Taguchi Optimization of Corrosion Resistance and Wettability of a-C Films on SS316L Deposited via Magnetron Sputtering Technique
Abstract
1. Introduction
2. Experimental
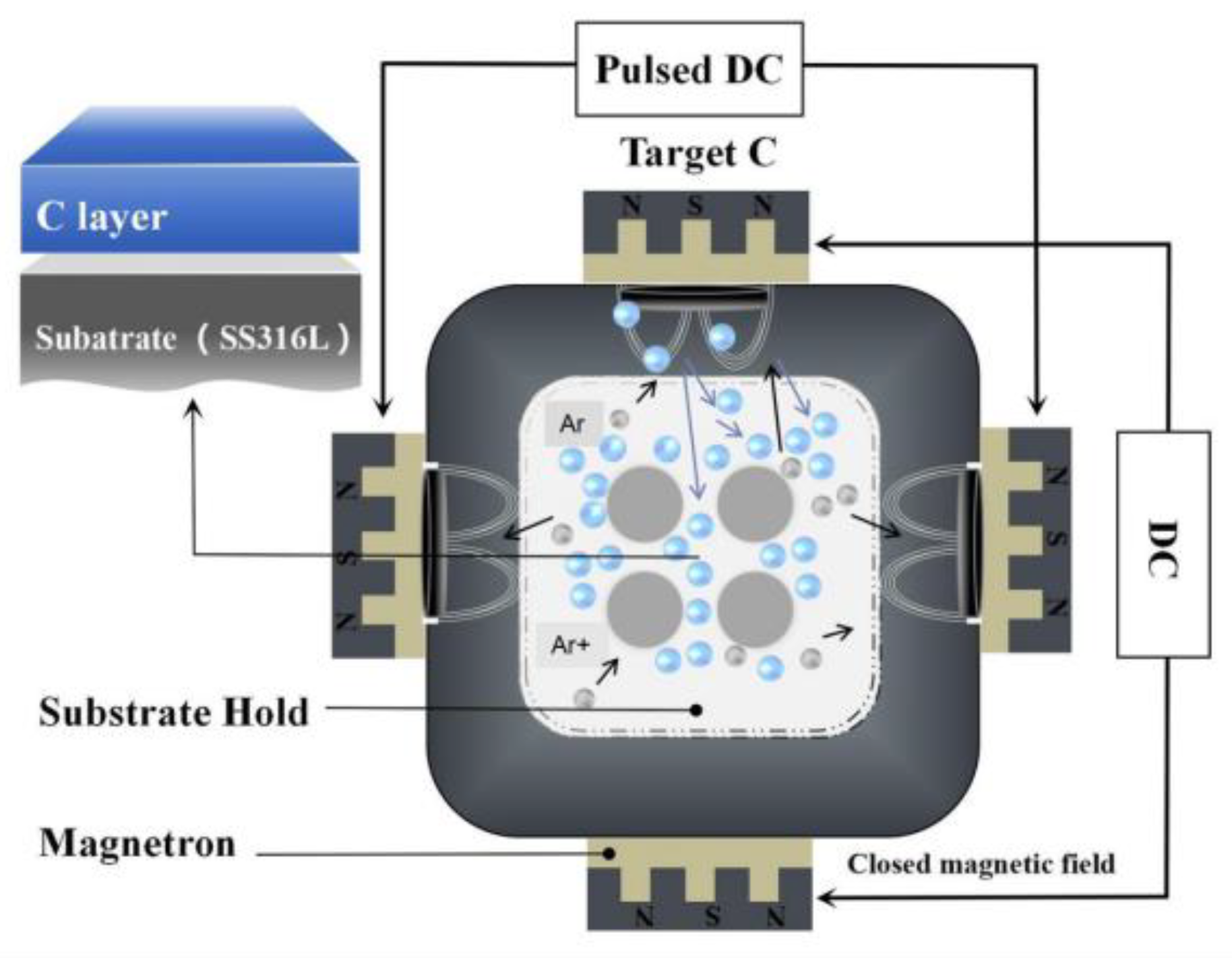
2.1. Materials and Preparation
2.2. Taguchi Design of Experiments
2.3. Film Evaluation
3. Thin Film Growth Morphology Analysis
3.1. Microstructural Characterization
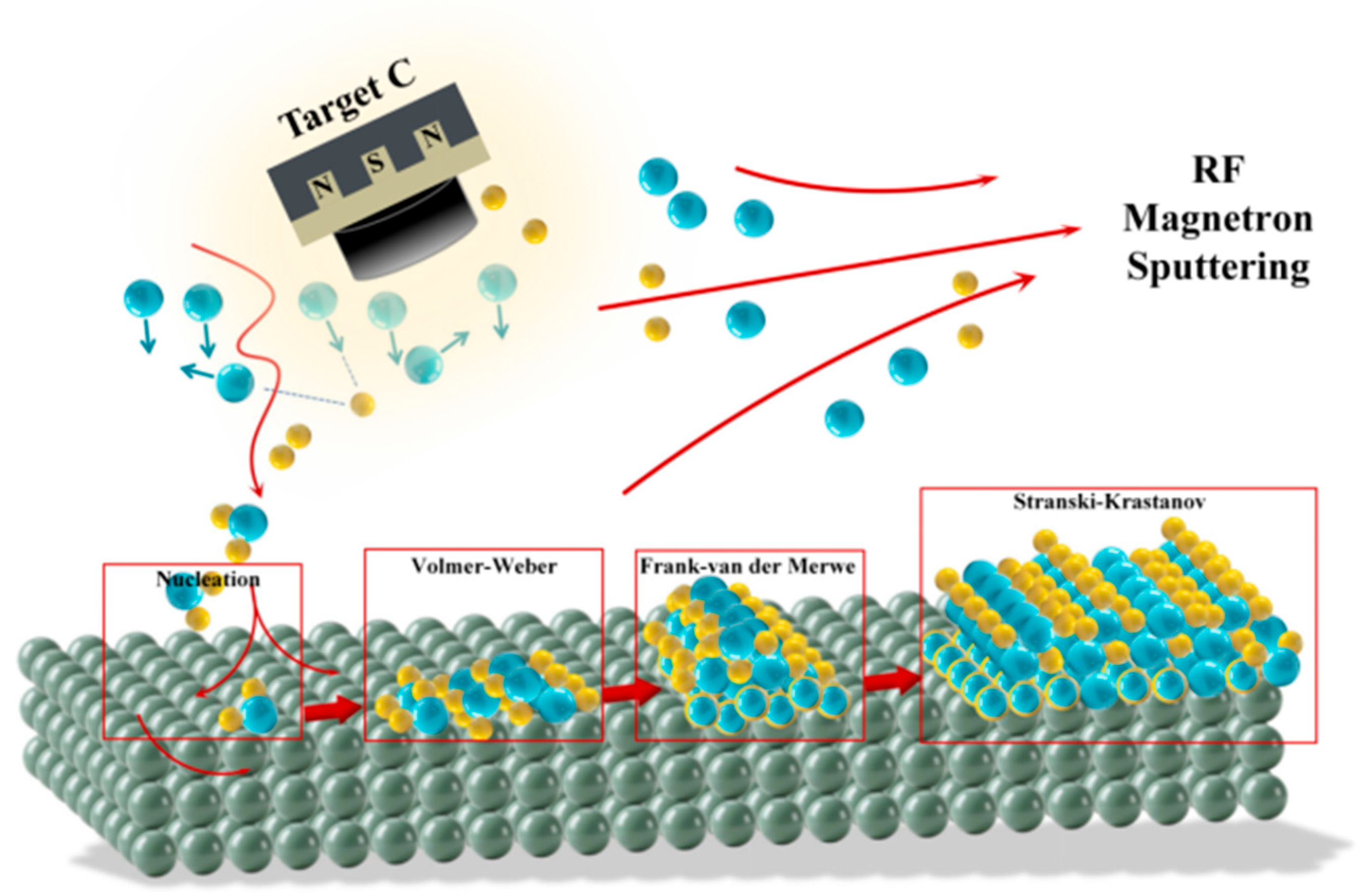
3.2. Mechanistic Insights into Film Formation
3.2.1. Initial Stage: Atomic Deposition, Diffusion, and Ducleation
3.2.2. Film Formation: Island Growth and Coalescence
3.2.3. Film Growth: Competition of Layer-by-Layer Growth
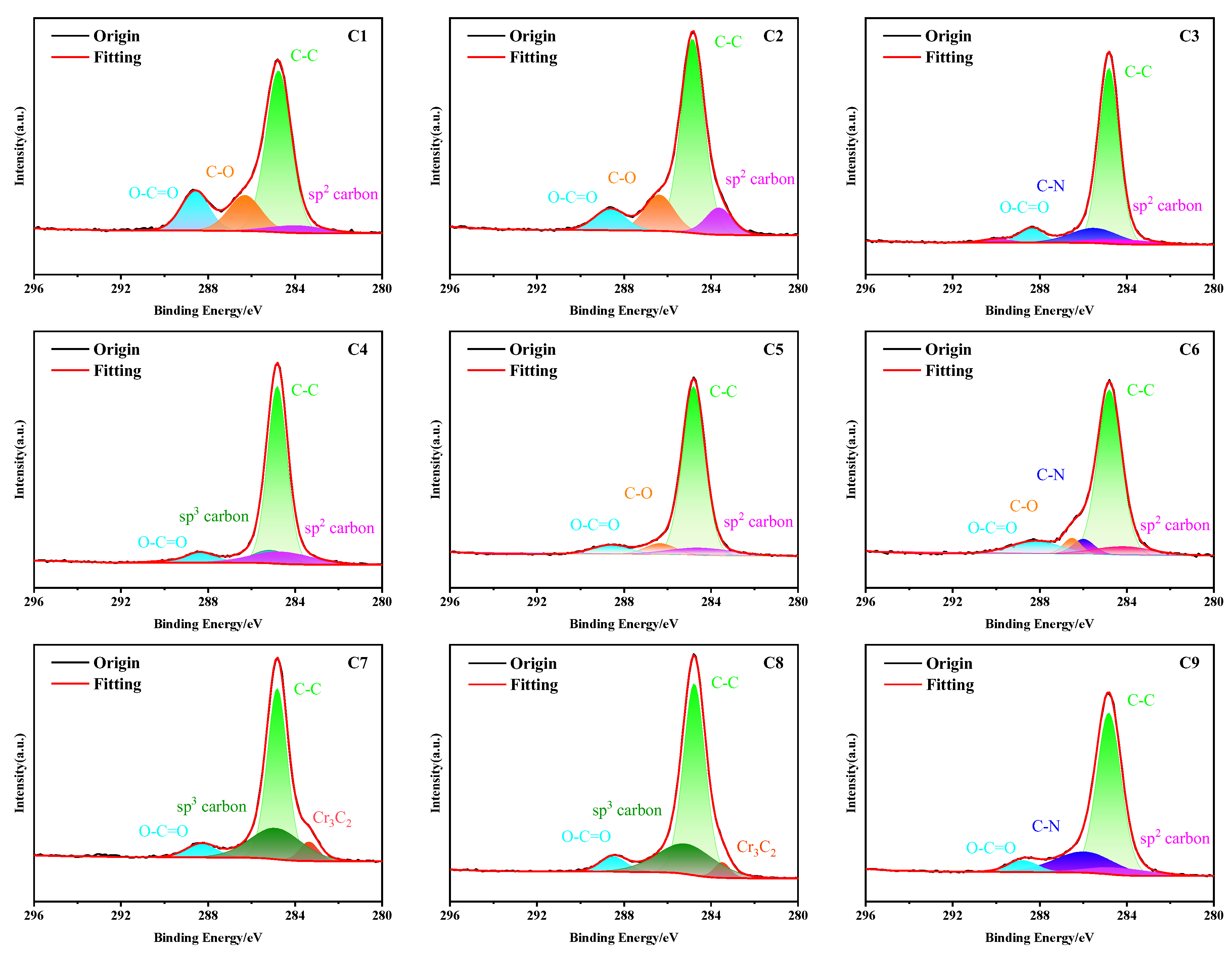
3.3. XPS Analysis
4. Results and Discussion
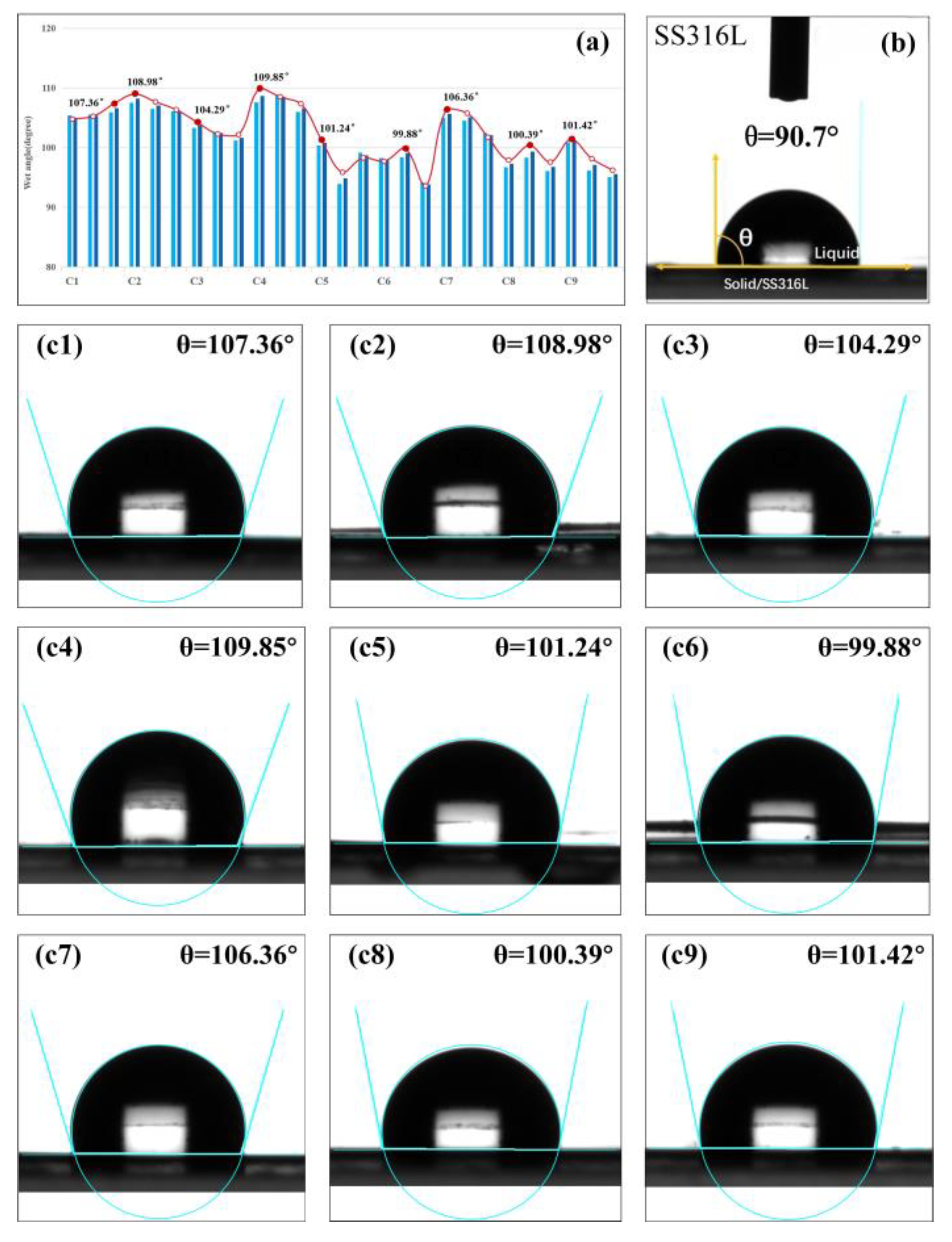
4.1. Surface Wettability of a-C Films
4.2. Electrochemical Corrosion Behavior
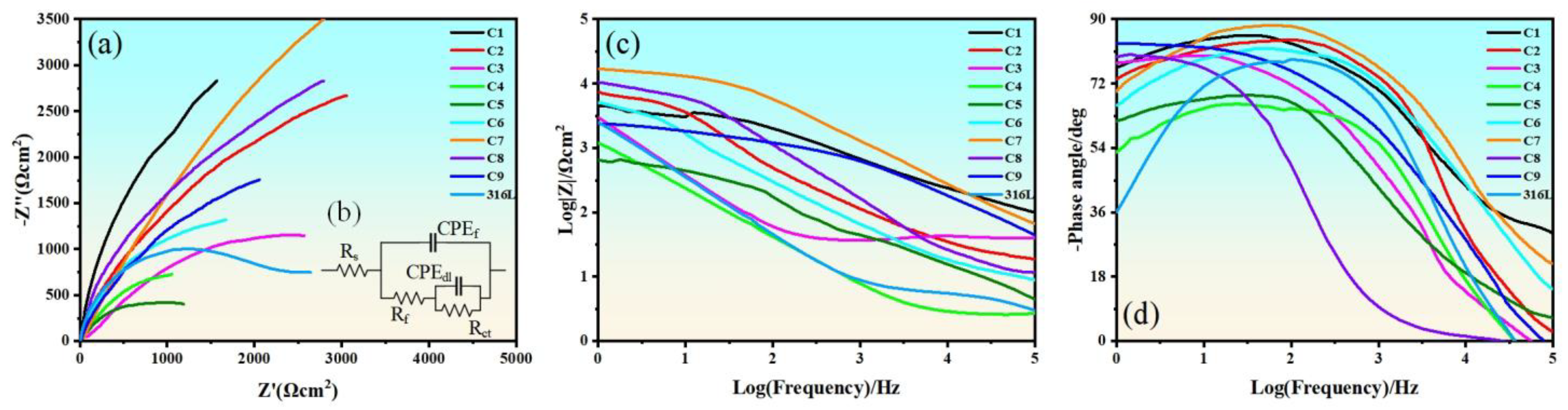
4.3. Electrochemical Impedance Spectroscopy
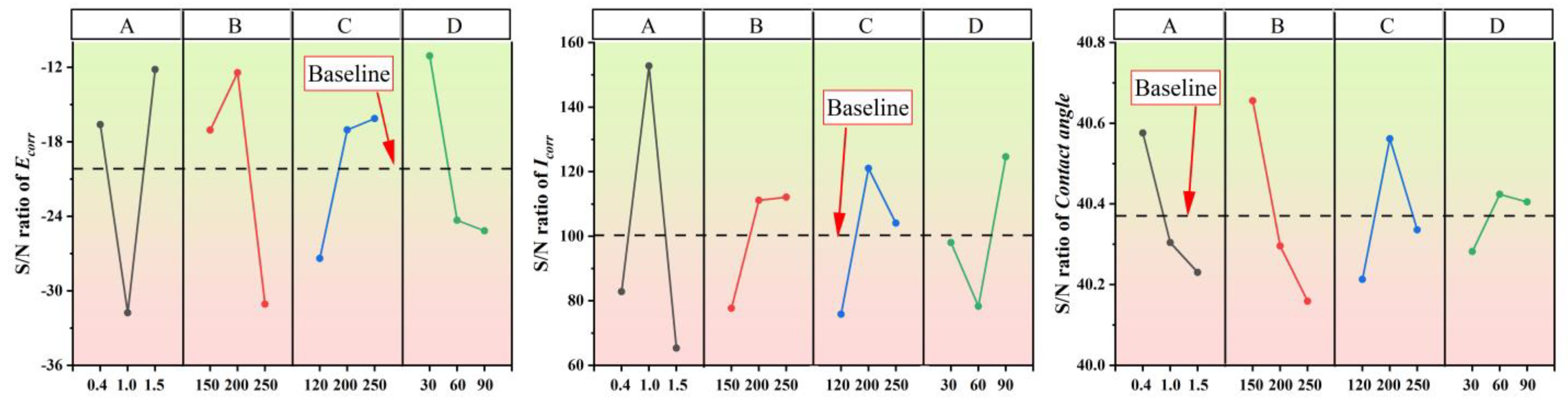
4.4. S/N Analysis
4.5. Comprehensive Performance Analysis
5. Conclusions
- Based on cross-sectional morphology observations by FE-SEM combined with atomic migration and collision theory, the physical growth mechanism of a-C films was systematically investigated. The study revealed the competitive mechanism between island-like and layer-by-layer growth modes, confirming that sputtered carbon atoms undergo a complex dynamic process involving surface diffusion, cluster aggregation, and structural evolution on the substrate surface. These findings provide a theoretical foundation for the structural regulation of thin films.
- The orthogonal experimental results demonstrated that deposition time predominantly influenced corrosion potential (45.53%), working pressure significantly affected corrosion current density (66.83%), and sputtering power was the primary factor governing contact angle (48.36%), providing quantitative insight into the coupled effects of process parameters on a-C films’ performance.
- A comprehensive performance evaluation of the a-C films was conducted using the TOPSIS method. The optimal set of process parameters was identified at a working pressure of 1.5 Pa, sputtering power of 150 W, substrate bias of −250 V, and a deposition time of 60 min. Under these conditions, the corrosion current density reached a minimum of 1.61 × 10−6 A·cm−2, and the corresponding corrosion rate was reduced to 0.018 mm/y. This study provides a reliable process optimization strategy and performance enhancement solution for the surface modification of fuel cell bipolar plates.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aono, M.; Terauchi, M.; Sato, Y.K.; Morita, K.; Inoue, T.; Kanda, K.; Yonezawa, K. Deposition of Amorphous Carbon Nitride Thin Films Using Pressure-Gradient RF Magnetron Sputtering and Their Chemical Bonding Structures. Appl. Surf. Sci. 2023, 635, 157677. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, C.; Wang, T.; Zhao, J. Construction and Electrochemical Corrosion Behavior of Carbon-Based Thin Films on SS316L Bipolar Plates by Magnetron Sputtering. Appl. Surf. Sci. 2025, 698, 163055. [Google Scholar] [CrossRef]
