Sustainable Edible Coatings Enriched with Bioactive Extracts from Exhausted Olive Pomace, Fucus Spiralis, and Limnospira sp. for the Postharvest Preservation of Strawberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Bioactive Compounds
2.2.1. Extraction of Bioactive Compounds from the EOP and Limnospira sp.
2.2.2. Extraction of Bioactive Compounds from Fucus spiralis
2.3. Extraction of Polysaccharides from Fucus spiralis
2.4. Extraction of Protein from Limnospira sp.
2.5. Edible Coating Formulations and Their Application on Strawberries
- Alginate (2%)
- Alginate (2%) + EOP bioactive-rich extract (0.25%)
- Alginate (2%) + Fucus spiralis bioactive-rich extract (0.25%) + Fucus spiralis polysaccharide-rich extract (0.5%)
- Alginate (2%) + Limnospira sp. bioactive-rich extract (0.25%) + Limnospira sp. protein-rich extract (0.5%)
2.6. Evaluation of Coated Strawberries
2.6.1. Decay
2.6.2. Moisture Loss
2.6.3. Color Analysis
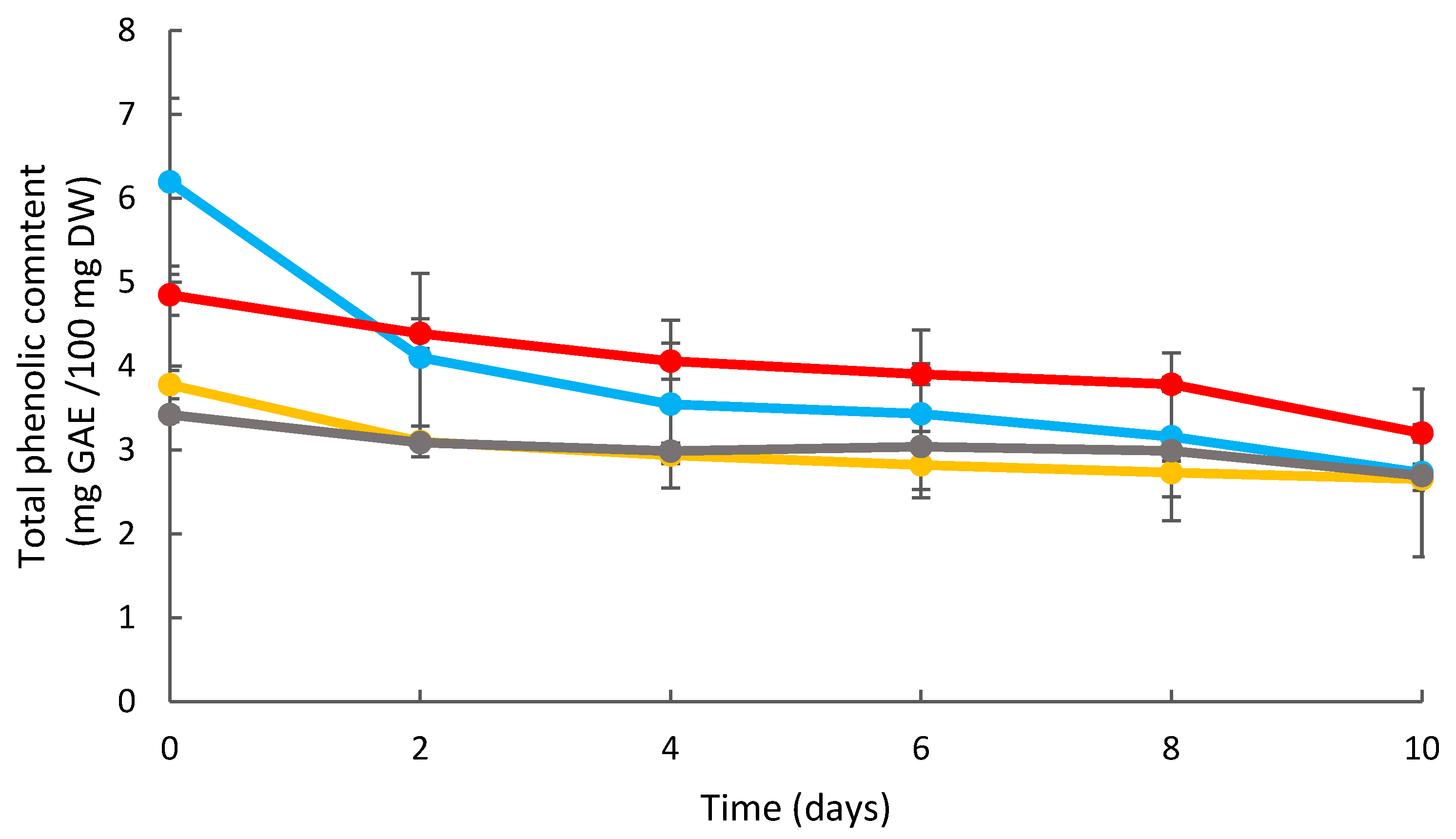
2.6.4. Determination of Total Phenolic Content Antioxidant Activity
TPC
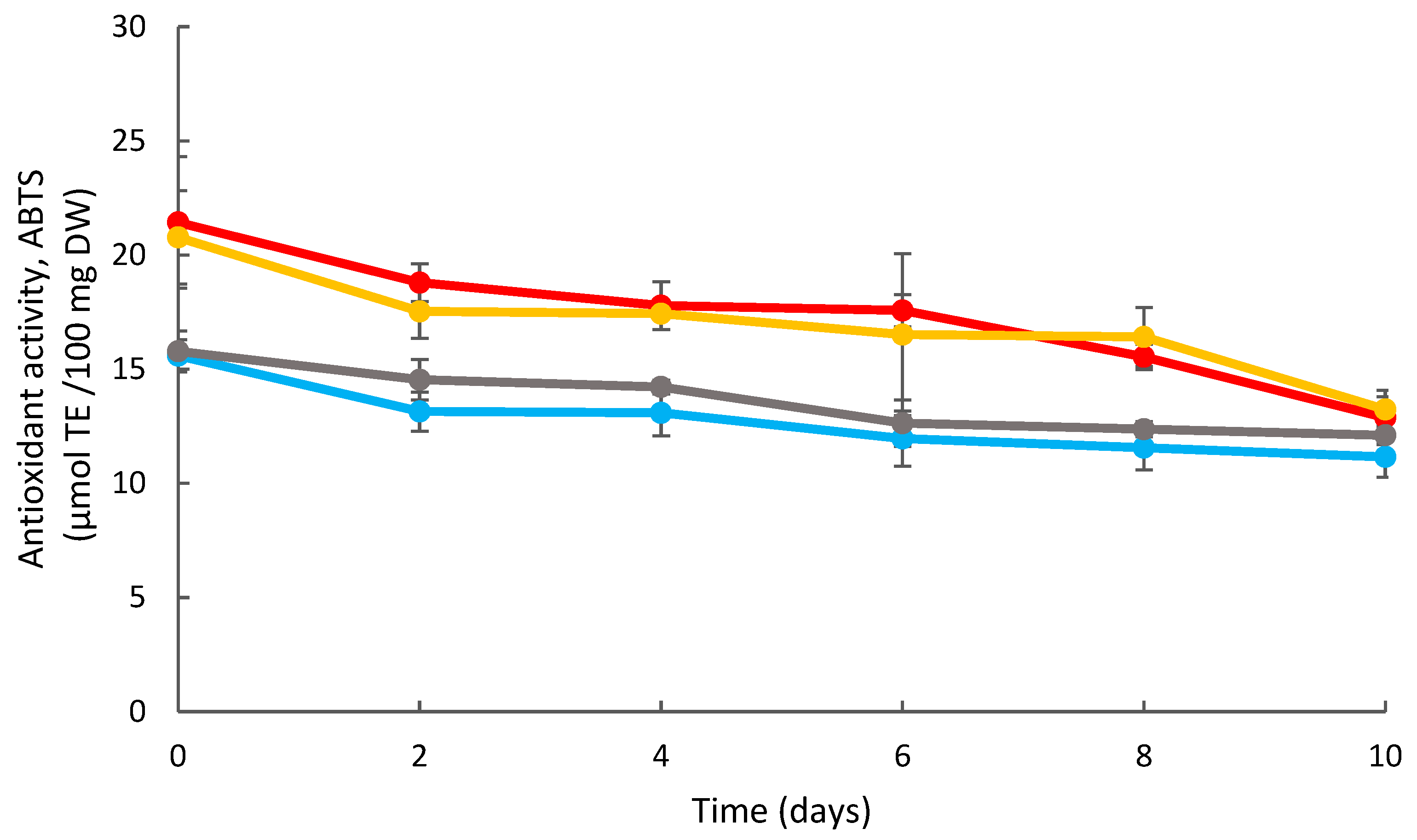
ABTS Radical Scavenging Assay
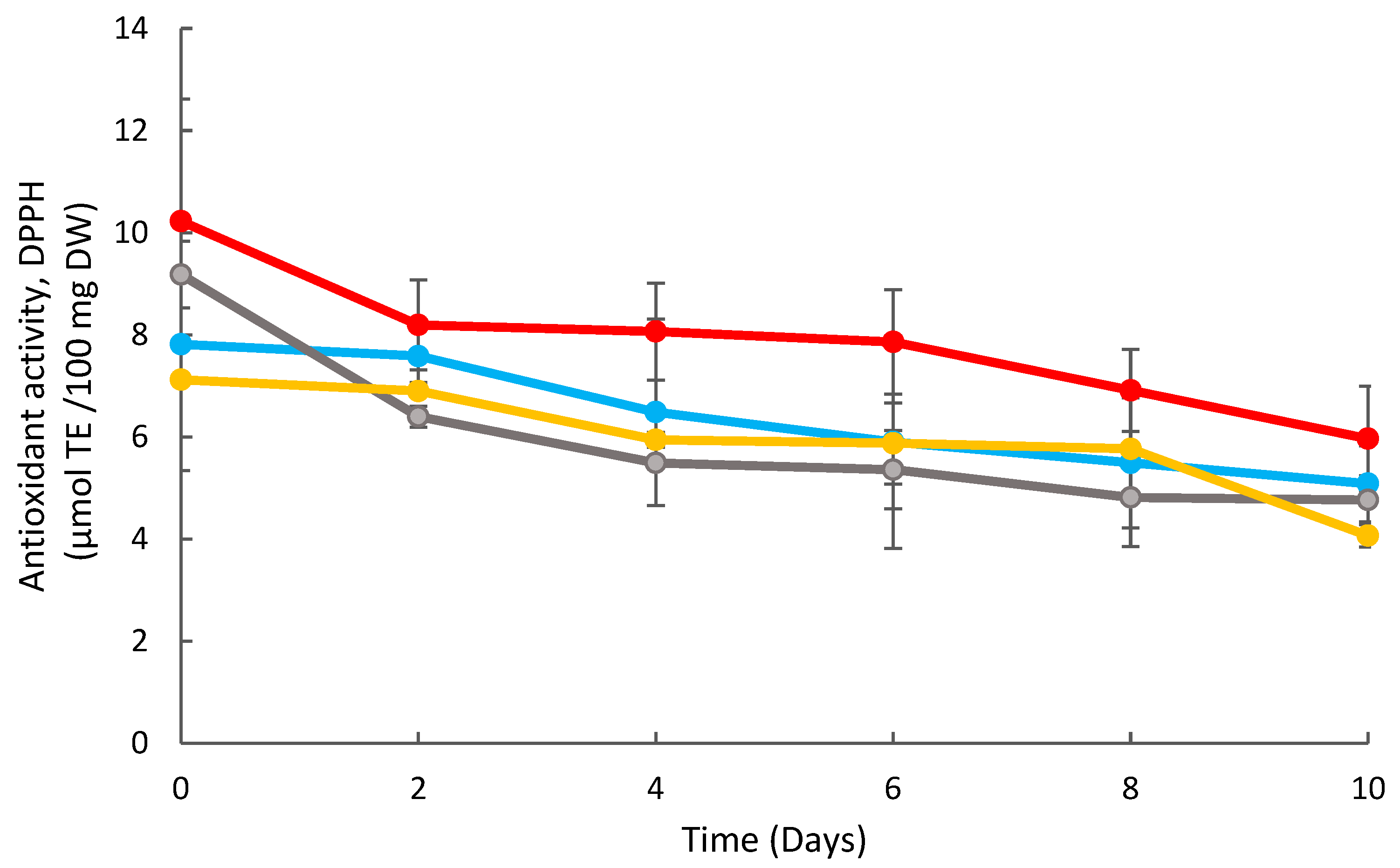
DPPH Radical Scavenging Assay
2.6.5. Determination of Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
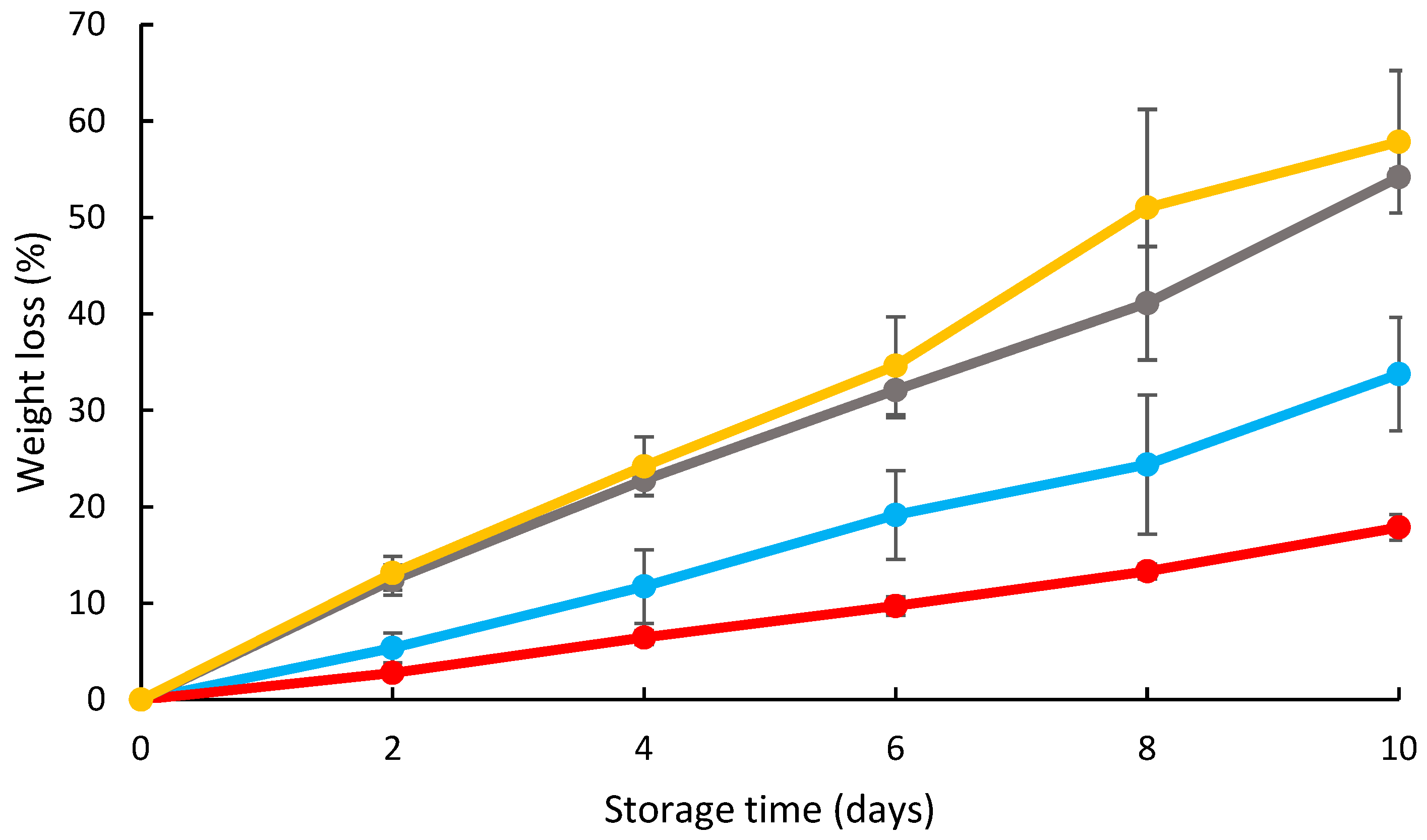
3.1. Effects of Coating on the Strawberry Moisture Loss
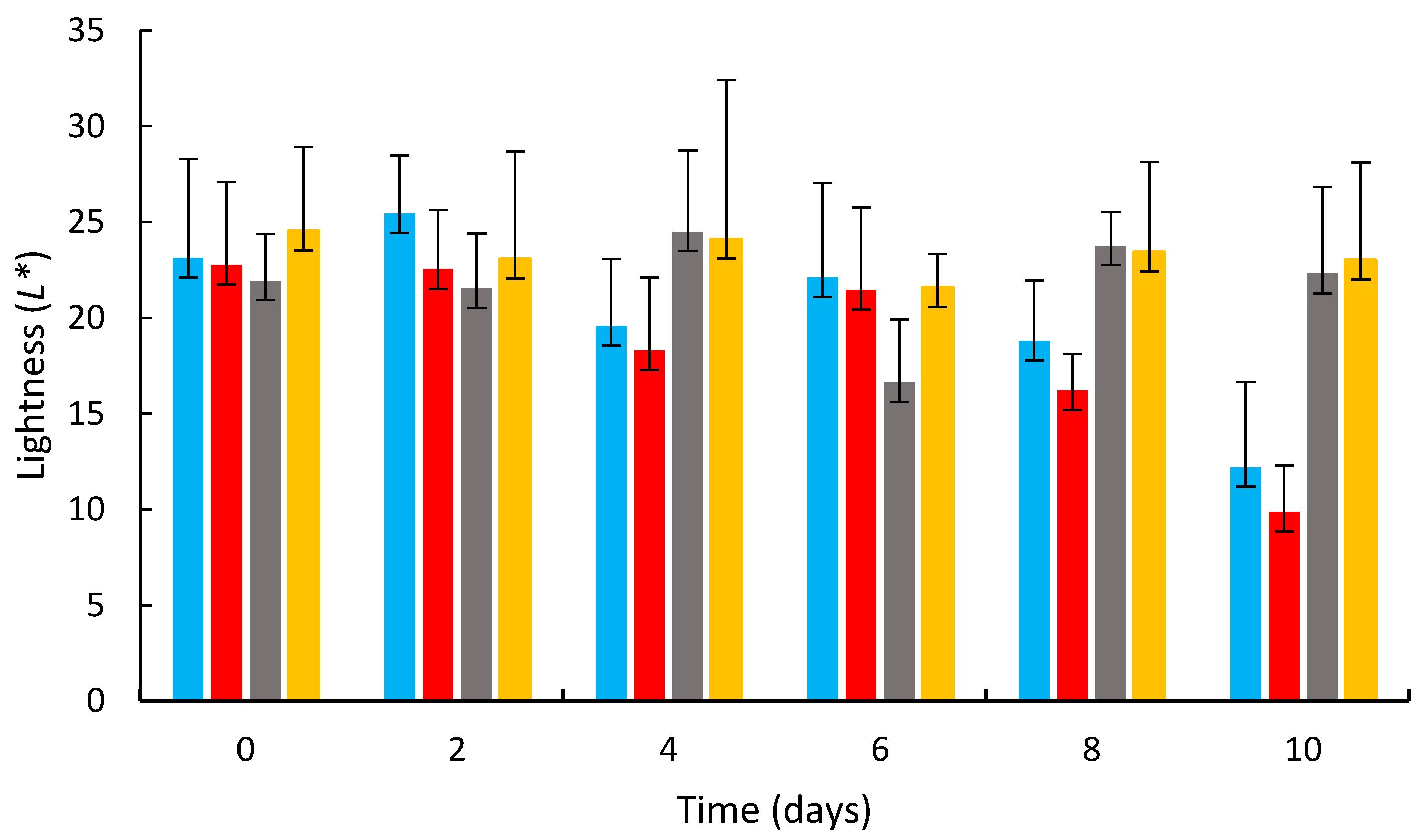
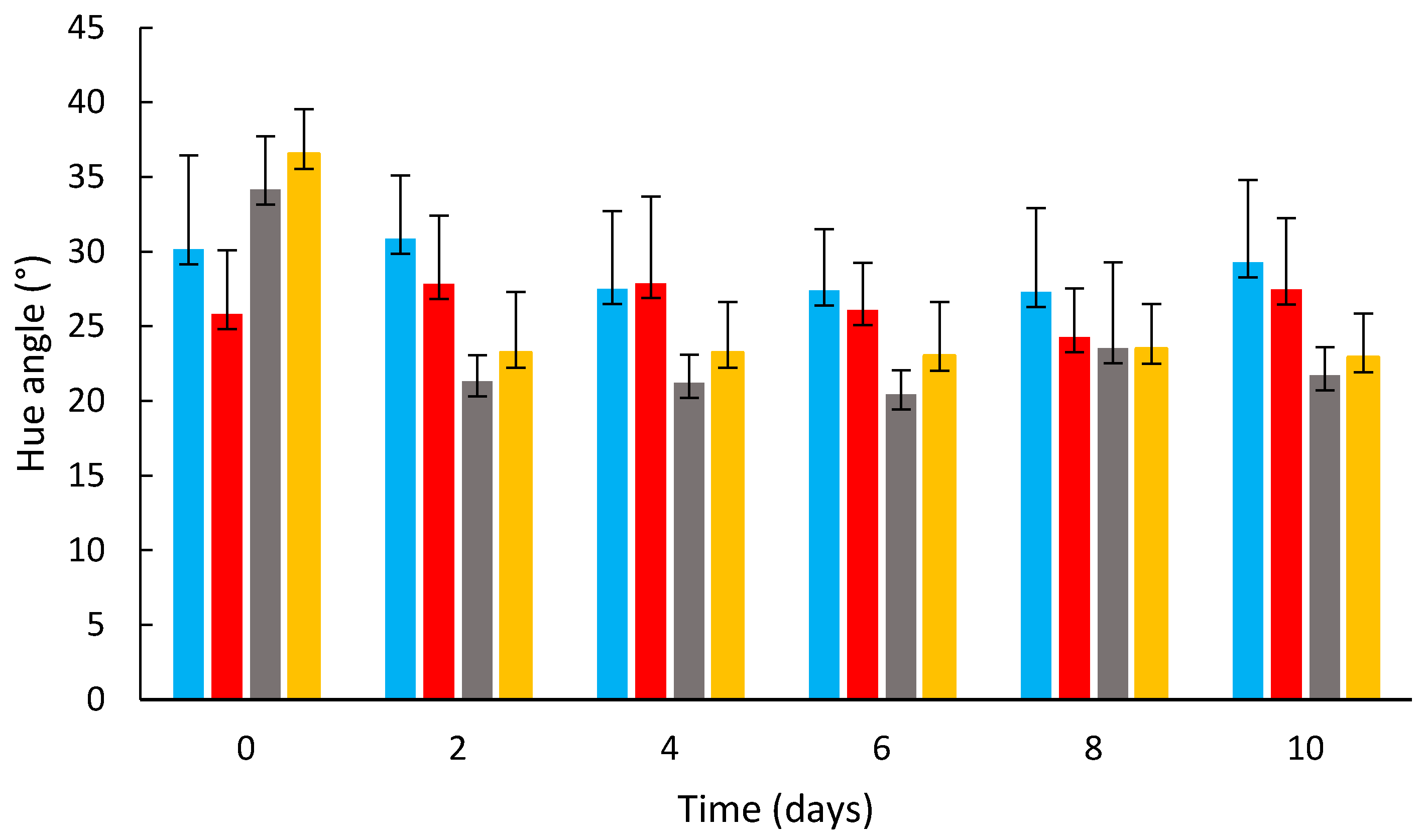
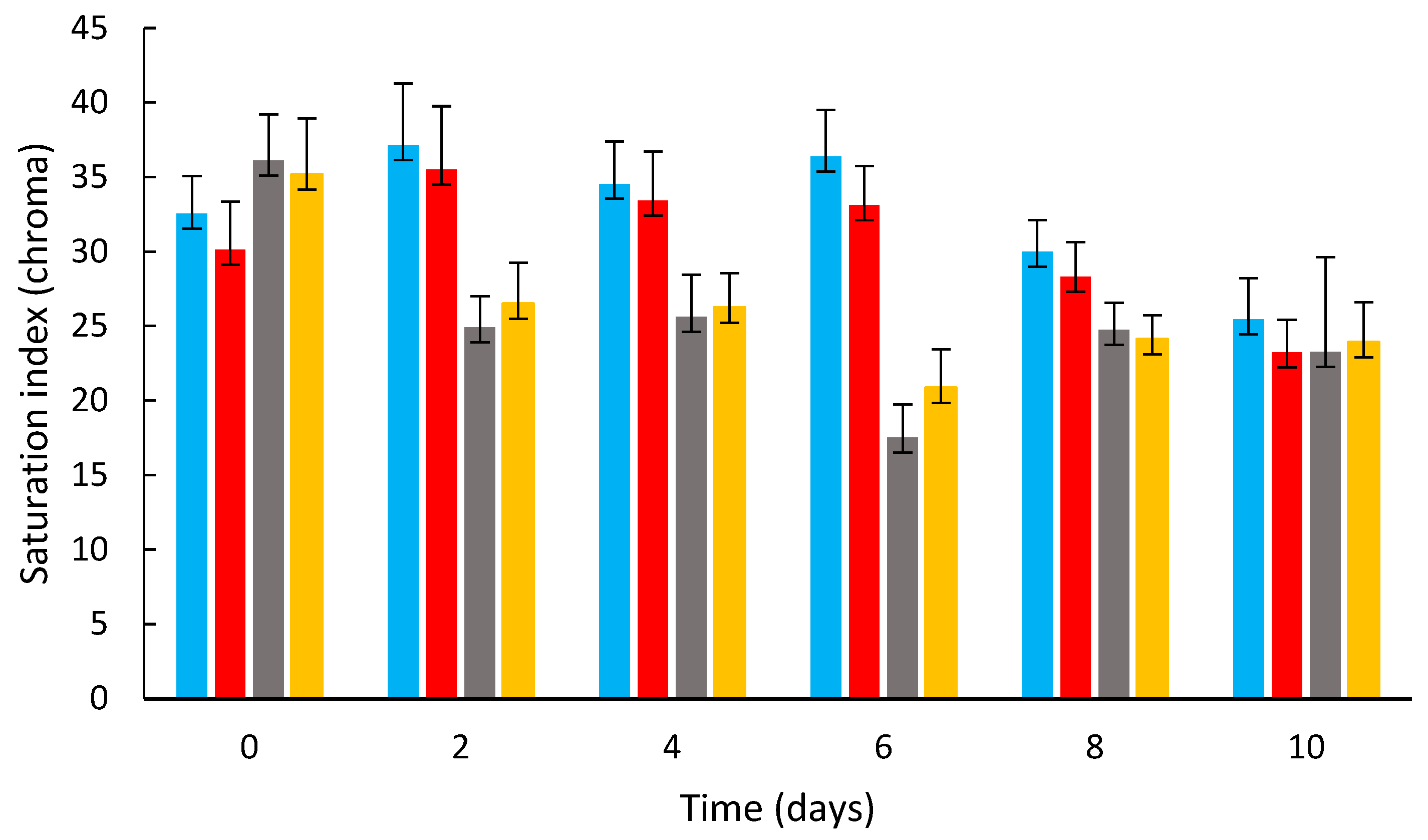
3.2. Effects of Coating on the Strawberry Color
3.3. Effects of Coating on the Strawberry Antioxidant Activity
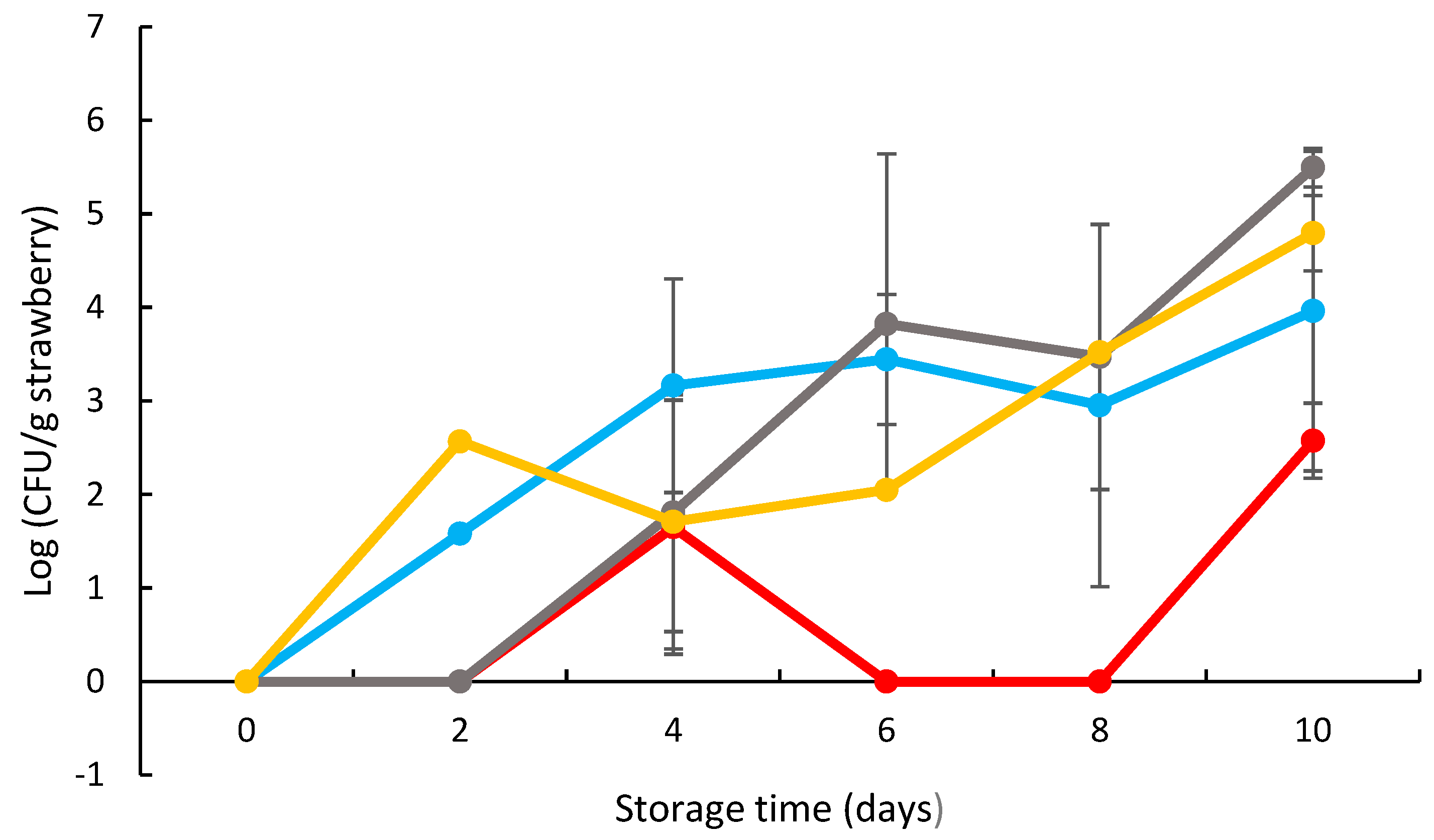
3.4. Effects of Coating on the Strawberry Decay
3.5. Effects of Coating on the Strawberry Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOP | Exhausted olive oil pomace |
| TPC | Total phenolic content |
| ABTS | 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| UNEP | United Nations Environment Program |
| TE | Trolox equivalent |
| HIV | Human immunodeficiency virus |
| RH | Relative humidity |
| CIIMAR | Interdisciplinary Center of Marine and Environmental Research |
| MGH | Microwave hydrodiffusion and gravity |
