Precision-Tuned Magnetron Sputtering for High-Performance Metallized Copper Films
Abstract
1. Introduction
2. Film Deposition and Characterization
2.1. Film Deposition
2.2. Film Characterization
3. Results and Discussion
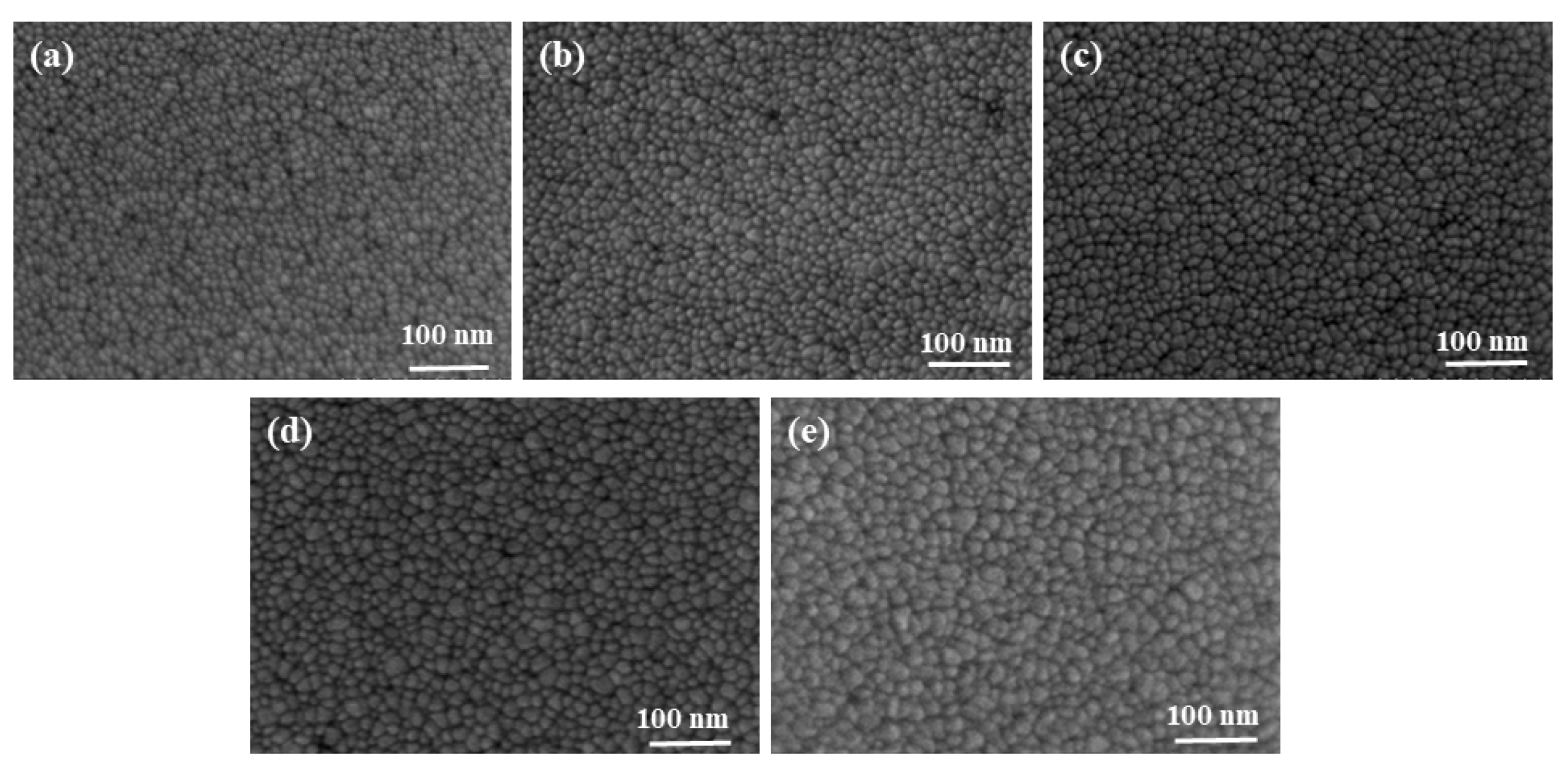
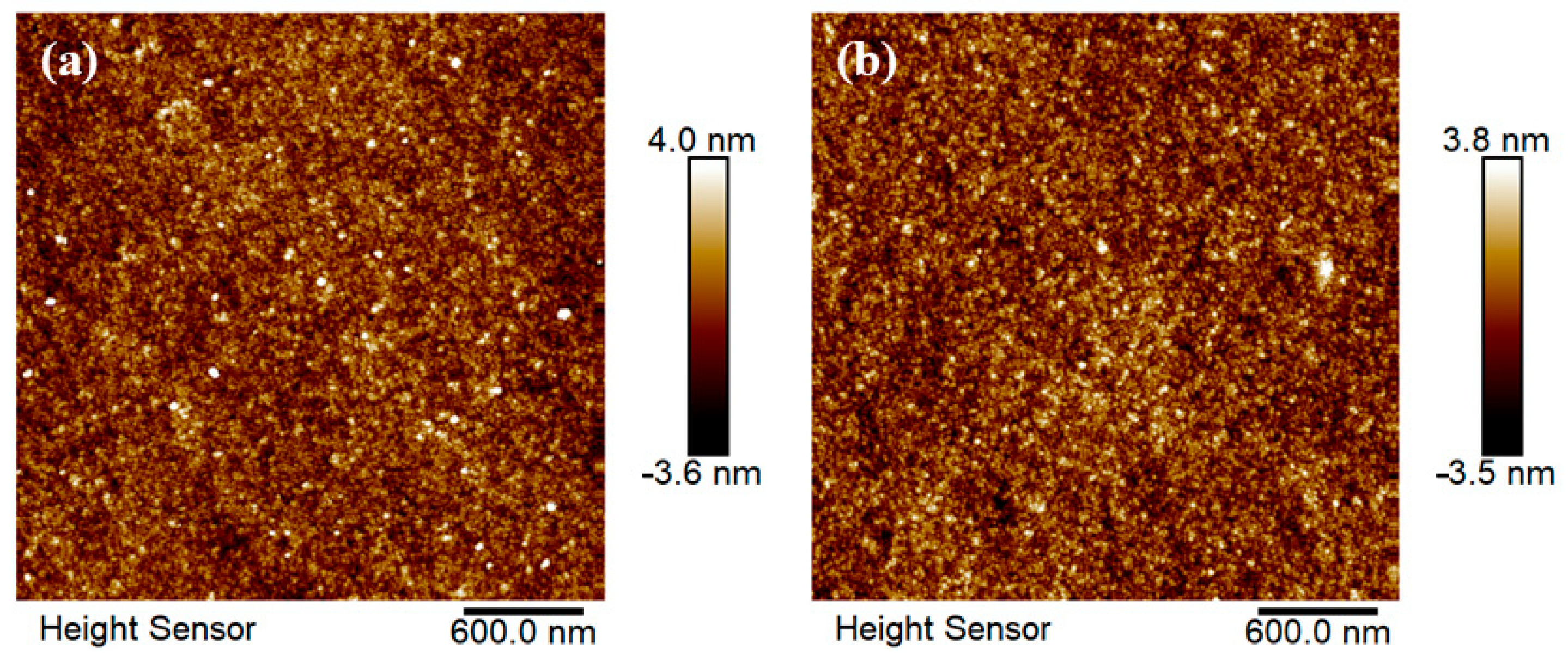
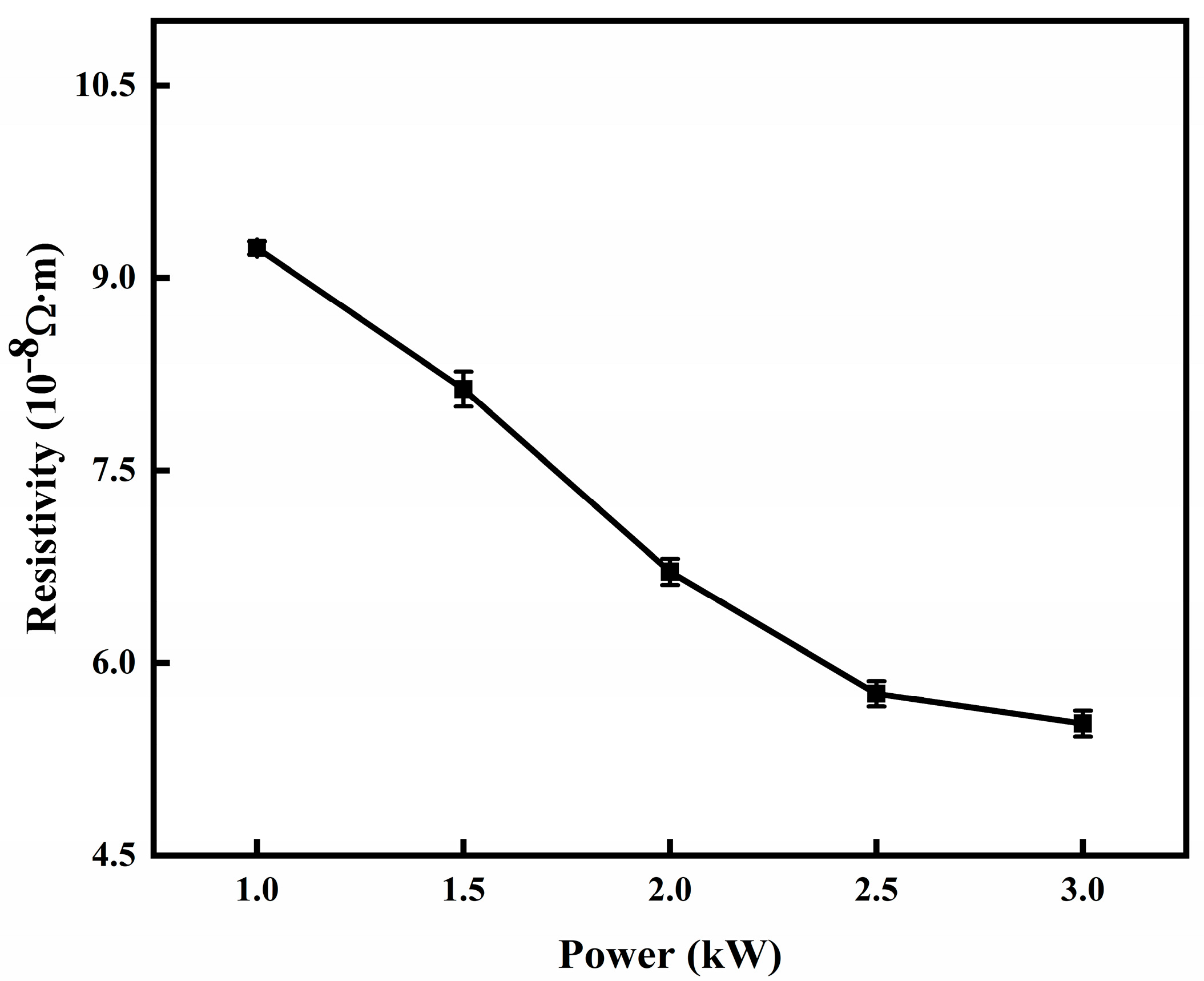
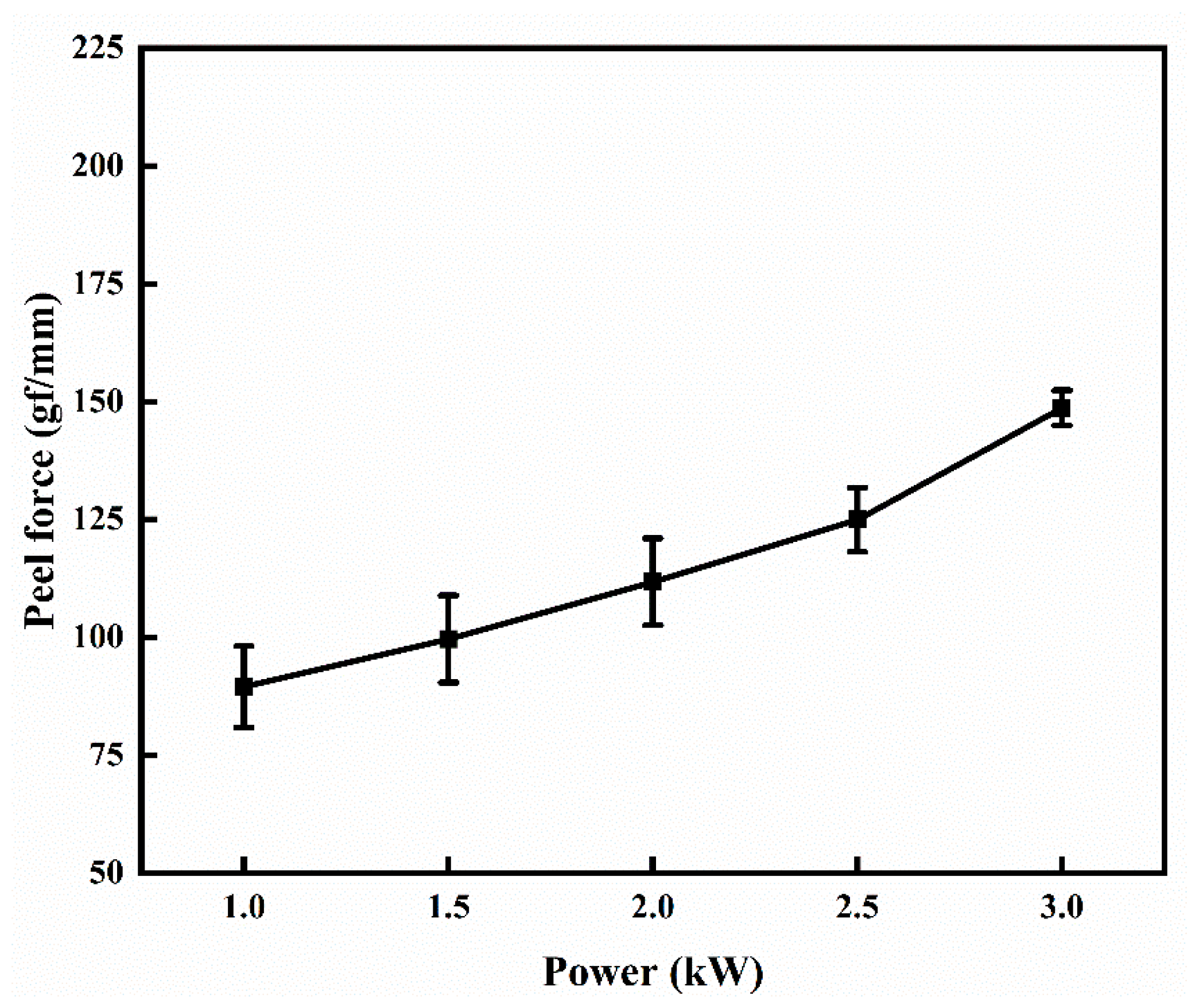
3.1. Effect of Sputtering Power
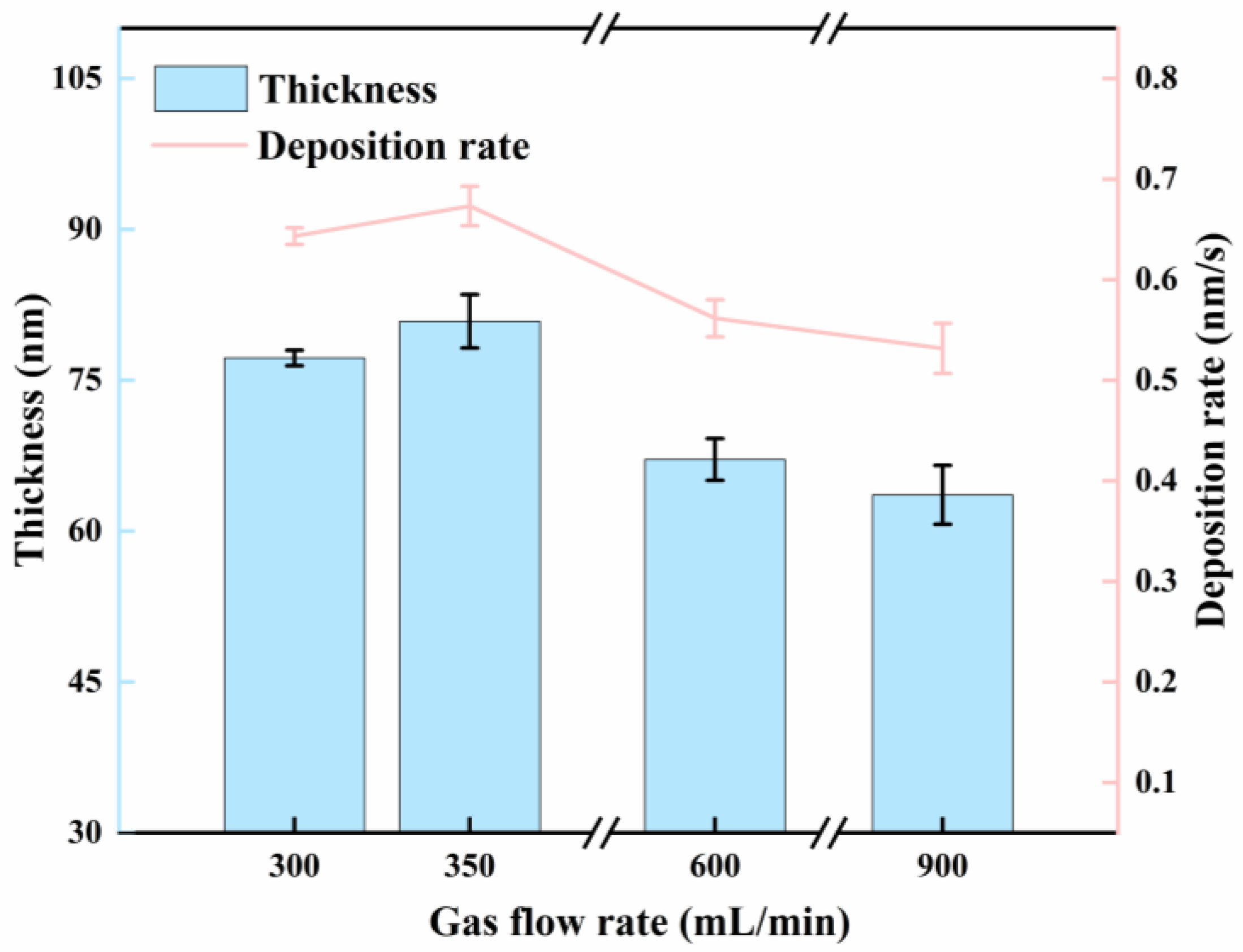
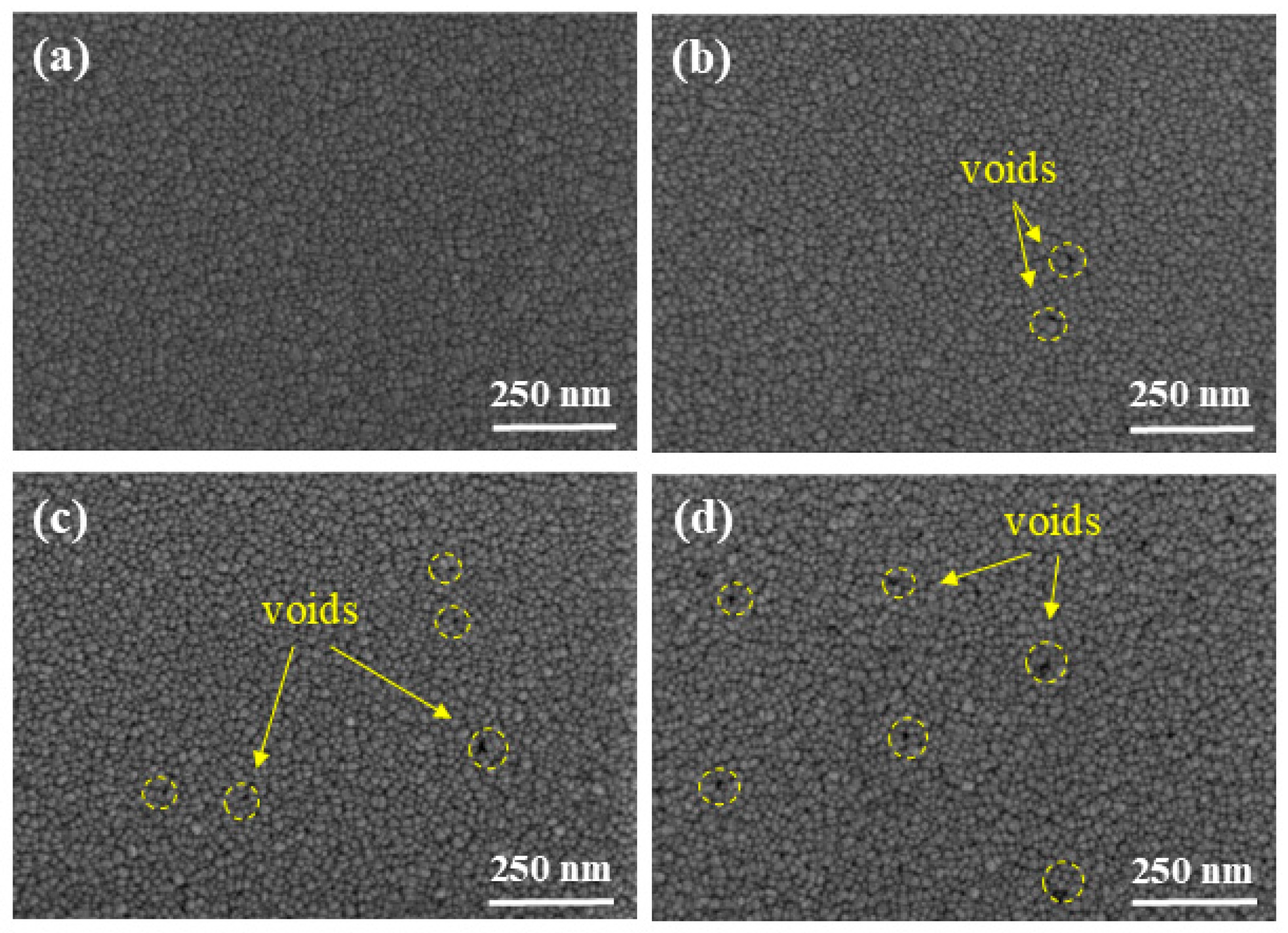
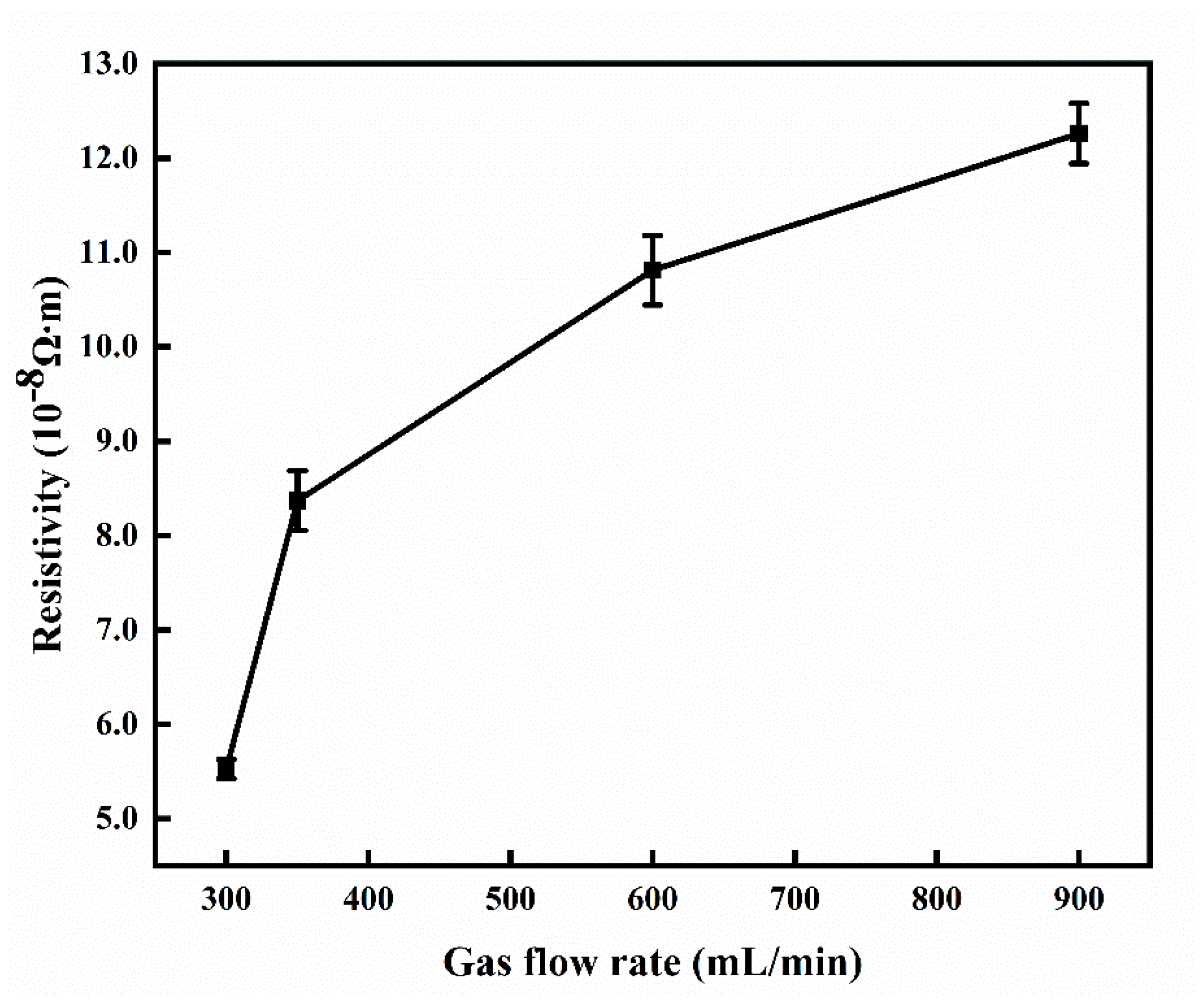
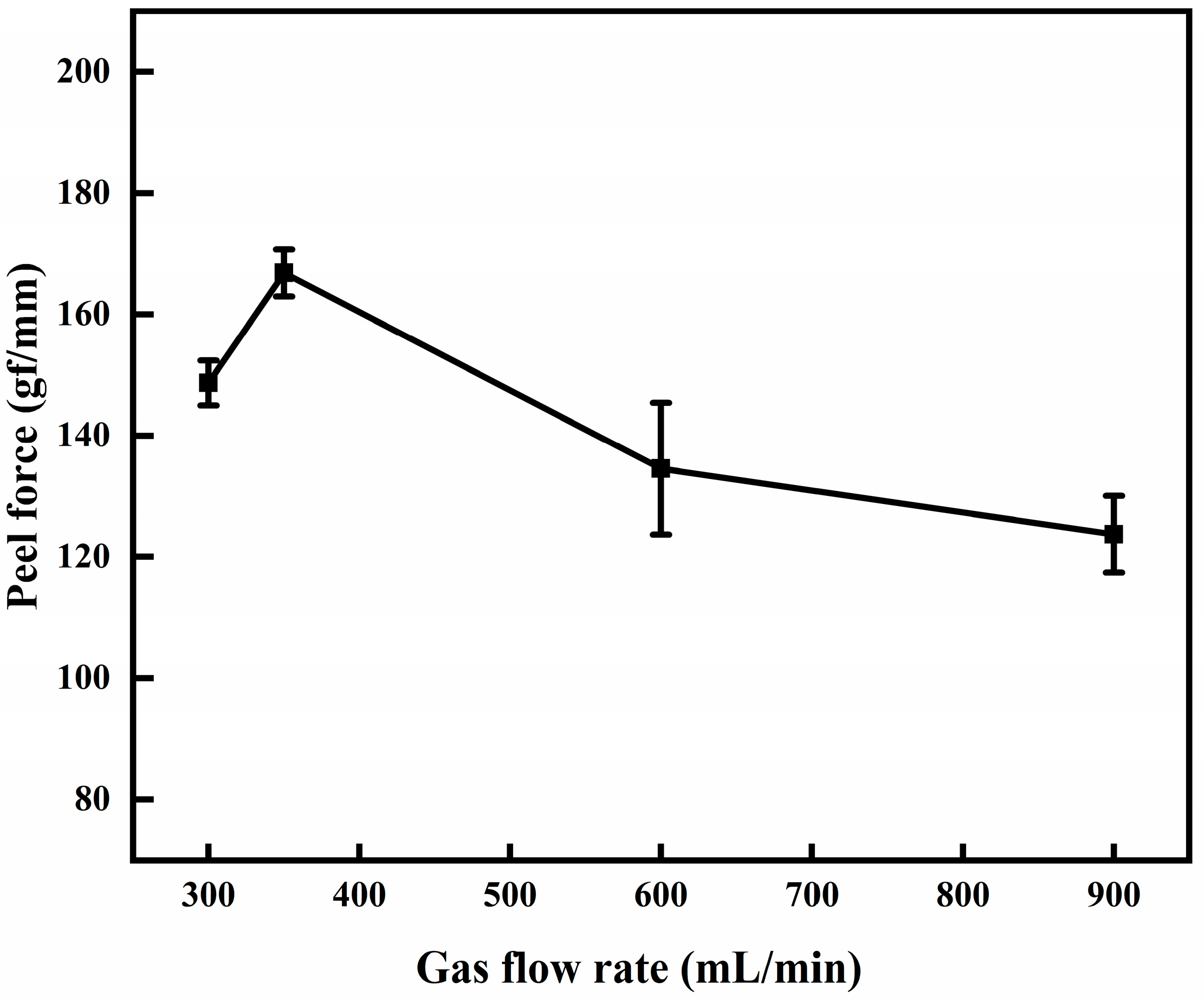
3.2. Effect of Gas Flow Rate
3.3. Effect of Substrate Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dun, X.; Wang, M.; Shi, H.; Xie, J.; Wei, M.; Dai, L.; Jiao, S. Composite copper foil current collectors with sandwich structure for high-energy density and safe lithium-ion batteries. Energy Storage Mater. 2025, 74, 103936. [Google Scholar] [CrossRef]
- Hur, J.; Lee, J.; Kim, B.Y.; Yoo, M.J.; Seo, J.H. Formation of a robust cu adhesive layer on poly (ether ether ketone) via self UV-initiated surface polymerization. J. Ind. Eng. Chem. 2022, 112, 307–315. [Google Scholar] [CrossRef]
