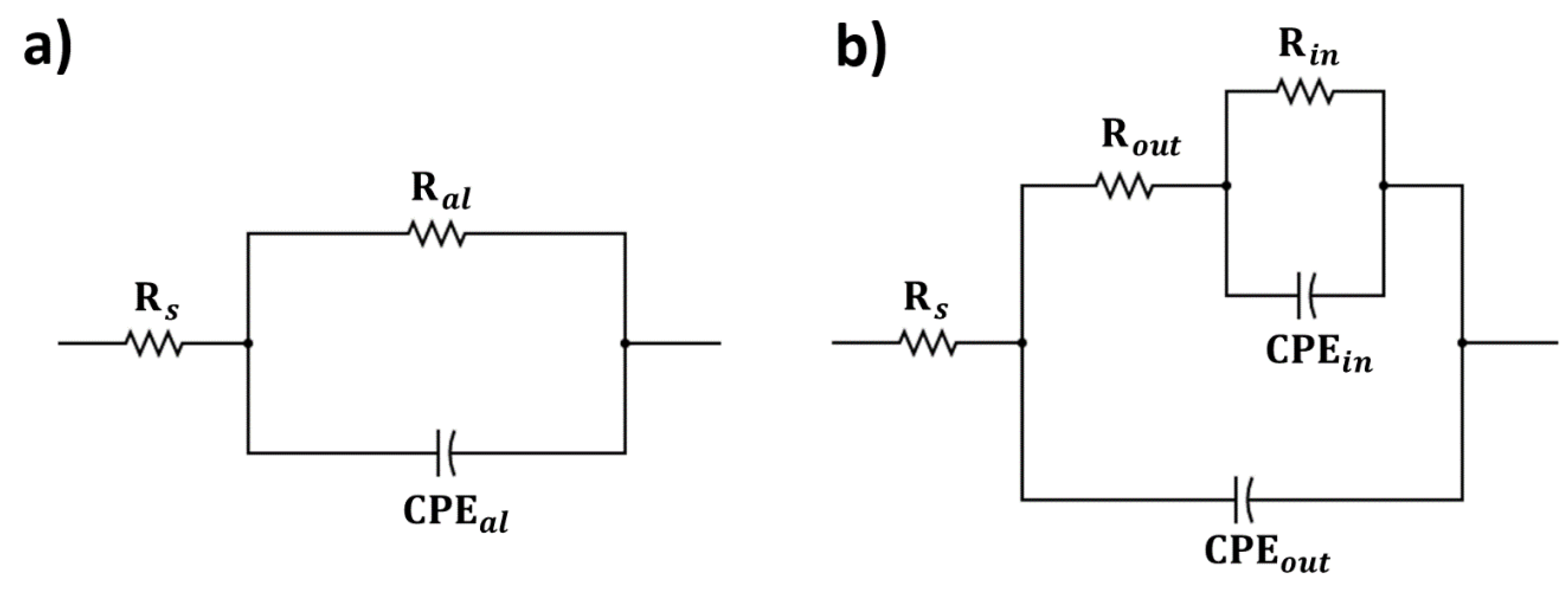
| AA 6061 | AA 6061 Base | AA 6061 5V | AA 6061 10V | AA 7075 | AA 7075 Base | AA 7075 5V | AA 7075 10V | |
|---|---|---|---|---|---|---|---|---|
| (Ω) | 17.0 | 32.9 | 30.6 | 122.6 | 16.1 | 18.4 | 33.2 | 45.2 |
| (kΩ) | 38.1 | - | - | - | 9.5 | - | - | - |
| ) | 10.2 | - | - | - | 1.0 | - | - | - |
| 0.92 | - | - | - | 0.93 | - | - | - | |
| (kΩ) | - | 3.2 | 1.0 | 4.7 | - | 3.0 | 6.6 | 215.0 |
| - | 47.3 | 117.4 | 36.1 | - | 64.1 | 98.6 | 73.0 | |
| - | 0.91 | 0.83 | 0.89 | - | 0.87 | 0.87 | 0.91 | |
| (kΩ) | - | 84.1 | 2446.0 | 1967.0 | - | 270.6 | 463.4 | 3190.0 |
| - | 3570 | 13.3 | 7.6 | - | 3220 | 552 | 109 | |
| - | 0.51 | 0.97 | 0.98 | - | 0.54 | 0.68 | 0.53 | |
| 18.9 | 1.1 | 3.4 | 7.3 | 21.9 | 2.5 | 1.3 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostelac, L.; Piccinotti, A.; Pezzato, L.; Colusso, E.; Pigato, M.; Pagot, G.; Di Noto, V.; Dabalà, M.; Brunelli, K. Formation of Black Coatings on AA7075 and AA6061 by Low-Voltage Plasma Electrolytic Oxidation for Use as Flat Solar Absorbers in the Aerospace. Coatings 2025, 15, 989. https://doi.org/10.3390/coatings15090989
Kostelac L, Piccinotti A, Pezzato L, Colusso E, Pigato M, Pagot G, Di Noto V, Dabalà M, Brunelli K. Formation of Black Coatings on AA7075 and AA6061 by Low-Voltage Plasma Electrolytic Oxidation for Use as Flat Solar Absorbers in the Aerospace. Coatings. 2025; 15(9):989. https://doi.org/10.3390/coatings15090989
Chicago/Turabian StyleKostelac, Lorena, Alberto Piccinotti, Luca Pezzato, Elena Colusso, Mirko Pigato, Gioele Pagot, Vito Di Noto, Manuele Dabalà, and Katya Brunelli. 2025. "Formation of Black Coatings on AA7075 and AA6061 by Low-Voltage Plasma Electrolytic Oxidation for Use as Flat Solar Absorbers in the Aerospace" Coatings 15, no. 9: 989. https://doi.org/10.3390/coatings15090989
APA StyleKostelac, L., Piccinotti, A., Pezzato, L., Colusso, E., Pigato, M., Pagot, G., Di Noto, V., Dabalà, M., & Brunelli, K. (2025). Formation of Black Coatings on AA7075 and AA6061 by Low-Voltage Plasma Electrolytic Oxidation for Use as Flat Solar Absorbers in the Aerospace. Coatings, 15(9), 989. https://doi.org/10.3390/coatings15090989