| PS | Polysaccharides |
| CIE | Commission Internationale de l’Eclairage |
| PCA | Plate count agar |
| PDA | Potato dextrose agar |
| ANOVA | Analysis of variance |
| WL | Weight loss |
| SCFA | Short-chain fatty acids |
| PPO | Polyphenol oxidase |
References
- FAO. The State of Food and Agriculture 2021; Food and Agriculture Organization of United Nation: Rome, Italy, 2021; Available online: https://www.fao.org/publications/sofa/2021/en/ (accessed on 24 July 2025).
- UNEP. Making Peace with Nature: A Scientific Blueprint to Tackle the Climate, Biodiversity and Pollution Emergencies; United Nations Environment Program: Nairobi, Kenya, 2021; Available online: https://www.unep.org/resources/making-peace-nature (accessed on 24 July 2025).
- Eurostat. Waste Statistics—Statistics Explained; European Commission: Luxembourg, 2023; Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics (accessed on 24 July 2025).
- Onwude, D.I.; Chen, G.; Eke-Emezie, N.; Kabutey, A.; Khaled, A.Y.; Sturm, B. Recent advances in reducing food losses in the supply chain of fresh agricultural produce. Processes 2020, 8, 1431. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest storage techniques and quality evaluation of fruits and vegetables for reducing food loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Martins, V.F.; Pintado, M.E.; Morais, R.M.; Morais, A.M. Recent highlights in sustainable bio-based edible films and coatings for fruit and vegetable applications. Foods 2024, 13, 318. [Google Scholar] [CrossRef]
- Popescu, P.A.; Palade, L.M.; Nicolae, I.C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-based edible coatings containing essential oils to preserve the shelf life and postharvest quality parameters of organic strawberries and apples during cold storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef]
- Abu Salha, B.; Gedanken, A. Extending the shelf life of strawberries by the sonochemical coating of their surface with nanoparticles of an edible anti-bacterial compound. Appl. Nano 2021, 2, 14–24. [Google Scholar] [CrossRef]