- Ye, Y.S.; Huang, W.X.; Xu, R.; Xiao, X.; Zhang, W.; Chen, H.; Wan, J.; Liu, F.; Lee, H.K.; Xu, J.; et al. Cold-starting all-solid-state batteries from room temperature by thermally modulated current collector in sub-minute. Adv. Mater. 2022, 34, 2202848. [Google Scholar] [CrossRef]
- Wang, E.; Wang, S.; Wang, F.; Zhang, G.; Shang, L. Adhesion improvement of sputtered Cu films on flexible polymer substrates through the design of metal interlayers. Phys. Scr. 2024, 99, 075926. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Strunskus, T.; Behnke, K.; Bechtolsheim, C.v.; Thran, A.; Faupel, F. Formation of metal-polymer interfaces by metal evaporation: Influence of deposition parameters and defects. Microelectron. Eng. 2000, 50, 465–471. [Google Scholar] [CrossRef]
- Chen, J.J.; An, Q.; Rodriguez, R.D.; Sheremet, E.; Wang, Y.; Sowade, E.; Baumann, R.R.; Feng, Z.Z. Surface modification with special morphology for the metallization of polyimide film. Appl. Surf. Sci. 2019, 487, 503–509. [Google Scholar] [CrossRef]
- Niu, J.; Huang, X.; Yang, H.; Li, D.; Gao, X.; Yang, L.; Chen, Q.; Sun, A.; Zheng, K.; Wang, T.; et al. Copper Polypropylene Metal Plastic Composite Copper Foil: Future Proof Anode Current Collector Solution for Lithium-Ion Batteries with High Energy Density. ACS Appl. Mater. Interfaces 2024, 16, 57603–57613. [Google Scholar] [CrossRef]
