Biocrusts Alter the Pore Structure and Water Infiltration in the Top Layer of Rammed Soils at Weiyuan Section of the Great Wall in China
Abstract
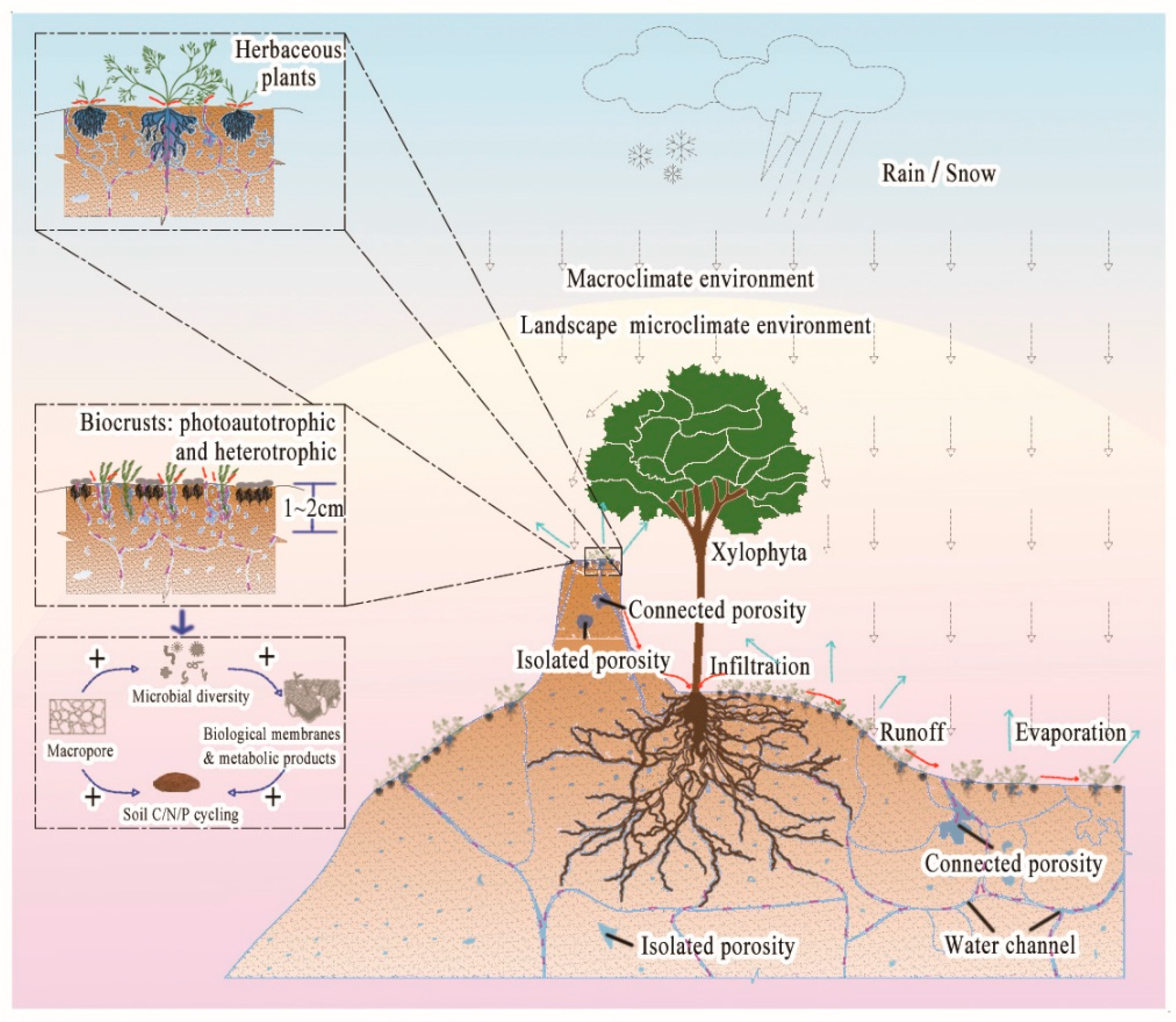
1. Introduction
- (1)
- to characterise and quantify the effects of biocrusts on the pore characteristics of rammed soil at sampled sites, and to reconstruct and visualise the three-dimensional structure of the soil pore network;
- (2)
- to clarify the differences in the effects of biocrusts on the pore parameters of rammed soil at different stages of succession; and
- (3)
- to reveal the key factors affecting the water infiltration into the rammed soil. The results of the study have important value for selecting bio-protective species in the soil sites and guiding the development of site restoration engineerings.