- Alharaty, G.; Ramaswamy, H.S. The effect of sodium alginate-calcium chloride coating on the quality parameters and shelf life of strawberry cut fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of edible coatings on the shelf-life of fresh strawberries: A comparative study using TOPSIS-Shannon entropy method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.; Faleiro, M.L.; Miguel, M.G.; Antunes, M.D. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Coelho, M.; Machado, M.; Costa, E.; Gomes, A.M.; Poças, F.; Sperotto, R.A.; Rosa-Martinez, E.; Vasconcelos, M.; Pintado, M.E.; et al. Integrated Valorization of Fucus Spiralis Alga: Polysaccharides and Bioactives for Edible Films and Residues as Biostimulants. Foods 2024, 13, 2938. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Lopes, A.I.; Gomes, D.; Parreira, C.; Badenes, S.M.; Costa, L.; Pintado, M.; Morais, A.M.M.B.; Morais, R.M.S.C. Unravelling the Potential of Seven Microalgae Species: Nutritional, Antioxidant, and Antimicrobial Properties and Application. Appl. Sci. 2025, 15, 6691. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Ribeiro, T.B.; Lopes, A.I.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules 2024, 29, 1935. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Lopes, A.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Edible coatings with polysaccharides and bioactive compounds from Exhausted olive oil pomace to extend the shelf life of strawberry. In Proceedings of the International Conference on Sustainable Foods—Achieving the Sustainable Development Goals (ICSF), Bragança, Portugal, 24–25 July 2024. [Google Scholar]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced Extraction of Hydroxytyrosol, Maslinic Acid and Oleanolic Acid from Olive Pomace: Process Parameters, Kinetics and Thermodynamics, and Greenness Assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Pereira, L. Guia Ilustrado das Macroalgas: Conhecer e Reconhecer Algumas Espécies da Flora Portuguesa; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2009. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic Compounds from Three Brown Seaweed Species Using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Cabral, E.M.; Mondala, J.R.M.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of Molecular Weight Fractionation on the Antimicrobial and Anticancer Properties of a Fucoidan Rich-Extract from the Macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef]