- Huang, J.; Tian, C.; Wang, J.; Liu, J.; Li, Y.; Liu, Y.; Chen, Z. Fabrication of selective electroless copper plating on PET sheet: Effect of PET surface structure on resolution and adhesion of copper coating. Appl. Surf. Sci. 2018, 458, 734–742. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, D.; Wang, F.; Long, Y.; Kang, Z.; Gui, Z. Super-high interface adhesion through silver/polyimide heterojunction. Mater. Des. 2023, 233, 112276. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Zhang, G. 2024 Copper Composite Current Collector Preparation Method and Application Thereof. CN115094384B, 28 July 2023. (In Chinese). [Google Scholar]
- Ma, Y.; Li, L.; Qian, J.; Qu, W.; Luo, R.; Wu, F.; Chen, R. Materials and structure engineering by magnetron sputtering for advanced lithium batteries. Energy Storage Mater. 2021, 39, 203–224. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of Apparatus Geometry and Deposition Conditions on the Structure and Topography of Thick Sputtered Coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of Substrate Temperature and Deposition Rate on Structure of Thick Sputtered Cu Coatings. J. Vac. Sci. Technol. 1975, 12, 830–835. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X.; Wang, Y.; Yang, Y.; Gu, H.; Chu, S.; Wu, J.; Zheng, J. Microstructure and Performance Regulation of Cu Seed Layer by Magnetron Sputtering for Composite Current Collector Used in Lithium Battery. Surf. Technol. 2025, 54, 1–12. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Wu, W.; Li, L.; Chen, J.; Zhang, J.; Zuo, Y.; Fu, J. Effect of sputtering power on surface topography of DC magnetron sputtered Ti thin films observed by AFM. Appl. Surf. Sci. 2008, 255, 4673–4679. [Google Scholar] [CrossRef]
- Ekpe, S.D.; Bezuidenhout, L.W.; Dew, S.K. Deposition rate model of magnetron sputtered particles. Thin Solid Film. 2005, 474, 330–336. [Google Scholar] [CrossRef]
- Chopra, K.L.; Randlett, M.R. Influence of deposition parameters on the coalescence stage of growth of metal films. J. Appl. Phys. 1968, 39, 1874–1881. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, L.; Dai, B.; Wei, X.; Ren, Y.; Ge, N.; Long, Z. Effects of microstructure of Cu thin films deposited by magnetron sputtering on electro-explosive performance. J. Funct. Mater. 2016, 47, 12152–12156. (In Chinese) [Google Scholar]