- Aghaei, A.A.; Eshaghi, A.; Ramazani, M.; Zabolian, H.; Abbasi-Firouzjah, M. Investigation of Silicon Carbon Oxynitride Thin Film Deposited by RF Magnetron Sputtering. Appl. Surf. Sci. Adv. 2024, 19, 100546. [Google Scholar] [CrossRef]
- Cheng, J.; Mi, B.; Wang, Q.; Wang, H.; Zhou, T.; Li, Y.; Hou, H.; Zhu, Y. Research on Magnetron Sputtering Thin Films as Electrode Materials for Supercapacitors. Chem. Eng. J. 2025, 509, 161242. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Xu, Z.; Toshiaki, N.; Xi, Y.; Ni, Q. Effect of Heat Treatment on Mechanical Property of Amorphous Carbon Films by Magnetron Sputtering. Diam. Relat. Mat. 2022, 129, 109328. [Google Scholar] [CrossRef]
- Kim, I.; Shim, C.; Kim, S.W.; Lee, C.; Kwon, J.; Byun, K.; Jeong, U. Amorphous Carbon Films for Electronic Applications. Adv. Mater. 2023, 35, 2204912. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Chen, Z.; Wang, Q.; Li, Y.; Qin, Z.; Wang, H. Properties of C-Doped CrTiN Films on the 316L Stainless Steel Bipolar Plate for PEMFC. Int. J. Hydrogen Energy 2021, 46, 32645–32654. [Google Scholar] [CrossRef]
- Liu, R.; Jia, Q.; Zhang, B.; Lai, Z.; Chen, L. Protective Coatings for Metal Bipolar Plates of Fuel Cells: A Review. Int. J. Hydrogen Energy 2022, 47, 22915–22937. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Zhou, T.; Wu, H.; Lai, X. Development and Characterization of Multilayered Cr–C/a-C:Cr Film on 316L Stainless Steel as Bipolar Plates for Proton Exchange Membrane Fuel Cells. J. Power Sources 2013, 230, 25–31. [Google Scholar] [CrossRef]
- Zhou, H.; Jiao, D.; Ding, H.; Qiu, W.; Zhong, X.; Liu, Z. Effect of Magnetron Sputtering C-Doped CrN Film on the Conductivity and Corrosion Resistance of 304 Stainless Steel Bipolar Plates. Surf. Coat. Technol. 2024, 483, 130769. [Google Scholar] [CrossRef]
- Mani, S.P.; Kalaiyarasan, M.; Agilan, P.; Ravichandran, K.; Rajendran, N.; Meng, Y. Evaluation of Anticorrosion and Contact Resistance Behavior of Poly(Orthophenylenediamine)-Coated 316L SS Bipolar Plate for Proton Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2022, 47, 41097–41110. [Google Scholar] [CrossRef]
- Chang, L.; Luo, X.; Ding, Y.; Zhang, J.; Gong, X.; Zhong, Y.; Yao, J.; Song, J.; Deng, Z.; Dong, C. Improved Corrosion Resistance and Interfacial Conductivity of A-C/(Ti:C)/Ti Nano-Thin Film for 316L Stainless Steel Bipolar Plates. Thin Solid Films 2024, 794, 140294. [Google Scholar] [CrossRef]
- Lin, C.H.; Tsai, S.Y. An Investigation of Coated Aluminium Bipolar Plates for PEMFC. Appl. Energy 2012, 100, 87–92. [Google Scholar] [CrossRef]