- Batista, P.; Cunha, S.A.; Ribeiro, T.; Borges, S.; Baptista-Silva, S.; Oliveira-Silva, P.; Pintado, M. Fucoidans: Exploring Its Neuroprotective Mechanisms and Therapeutic Applications in Brain Disorders. Trends Food Sci. Technol. 2024, 143, 104300. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling Phlorotannins from Fucus spp. of the Northern Portuguese Coastline: Chemical Approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the Brown Seaweed Sargassum latifolium in the Design of Alginate-Fucoidan Based Films with Natural Antioxidant Properties and Kinetic Modeling of Moisture Sorption and Polyphenolic Release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Xu, B.; Wu, S. Preservation of Mango Fruit Quality Using Fucoidan Coatings. LWT 2021, 143, 111150. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Chatzikonstantinou, A.V.; Mataragas, M.; Kondyli, E.; Stamatis, H.; Bosnea, L. Evaluation of the Antioxidant and Physicochemical Properties of Microalgae/Whey Protein-Based Edible Films. Biol. Life Sci. Forum 2021, 6, 97. [Google Scholar]
- Martins, V.F.R.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Edible Films with Protein and Bioactive Compounds from Arthrospira sp. Biol. Life Sci. Forum 2024, 40, 6. [Google Scholar]
- Parreidt, T.S.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The development of a uniform alginate-based coating for cantaloupe and strawberries and the characterization of water barrier properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT 2013, 51, 9–15. [Google Scholar] [CrossRef]
- Zinina, O.; Merenkova, S.; Galimov, D. Development of biodegradable alginate-based films with bioactive properties and optimal structural characteristics with incorporation of protein hydrolysates. Sustainability 2023, 15, 15086. [Google Scholar] [CrossRef]