- Le, M.T.; Sohn, Y.U.; Lim, J.W.; Choi, G.S. Effect of sputtering power on the nucleation and growth of Cu films deposited by magnetron sputtering. Mater. Trans. 2010, 51, 116–120. [Google Scholar] [CrossRef]
- Fuchs, K. The conductivity of thin metallic films according to the electron theory of metals. Math. Proc. Camb. Philos. Soc. 2008, 34, 100–108. [Google Scholar] [CrossRef]
- Sondheimer, E.H. The mean free path of electrons in metals. Adv. Phys. 2001, 50, 499–537. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M.; Wu, X.; Li, C.; Luo, X. Research Progress on Effect of Length Scale on Electrical Resistivity of Metals. Chin. J. Mater. Res. 2014, 28, 81–87. (In Chinese) [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Zhang, G.P.; Yu, Q.Y. Effects of interface and grain boundary on the electrical resistivity of Cu/Ta multilayers. J. Mater. Sci. Technol. 2009, 25, 699–702. [Google Scholar] [CrossRef]
- Mayadas, A.F.; Shatzkes, M. Electrical-resistivity model for polycrystalline films: The case of arbitrary reflection at external surfaces. Phys. Rev. B 1970, 1, 1382–1389. [Google Scholar] [CrossRef]