- Pugal Mani, S.; Srinivasan, A.; Rajendran, N. Effect of Nitrides on the Corrosion Behaviour of 316L SS Bipolar Plates for Proton Exchange Membrane Fuel Cell (PEMFC). Int. J. Hydrogen Energy 2015, 40, 3359–3369. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, G.; Hou, M.; Wu, B.; Shao, Z.; Yi, B. Carbon-Based Films Coated 316L Stainless Steel as Bipolar Plate for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 405–409. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.T.; Li, Z.X.; Wang, Y.F.; Li, H.Z.; Lei, J.J. Corrosion and Conductivity Behavior of Titanium-Doped Amorphous Carbon Film Coated SS316L in the Environment of PEMFCs. Mater. Chem. Phys. 2022, 276, 125234. [Google Scholar] [CrossRef]
- Mingge, W.; Congda, L.; Dapeng, T.; Tao, H.; Guohai, C.; Donghui, W. Effects of Metal Buffer Layer for Amorphous Carbon Film of 304 Stainless Steel Bipolar Plate. Thin Solid Films 2016, 616, 507–514. [Google Scholar] [CrossRef]
- Bi, F.; Li, X.; Yi, P.; Hou, K.; Peng, L.; Lai, X. Characteristics of Amorphous Carbon Films to Resist High Potential Impact in PEMFCs Bipolar Plates for Automotive Application. Int. J. Hydrogen Energy 2017, 42, 14279–14289. [Google Scholar] [CrossRef]
- Jia, Q.; Mu, Z.; Zhang, X.; Zhang, B.; Liu, R.; Gao, K.; Yu, Y.; Lai, Z.; Zhang, J. Electronic Conductive and Corrosion Mechanisms of Dual Nanostructure CuCr-Doped Hydrogenated Carbon Films for SS316L Bipolar Plates. Mater. Today Chem. 2021, 21, 100521. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.T.; Li, Z.X.; Wang, Y.F.; Li, H.Z.; Lei, J.J. Corrosion Resistance and Conductivity of Amorphous Carbon Coated SS316L and TA2 Bipolar Plates in Proton-Exchange Membrane Fuel Cells. Diam. Relat. Mat. 2021, 118, 108503. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Feng, L.; Gan, P.; Lai, X. Performance of a Proton Exchange Membrane Fuel Cell Stack Using Conductive Amorphous Carbon-Coated 304 Stainless Steel Bipolar Plates. J. Power Sources 2010, 195, 7061–7066. [Google Scholar] [CrossRef]
- Jin, W.; Feng, K.; Li, Z.; Cai, X.; Yu, L.; Zhou, D. Improvement of Corrosion Resistance and Electrical Conductivity of 304 Stainless Steel Using Close Field Unbalanced Magnetron Sputtered Carbon Film. J. Power Sources 2011, 196, 10032–10037. [Google Scholar] [CrossRef]
- Larijani, M.M.; Yari, M.; Afshar, A.; Jafarian, M.; Eshghabadi, M. A Comparison of Carbon Coated and Uncoated 316L Stainless Steel for Using as Bipolar Plates in PEMFCs. J. Alloys Compd. 2011, 509, 7400–7404. [Google Scholar] [CrossRef]
- Li, X.; Peng, L.; Zhang, D.; Yi, P.; Lai, X. The Frequency of Pulsed DC Sputtering Power Introducing the Graphitization and the Durability Improvement of Amorphous Carbon Films for Metallic Bipolar Plates in Proton Exchange Membrane Fuel Cells. J. Power Sources 2020, 466, 228346. [Google Scholar] [CrossRef]