- Sun, K.; Wang, Z.; Xiang, W.; Ye, S.; Wan, H. Construction of sodium alginate/ginkgo biloba polysaccharide based films embedded with ginkgo biloba extract and its application in postharvest quality stabilization of sweet cherries. Food Chem. 2025, 485, 144533. [Google Scholar] [CrossRef]
- Li, A.M.; Li, G.; Shang, Q.; Chen, X.; Liu, W.; Pi, X.; Zhu, L.; Yin, Y.; Yu, G.; Wang, X. In vitro fermentation of alginate and its derivatives by human gut microbiota. Anaerobe 2016, 39, 19–25. [Google Scholar] [CrossRef]
- Nunzio, M.D.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Francesco Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Guan, F.; Fu, G.; Ma, Y.; Zhou, L.; Li, G.; Sun, C.; Zhang, T. Spirulina polysaccharide-based prebiotic foods preparations-a promising approach for modulating gut microbiota and improving health. J. Funct. Foods 2024, 116, 106158. [Google Scholar] [CrossRef]
- Can, S.S.; Seyhaneyildiz, E.; Dorucu, M. The Effects of Probiotic-Prebiotic on the Biomass and Protein Content of Spirulina platensis in Different Temperatures and Illuminations. Turk. J. Fish. Aquat. Sci. 2022, 22, 10. [Google Scholar] [CrossRef]
- Yu, J.; Ma, D.; Qu, S.; Liu, Y.; Xia, H.; Bian, F.; Zhang, Y.; Huang, C.; Wu, R.; Wu, J.; et al. Effects of different probiotic combinations on the components and bioactivity of Spirulina. J. Basic Microbiol. 2020, 60, 543–557. [Google Scholar] [CrossRef]
- Pérez, L.; Conde, E.; Domínguez, H. Microwave Hydrodiffusion and Gravity Processing of Sargassum muticum. Process Biochem. 2014, 49, 981–988. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of Intensification Techniques for the Extraction and Depolymerization of Fucoidan. Algal Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Walton, J.; Viñas, I.; Tiwari, B.K. Ultrasound Assisted Extraction of Polysaccharides from Mushroom by Products. LWT 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Zaim, S.; Mortadi, A.; Chibi, F.; Benchennouf, E.H.; Arsalane, W.; Cherkaoui, O.; Rchid, H.; Nmila, R.; El Moznine, R. Extraction of Polysaccharides from Brown Algae: Rheological Studies. Iran. Polym. J. (Engl. Ed.) 2020, 29, 1137–1145. [Google Scholar] [CrossRef]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Lopes, A.I.; Machado, M.; Costa, E.M.; Ribeiro, T.B.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicro bial Activities. Foods 2025, 14, 1327. [Google Scholar] [CrossRef]