- Doerner, M.F.; Nix, W.D. Stresses and deformation processes in thin films on substrates. Crit. Rev. Solid State Mater. Sci. 1988, 14, 225–268. [Google Scholar] [CrossRef]
- Jiang, P. Effect of deposition pressure on structural and optical properties of ZnO films. Master’s Thesis, Soochow University, Suzhou, China, May 2009. [Google Scholar]
- Kusano, E. Structure-Zone Modeling of Sputter-Deposited Thin Films: A Brief Review. Appl. Sci. Converg. Technol. 2019, 28, 179–185. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Thinsoongnoen, P.; Misut, P.; Autama, S.; Kulsetthanchalee, C. Preparation and characterization of PET/SEBS incorporated with organomontmorillonite. IOP Conf. Ser. Mater. Sci. Eng. 2020, 965, 012019. [Google Scholar] [CrossRef]
- Aradhya, S.; Frei, M.; Hybertsen, M.; Venkataraman, L. Van der Waals interactions at metal/organic interfaces at the single-molecule level. Nat. Mater. 2012, 11, 872–876. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Wang, H.; Wang, Y.; Xiong, Y.; Wu, C.; Li, K.; Zhou, H. Recent Research Progress of Generation Mechanism and Influence Factors of Intrinsic Stress in Vacuum Deposited Copper Films. Vac. Cryog. 2021, 27, 103–114. (In Chinese) [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]