- Ahmad, I.; Roy, S.S.; Maguire, P.D.; Papakonstantinou, P.; McLaughlin, J.A. Effect of Substrate Bias Voltage and Substrate on the Structural Properties of Amorphous Carbon Films Deposited by Unbalanced Magnetron Sputtering. Thin Solid Films 2005, 482, 45–49. [Google Scholar] [CrossRef]
- Bi, F.; Hou, K.; Yi, P.; Peng, L.; Lai, X. Mechanisms of Growth, Properties and Degradation of Amorphous Carbon Films by Closed Field Unbalanced Magnetron Sputtering on Stainless Steel Bipolar Plates for PEMFCs. Appl. Surf. Sci. 2017, 422, 921–931. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, W.; Bi, F.; Peng, L.; Lai, X. Microstructure and Properties of A-C Films Deposited under Different Argon Flow Rate on Stainless Steel Bipolar Plates for Proton Exchange Membrane Fuel Cells. J. Power Sources 2019, 410–411, 188–195. [Google Scholar] [CrossRef]
- Do, N.T.; Dinh, V.H.; Lich, L.V.; Dang-Thi, H.H.; Nguyen, T.G. Effects of Substrate Bias Voltage on Structure of Diamond-Like Carbon Films on AISI 316L Stainless Steel: A Molecular Dynamics Simulation Study. Materials 2021, 14, 4925. [Google Scholar] [CrossRef]
- Alaefour, I.; Shahgaldi, S.; Zhao, J.; Li, X. Synthesis and Ex-Situ Characterizations of Diamond-like Carbon Coatings for Metallic Bipolar Plates in PEM Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 11059–11070. [Google Scholar] [CrossRef]
- Feng, K.; Shen, Y.; Sun, H.; Liu, D.; An, Q.; Cai, X.; Chu, P.K. Conductive Amorphous Carbon-Coated 316L Stainless Steel as Bipolar Plates in Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 6771–6777. [Google Scholar] [CrossRef]
- Yari, M.; Larijani, M.M.; Afshar, A.; Eshghabadi, M.; Shokouhy, A. Physical Properties of Sputtered Amorphous Carbon Coating. J. Alloys Compd. 2012, 513, 135–138. [Google Scholar] [CrossRef]
- Afshar, A.; Yari, M.; Larijani, M.M.; Eshghabadi, M. Effect of Substrate Temperature on Structural Properties and Corrosion Resistance of Carbon Thin Films Used as Bipolar Plates in Polymer Electrolyte Membrane Fuel Cells. J. Alloys Compd. 2010, 502, 451–455. [Google Scholar] [CrossRef]
- Dong, H.; He, Z.; Zhang, S.; Sun, D. Effect of Temperature and Bias Voltage on Electrical and Electrochemical Properties of Diamond-like Carbon Films Deposited with HiPIMS. Surf. Coat. Technol. 2019, 358, 987–993. [Google Scholar] [CrossRef]
- Hu, R.; Tang, J.; Zhu, G.; Deng, Q.; Lu, J. The Effect of Duty Cycle and Bias Voltage for Graphite-like Carbon Film Coated 304 Stainless Steel as Metallic Bipolar Plate. J. Alloys Compd. 2019, 772, 1067–1078. [Google Scholar] [CrossRef]
- Li, H.; Guo, P.; Zhang, D.; Liu, L.; Wang, Z.; Ma, G.; Xin, Y.; Ke, P.; Saito, H.; Wang, A. Interface-Induced Degradation of Amorphous Carbon Films/Stainless Steel Bipolar Plates in Proton Exchange Membrane Fuel Cells. J. Power Sources 2020, 469, 228269. [Google Scholar] [CrossRef]