- Khemira, H.; Amri, I.; Souissi, N.; Mejri, M. Effect of sodium alginate edible coatings enriched with green tea extract on postharvest quality of strawberries. Postharvest Biol. Technol. 2019, 153, 48–57. [Google Scholar]
- Agustini, N.W.S.; Kusmiati, K.; Admirasari, R.; Nurcahyanto, D.A.; Hidhayati, N.; Apriastini, M.; Afiati, F.; Priadi, D.; Fitriani, B.M.; Adalina, Y.; et al. Characterization of Corn-Starch Edible Film with the Addition of Microalgae Extract Chlorella vulgaris as an Antioxidant Applied to Dodol (Glutinous-Rice Cake) Products. Case Stud. Chem. Environ. Eng. 2023, 8, 100511. [Google Scholar] [CrossRef]
- Commission Internationale de l’Eclairage (CIE). Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; C.I.E.: Paris, France, 1978; TC 1. [Google Scholar]
- Moreira, M.R.; Roura, S.I.; Ponce, A. Effectiveness of chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. Food Sci. Technol. 2011, 44, 2335–2341. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; del Socorro Flores-González, M.; Arévalo-Niño, K. Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (Fragaria ananassa). J. Food Sci. 2015, 80, M1823–M1830. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of hydroxyethyl cellulose and sodium alginate edible coating containing asparagus waste extract on postharvest quality of strawberry fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- Jamesa, K.; Longa, H.; O’Haraa, I.; Williamsa, B.; Moghaddama, L. Bio-based film development: Harnessing alginate and fucoidan extracted from Ascophyllum nodosum with glycerol and choline chloride-based solvent. Food Hydrocoll. 2024, 153, 110013. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Hornero-Méndez, D. Identification and quantitative analysis of carotenoids and their esters from strawberries (Fragaria × ananassa). J. Agric. Food Chem. 2012, 60, 9607–9615. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Popa, E.E.; Drăghici, M.C.; Popescu, P.A.; Popa, V.I.; Bujor, O.C.; Ion, V.A.; Popa, M.E. Latest developments in edible coatings on minimally processed fruits and vegetables: A review. Foods 2021, 10, 2821. [Google Scholar] [CrossRef]