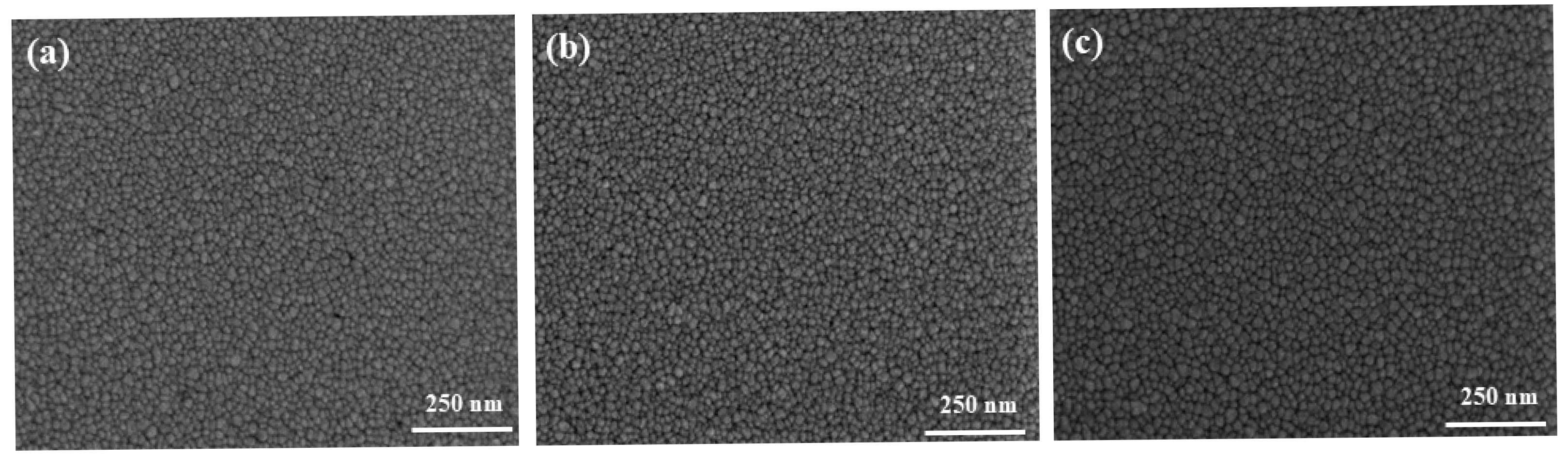
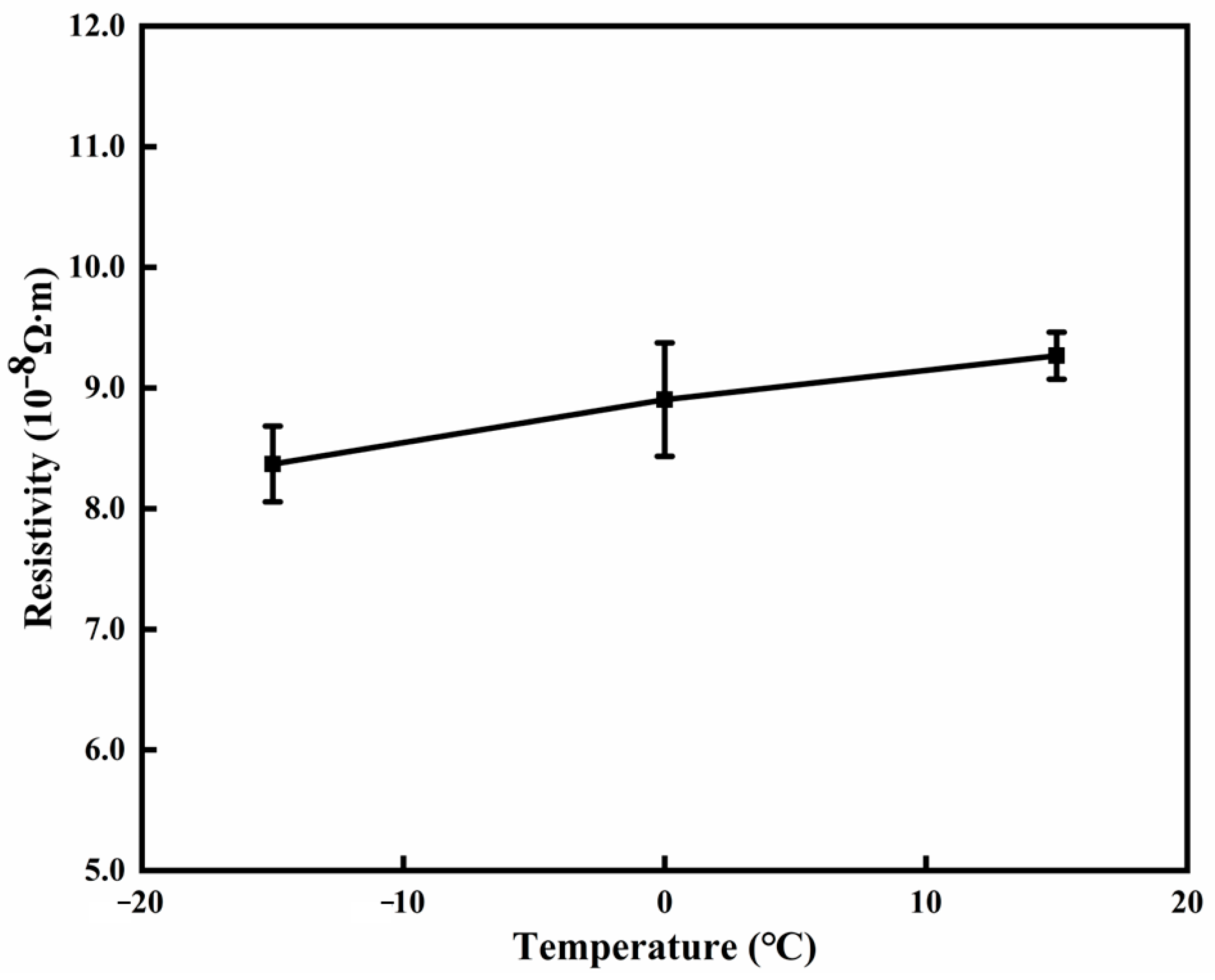
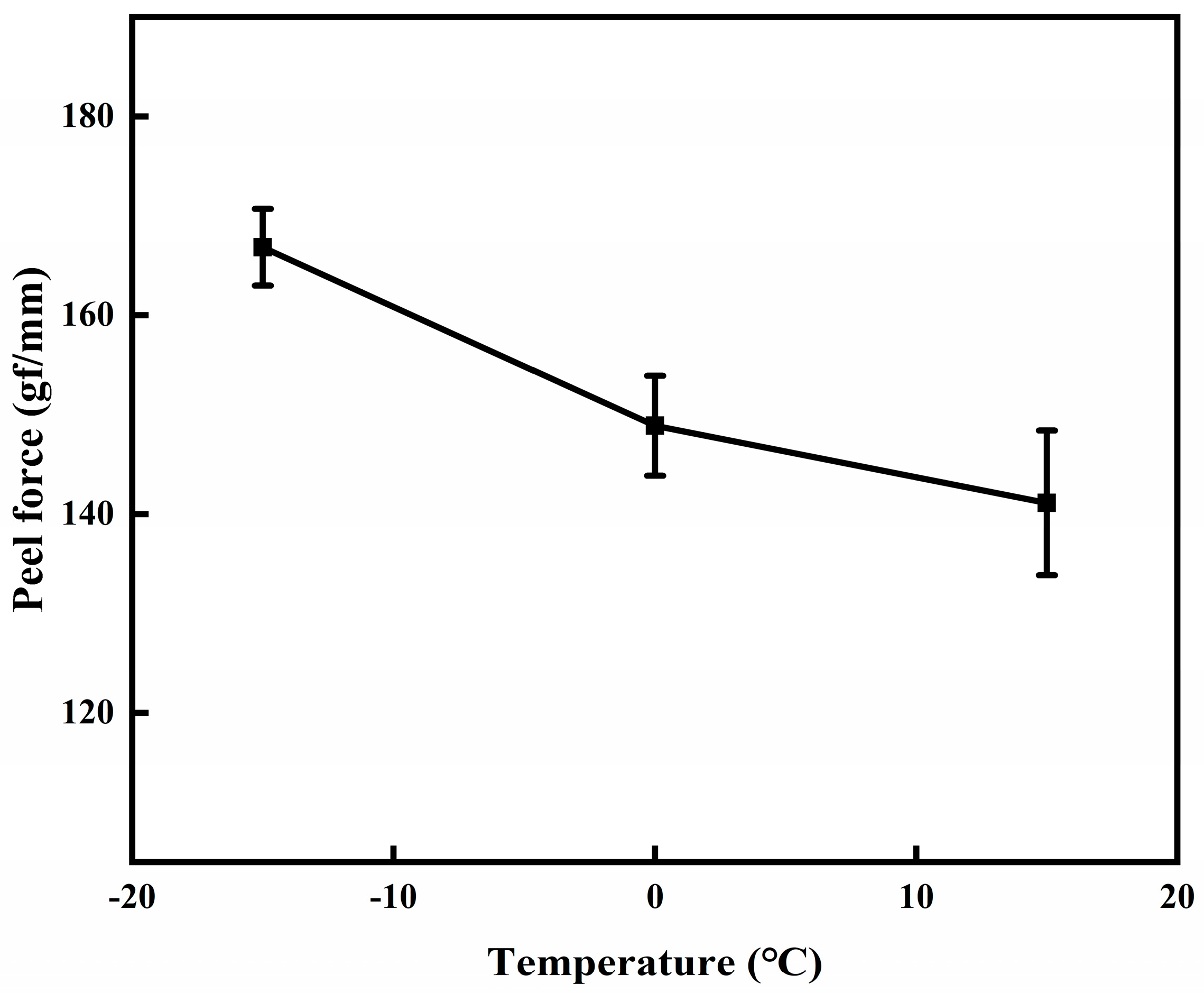
| Number |
Target Power
/kW |
Gas Flow Rate /(mL·min−1) |
Working Pressure
/Pa |
Deposition time/min | Temperature of Cooling Roll /℃ |
Thickness
/nm |
|---|---|---|---|---|---|---|
| Series 1: Different target power with identical deposition times | ||||||
| 1# | 1 | 300 | 0.1 | 3 | −15 | 40.8 |
| 2# | 1.5 | 61.4 | ||||
| 3# | 2 | 91 | ||||
| 4# | 2.5 | 102 | ||||
| 5# | 3 | 115 | ||||
| Series 2: Different target power with nearly identical film thickness | ||||||
| 6# | 1 | 300 | 0.1 | 6 | −15 | 80 |
| 7# | 1.5 | 4 | 85 | |||
| 3# | 2 | 3 | 91 | |||
| 8# | 2.5 | 2.5 | 79 | |||
| 9# | 3 | 2 | 77.2 | |||
| Series 3: Different gas flow rates with identical deposition times | ||||||
| 9# | 3 | 300 | 0.1 | 2 | −15 | 77.2 |
| 10# | 350 | 0.2 | 80.8 | |||
| 11# | 600 | 0.3 | 67.1 | |||
| 12# | 900 | 0.4 | 63.6 | |||
| Series 4: Different gas flow rates with nearly identical film thickness | ||||||
| 9# | 3 | 300 | 0.1 | 2 | −15 | 77.2 |