- Efeoglu, I.; Totik, Y.; Gulten, G.; Yaylali, B.; Yesilyurt, M.; Parvin, B.G. Friction and Corrosion Characterization and Parametric Optimization of Nb-Doped Hydrogenated Amorphous Carbon (a-C:H) Thin Films. Surf. Coat. Technol. 2025, 498, 131816. [Google Scholar] [CrossRef]
- Efeoglu, I.; Totik, Y.; Gulten, G.; Yaylali, B.; Yesilyurt, M. Adhesion and Friction-Wear Characterization of W-Doped Hydrogenated Diamond-like Carbon (a-C:H) Coatings. Surf. Coat. Technol. 2025, 495, 131578. [Google Scholar] [CrossRef]
- Arudi, I.S.; Hamzah, E.; Mat Yajid, M.A.; Bushroa, A.R.; Munshi, S.M.; Al-Ashhab, M.S.; Ibrahim, M.Z. Taguchi Optimization of Hardness and Scratch Adhesion Strength of Multilayer Ti/TiN Coatings on Ti- 51 at%Ni Alloy Deposited via Magnetron Sputtering Technique. J. Mater. Res. Technol. 2023, 27, 7664–7672. [Google Scholar] [CrossRef]
- Shanian, A.; Savadogo, O. TOPSIS Multiple-Criteria Decision Support Analysis for Material Selection of Metallic Bipolar Plates for Polymer Electrolyte Fuel Cell. J. Power Sources 2006, 159, 1095–1104. [Google Scholar] [CrossRef]
- Santiago, Ó.; Raso, M.A.; Navarro, E.; Leo, T.J. Selection of Thermoplastic Polymers for Use as Bipolar Plates in Direct Methanol Fuel Cell Applications. Mater. Design 2019, 183, 108148. [Google Scholar] [CrossRef]
- Cai, L.; Liang, C.; Zhang, C.; Wang, Y.; Peng, T.; Fan, M.; Li, R.; Chin, C.S. Design and Investigation of Novel Gradient Flow Fields for Proton Exchange Membrane Fuel Cell. Int. J. Heat Mass Transfer 2024, 224, 125310. [Google Scholar] [CrossRef]
- Lee, T.; Park, J.S.; Oh, S. Effect of sputtering working pressure on the reliability and performance of amorphous indium gallium zinc oxide thin film transistors. AIP Adv. 2024, 14. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kwon, J.H.; Jang, G.S.; Hwang, N.M. Effect of Pressure on the Film Deposition during RF Magnetron Sputtering Considering Charged Nanoparticles. Coatings 2021, 11, 132. [Google Scholar] [CrossRef]
- Jain, H.; Creatore, M.; Poodt, P. Molecular layer deposition of alucone in high aspect ratio trenches: The effect of TMA outgassing on step-coverage. J. Vac. Sci. Technol. A 2022, 41, 012401. [Google Scholar] [CrossRef]
- Kijima, Y.; Hanada, T. Effect of the pressure of sputtering atmosphere on the physical properties of amorphous aluminum oxide films. J. Mater. Sci. 2000, 35, 2193–2199. [Google Scholar] [CrossRef]
- Mitin, D.M.; Serdobintsev, A.A. Effect of scattering of sputtered atoms on the growth rate of films fabricated by magnetron sputtering. Tech. Phys. Lett. 2017, 43, 814–816. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Nguyen, T.A.K.; Dang, N.M.; Lin, C.H.; Lee, M.C.; Wang, Z.Y.; Tsai, Y.C.; Lin, M.T. Effects of RF Magnetron Sputtering Power on the Mechanical Behavior of Zr-Cu-Based Metallic Glass Thin Films. Nanomaterials 2023, 13, 2677. [Google Scholar] [CrossRef]