- Natalia, G.; Melián, E.; Acebal, C.C.; Domini, C.E.; Faccio, R.; López, O.V. Harnessing antioxidant-rich byproducts from olive and wine industries to produce corn starch films as edible coatings for minimally processed foods. Food Biosci. 2025, 69, 106802. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michałowska, A. Enzymatic browning in apple products and its inhibition treatments: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5038–5076. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Athiyappan, K.D.; Routray, W.; Paramasivan, B. Phycocyanin from Spirulina: A comprehensive re-view on cultivation, extraction, purification, and its application in food and allied industries. Food Hum. 2024, 2, 100235. [Google Scholar] [CrossRef]
- Martínez-Burgos, W.J.; Pozzan, R.; Severo, I.A.; Ordonez, J.C. Chapter 1—Introductory Chapter: Cyanobacteria—An Overview. In Insights into Algae—Fundamentals, Culture Techniques and Biotechnological Uses of Microalgae and Cyanobacteria; Severo, I.A., Martinez-Burgos, W.J., Ordonez, J., Eds.; IntechOpen: London, UK, 2024. [Google Scholar]
- Valero, D.; Serrano, M. Postharvest biology and technology for preserving fruit quality. Food Sci. Technol. Int. 2010, 16, 385–395. [Google Scholar]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Raymundo, A. Microalgae biomass incorporation in edible films and coatings: A new strategy toward improving food shelf life. Foods 2020, 9, 571. [Google Scholar]
- Silva, S.B.; Rocha, C.M.R.; Coimbra, M.A. Seaweed polysaccharides in edible coatings and films for the preservation of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2021, 61, 1563–1579. [Google Scholar]
- Margesin, R.; Bergauer, P.; Gander, S. Degradation of phenol and toxicity of phenolic compounds: A comparison of cold-tolerant Arthrobacter sp. and mesophilic Pseudomonas putida. Extremophiles 2004, 8, 201–207. [Google Scholar] [CrossRef] [PubMed]
- ASTM S500 Third Edition; Standard & Reference Guide for Professional Water Damage Restoration. ASTM International: West Conshohocken, PA, USA, 2021.