| 10# | 350 | 0.2 | 80.8 | |||
| 13# | 600 | 0.3 | 3 | 80.5 | ||
| 14# | 900 | 0.4 | 80.1 | |||
| Series 5: Different substrate temperature with identical deposition times | ||||||
| 10# | 3 | 350 | 0.2 | 2 | −15 | 80.8 |
| 15# | 0 | 79 | ||||
| 1 6# | 15 | 82 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Hao, X.; Zhang, L.; Ding, J.; Geng, L.; Zheng, J. Precision-Tuned Magnetron Sputtering for High-Performance Metallized Copper Films. Coatings 2025, 15, 1089. https://doi.org/10.3390/coatings15091089
Yang Y, Hao X, Zhang L, Ding J, Geng L, Zheng J. Precision-Tuned Magnetron Sputtering for High-Performance Metallized Copper Films. Coatings. 2025; 15(9):1089. https://doi.org/10.3390/coatings15091089
Chicago/Turabian StyleYang, Ying, Xiaoyu Hao, Liuyan Zhang, Jicheng Ding, Lanfang Geng, and Jun Zheng. 2025. "Precision-Tuned Magnetron Sputtering for High-Performance Metallized Copper Films" Coatings 15, no. 9: 1089. https://doi.org/10.3390/coatings15091089
APA StyleYang, Y., Hao, X., Zhang, L., Ding, J., Geng, L., & Zheng, J. (2025). Precision-Tuned Magnetron Sputtering for High-Performance Metallized Copper Films. Coatings, 15(9), 1089. https://doi.org/10.3390/coatings15091089