- Zhou, C.; Li, T.; Wei, X.; Yan, B. Effect of the Sputtering Power on the Structure, Morphology and Magnetic Properties of Fe Films. Metals 2020, 10, 896. [Google Scholar] [CrossRef]
- Mars, K.; Sałęga-Starzecki, M.; Zawadzka, K.M.; Godlewska, E. Influence of Sputtering Power on the Properties of Magnetron Sputtered Tin Selenide Films. Materials 2024, 17, 3132. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, E.; Pedroni, M.; Saleh, M.; Minelli, D.; Firpo, G.; Miorin, E.; Deambrosis, S.M.; Zin, V.; Ripamonti, D.; Origo, L. Effect of Negative Substrate Bias Voltage and Pressure on the Structure and Properties of Tungsten Films Deposited by Magnetron Sputtering Technique. Coatings 2025, 15, 319. [Google Scholar] [CrossRef]
- Mei, H.; Wang, W.; Zhao, J.; Zhong, W.; Qiu, M.; Xu, J.; Gao, K.; Liu, G.; Liang, J.; Gong, W. Effect of Bias Voltage on the Microstructure and Photoelectric Properties of W-Doped ZnO Films. Nanomaterials 2024, 14, 2050. [Google Scholar] [CrossRef] [PubMed]
- Ataie, S.A.; Qashqay, S.M.; Zamani-Meymian, M.R.; Ferreira, F. Effect of Substrate Bias Voltage on Microstructure and Mechanical Properties of Cr-Nb-Ti-Zr-N-O Ceramic Thin Films Produced by Reactive Sputtering. Coatings 2023, 13, 1141. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, K.; Li, Z.; Lu, F.; Huang, J.; Wu, Y.; Chu, P.K. Self-Passivating Carbon Film as Bipolar Plate Protective Coat-ing in Polymer Electrolyte Membrane Fuel Cell. Int. J. Hydrogen Energy 2016, 41, 5783–5792. [Google Scholar] [CrossRef]
- Bi, F.; Peng, L.; Yi, P.; Lai, X. Multilayered Zr–C/a-C Film on Stainless Steel 316L as Bipolar Plates for Proton Exchange Membrane Fuel Cells. J. Power Sources 2016, 314, 58–65. [Google Scholar] [CrossRef]
- Luo, H.; Lü, Y.; Zhao, X.; Zhang, Y. Modulation of Bonding Structure and Mechanical Properties of DLC Coatings by Binary Doping: Molecular Dynamics Simulation. Diam. Relat. Mat. 2025, 157, 112465. [Google Scholar] [CrossRef]
- Parrott, E.S.; Patel, J.B.; Haghighirad, A.-A.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Growth Modes and Quantum Confine-ment in Ultrathin Vapour-Deposited MAPbI3 Films. Nanoscale 2019, 11, 14276–14284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.L.; Wang, Q.; Han, S.; Cao, P.J.; Liu, W.J.; Jia, F.; Zeng, Y.X.; Ma, X.C.; Lu, Y.M. Optimization of Process Parameters for the Electrical Properties in Ga-Doped ZnO Thin Films Prepared by r.f. Magnetron Sputtering. Appl. Surf. Sci. 2014, 298, 208–213. [Google Scholar] [CrossRef]
- Gulten, G.; Yaylali, B.; Efeoglu, I.; Totik, Y.; Kelly, P.; Kulczyk-Malecka, J. Effect of Nb and V Doped Elements on the Me-chanical and Tribological Properties of CrYN Coatings. Surf. Coat. Technol. 2024, 477, 130297. [Google Scholar] [CrossRef]