- Hamad, G.M.; El-Baky, N.A.; Sharaf, M.M.; Amara, A.A. Volatile Compounds, Fatty Acids Constituents, and Antimicrobial Activity of Cultured Spirulina (Arthrospira fusiformis) Isolated from Lake Mariout in Egypt. Sci. World J. 2023, 2023, 9919814. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Castillo, G.C.; Rodrigo, D.; Martínez, A.; Pina-Pérez, M.C. Bioactivity of Fucoidan as an Antimicrobial Agent in a New Functional Beverage. Beverages 2018, 4, 64. [Google Scholar] [CrossRef]
- Kurtböke, D.I.; Palk, A.; Marker, A.; Neuman, C.; Moss, L.; Streeter, K.; Katouli, M. Isolation and characterization of Enterobacteriaceae species infesting post-harvest strawberries and their biological control using bacteriophages. Environ. Biotechnol. 2016, 100, 8593–8606. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, V.F.R.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Sustainable Edible Coatings Enriched with Bioactive Extracts from Exhausted Olive Pomace, Fucus Spiralis, and Limnospira sp. for the Postharvest Preservation of Strawberries. Coatings 2025, 15, 1085. https://doi.org/10.3390/coatings15091085
Martins VFR, Pintado M, Morais RMSC, Morais AMMB. Sustainable Edible Coatings Enriched with Bioactive Extracts from Exhausted Olive Pomace, Fucus Spiralis, and Limnospira sp. for the Postharvest Preservation of Strawberries. Coatings. 2025; 15(9):1085. https://doi.org/10.3390/coatings15091085
Chicago/Turabian StyleMartins, Valter F. R., Manuela Pintado, Rui M. S. C. Morais, and Alcina M. M. B. Morais. 2025. "Sustainable Edible Coatings Enriched with Bioactive Extracts from Exhausted Olive Pomace, Fucus Spiralis, and Limnospira sp. for the Postharvest Preservation of Strawberries" Coatings 15, no. 9: 1085. https://doi.org/10.3390/coatings15091085
APA StyleMartins, V. F. R., Pintado, M., Morais, R. M. S. C., & Morais, A. M. M. B. (2025). Sustainable Edible Coatings Enriched with Bioactive Extracts from Exhausted Olive Pomace, Fucus Spiralis, and Limnospira sp. for the Postharvest Preservation of Strawberries. Coatings, 15(9), 1085. https://doi.org/10.3390/coatings15091085