- Mahmoodnezhad, M.; Abbas Nejad, A.; Ahmadi, M.H. Multi-Objective Optimization and Exergoeconomic Analysis of a Novel Solar Desalination System with Absorption Cooling. Energy 2024, 312, 133702. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Qiao, J.; Wei, J.; Wang, L.; Li, Z.; Zhuo, J. Safety Resilience Evaluation of Hydrogen Refueling Stations Based on Improved TOPSIS Approach. Int. J. Hydrogen Energy 2024, 66, 396–405. [Google Scholar] [CrossRef]


| Component | C | Si | Mn | P | S | Ni | Cr | Mo |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 0.027 | 0.38 | 1.33 | 0.035 | 0.002 | 10.2 | 18.2 | 2.01 |
| Sample | Parameters and Levels 1 | |||
|---|---|---|---|---|
| A | B | C | D | |
| C1 | 1 | 1 | 1 | 1 |
| C2 | 1 | 2 | 2 | 2 |
| C3 | 1 | 3 | 3 | 3 |
| C4 | 2 | 1 | 2 | 3 |
| C5 | 2 | 2 | 3 | 1 |
| C6 | 2 | 3 | 1 | 2 |
| C7 | 3 | 1 | 3 | 2 |
| C8 | 3 | 2 | 1 | 3 |
| C9 | 3 | 3 | 2 | 1 |
| Substrate | SS316L | |||
| Target | Graphite Target | |||
| Gas | Argon | |||
| Substrate-to-Target Distance | 100 mm | |||
| Substrate Holder Rotation Frequency | 5 Hz | |||
| Factor | Process Parameter | Level 1 | Level 2 | Level 3 |
| A | Working pressure (Pa) | 0.4 | 1.0 | 1.5 |
| B | Sputtering power (W) | 150 | 200 | 250 |
| C | Bias voltage (V) | −120 | −200 | −250 |
| D | Deposition time (min) | 30 | 60 | 90 |
| Specimens | OCP (V vs. Ag/AgCl) | Ecorr (V vs. Ag/AgCl) | Icorr (A/cm2) | r (mm/y) | βa (V/dec) | βc (V/dec) |
|---|---|---|---|---|---|---|
| SS316L | 0.026 | −0.040 | 1.58 × 10−5 | 0.183 | 0.204 | 0.140 |
| C1 | 0.224 | −0.036 | 1.90 × 10−6 | 0.021 | 0.081 | 0.177 |
| C2 | −0.257 | 0.023 | 5.89 × 10−6 | 0.066 | 0.182 | 0.158 |
| C3 | 0.007 | −0.262 | 1.05 × 10−5 | 0.119 | 0.289 | 0.130 |
| C4 | 0.004 | −0.27 | 2.88 × 10−5 | 0.326 | 0.315 | 0.119 |
| C5 | 0.197 | −0.013 | 2.37 × 10−5 | 0.268 | 0.139 | 0.197 |
| C6 | −0.036 | −0.298 | 9.63 × 10−6 | 0.108 | 0.318 | 0.144 |
| C7 | 0.082 | 0.05 | 1.61 × 10−6 | 0.018 | 0.162 | 0.200 |
| C8 | 0.003 | −0.152 | 4.30 × 10−6 | 0.049 | 0.171 | 0.162 |
| C9 | 0.043 | −0.011 | 6.26 × 10−6 | 0.071 | 0.118 | 0.252 |
| Sample | Rs (Ωcm2) | CPEf (F/cm2) | Rf (Ωcm2) | CPEdl (F/cm2) | Rct (Ωcm2) |
|---|---|---|---|---|---|
| C1 | 2.68 | 5.42 × 10−8 | 1172 | 1.66 × 10−7 | 7.56 × 10−3 |
| C2 | 2.43 | 6.11 × 10−8 | 1105 | 2.14 × 10−7 | 6.42 × 10−3 |
| C3 | 2.56 | 2.26 × 10−7 | 965 | 3.31 × 10−6 | 1.85 × 10−3 |
| C4 | 2.78 | 1.84 × 10−7 | 997 | 7.24 × 10−7 | 2.78 × 10−3 |
| C5 | 2.95 | 2.07 × 10−7 | 986 | 8.01 × 10−7 | 2.26 × 10−3 |
| C6 | 2.41 | 1.32 × 10−7 | 1023 | 5.23 × 10−7 | 4.83 × 10−3 |
| C7 | 2.54 | 5.51 × 10−8 | 1202 | 1.71 × 10−7 | 7.64 × 10−3 |
| C8 | 2.61 | 5.97 × 10−8 | 1124 | 2.02 × 10−7 | 6.57 × 10−3 |
| C9 | 2.75 | 9.56 × 10−8 | 1054 | 5.09 × 10−7 | 5.32 × 10−3 |
| No. | Parameters and Levels | Ecorr | S/N (dB) | Icorr (×10−6) | S/N (dB) | Contact Angle | S/N (dB) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | |||||||
| C1 | 1 | 1 | 1 | 1 | −0.036 | −11.568 | 1.9 | 33.450 | 107.36 | 40.617 |
| C2 | 1 | 2 | 2 | 2 | 0.023 | −9.816 | 5.89 | 92.414 | 108.98 | 40.747 |
| C3 | 1 | 3 | 3 | 3 | −0.262 | −28.404 | 10.52 | 122.642 | 104.29 | 40.365 |
| C4 | 2 | 1 | 2 | 3 | −0.27 | −30.458 | 28.88 | 175.272 | 109.85 | 40.816 |
| C5 | 2 | 2 | 3 | 1 | −0.013 | −10.842 | 23.72 | 165.014 | 101.24 | 40.107 |
| C6 | 2 | 3 | 1 | 2 | −0.298 | −53.979 | 9.63 | 118.035 | 99.88 | 39.990 |
| C7 | 3 | 1 | 3 | 2 | 0.05 | −9.119 | 1.60 | 24.494 | 106.36 | 40.536 |
| C8 | 3 | 2 | 1 | 3 | −0.152 | −16.595 | 4.30 | 76.016 | 100.39 | 40.034 |
| C9 | 3 | 3 | 2 | 1 | −0.011 | −10.782 | 6.26 | 95.589 | 101.42 | 40.122 |
| Factor | Degree of Freedom | Sum of Fquare | Variance | Contribution (P%) |
|---|---|---|---|---|
| Contact Angle | ||||
| A | 2 | 28.033 | 14.017 | 24.01% |
| B | 2 | 53.098 | 26.549 | 48.36% |
| C | 2 | 34.990 | 17.495 | 23.16% |
| D | 2 | 2.646 | 1.323 | 4.48% |
| Total | 8 | 100 | ||
| Ecorr | ||||
| A | 2 | 0.038 | 0.019 | 24.49% |
| B | 2 | 0.033 | 0.016 | 21.41% |
| C | 2 | 0.013 | 0.007 | 8.76% |
| D | 2 | 0.070 | 0.035 | 45.34% |
| Total | 8 | 100 | ||
| Icorr | ||||
| A | 2 | 497.088 | 248.544 | 66.83% |
| B | 2 | 10.470 | 5.235 | 1.41% |
| C | 2 | 118.042 | 59.021 | 15.87% |
| D | 2 | 118.230 | 59.115 | 15.89% |
| Total | 8 | 100 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Comprehensive Performance | 0.803 | 0.478 | 0.103 | 0.048 | 0.372 | 0.093 | 0.995 | 0.365 | 0.443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhou, C.; Jiang, Z.; Zhao, J.; Wang, T.; Duan, H. Taguchi Optimization of Corrosion Resistance and Wettability of a-C Films on SS316L Deposited via Magnetron Sputtering Technique. Coatings 2025, 15, 1084. https://doi.org/10.3390/coatings15091084
Yang X, Zhou C, Jiang Z, Zhao J, Wang T, Duan H. Taguchi Optimization of Corrosion Resistance and Wettability of a-C Films on SS316L Deposited via Magnetron Sputtering Technique. Coatings. 2025; 15(9):1084. https://doi.org/10.3390/coatings15091084
Chicago/Turabian StyleYang, Xiaoxing, Cunlong Zhou, Zhengyi Jiang, Jingwei Zhao, Tianxiang Wang, and Haojie Duan. 2025. "Taguchi Optimization of Corrosion Resistance and Wettability of a-C Films on SS316L Deposited via Magnetron Sputtering Technique" Coatings 15, no. 9: 1084. https://doi.org/10.3390/coatings15091084
APA StyleYang, X., Zhou, C., Jiang, Z., Zhao, J., Wang, T., & Duan, H. (2025). Taguchi Optimization of Corrosion Resistance and Wettability of a-C Films on SS316L Deposited via Magnetron Sputtering Technique. Coatings, 15(9), 1084. https://doi.org/10.3390/coatings15091084