2. Materials and Methods
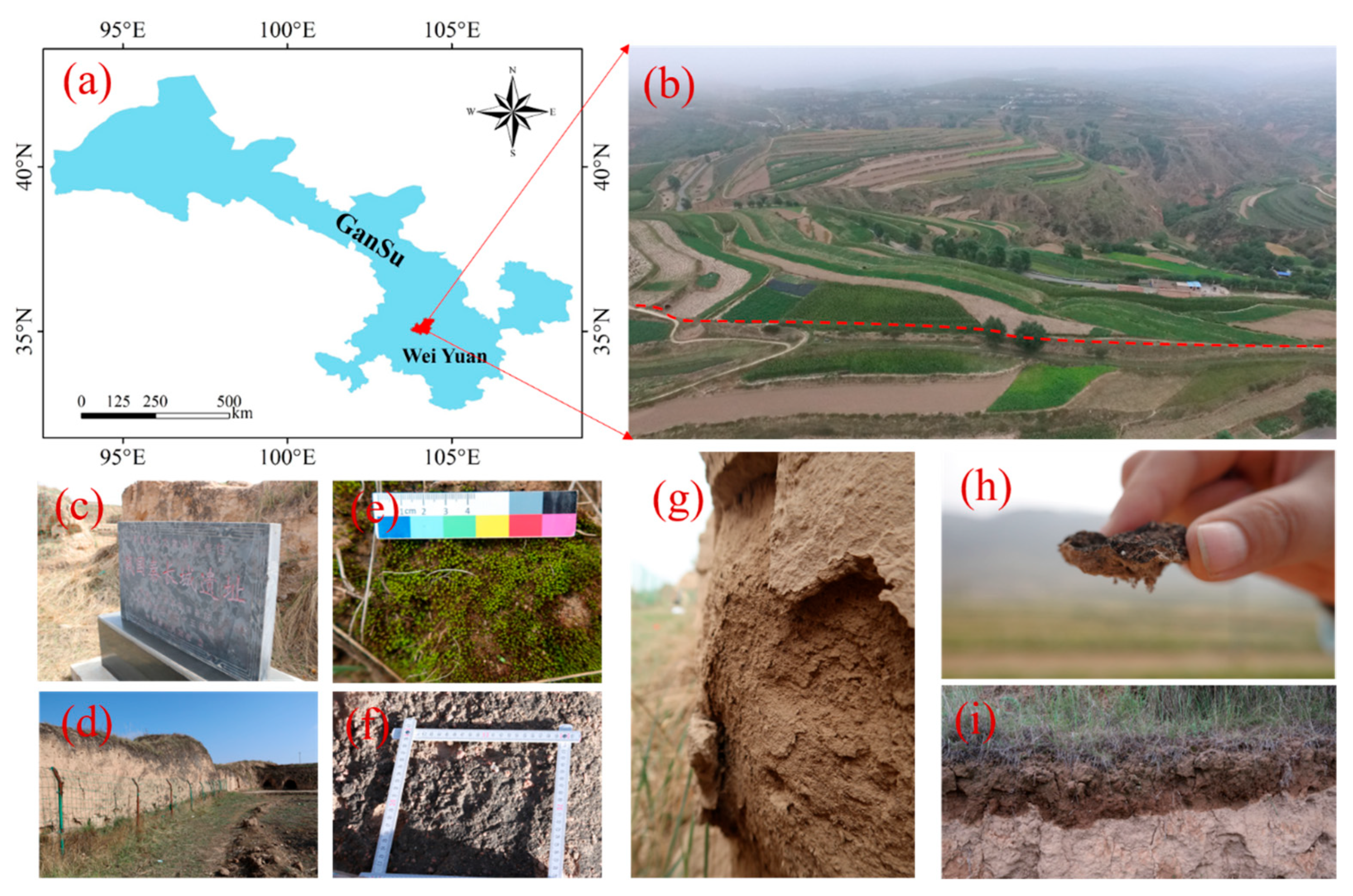
2.1. Study Site
2.2. Experimental Design and Sampling
2.3. Determination of Soil Physical and Chemical Properties
2.4. Characterisation of Soil Microstructure
2.5. Determination of Ct Pore Characterisation Parameters
2.6. Simulation Test of Soil Moisture Infiltration
2.7. Data Analysis and Mapping
3. Results
3.1. Characteristics of Biocrusts and Their Impact on Soil Properties
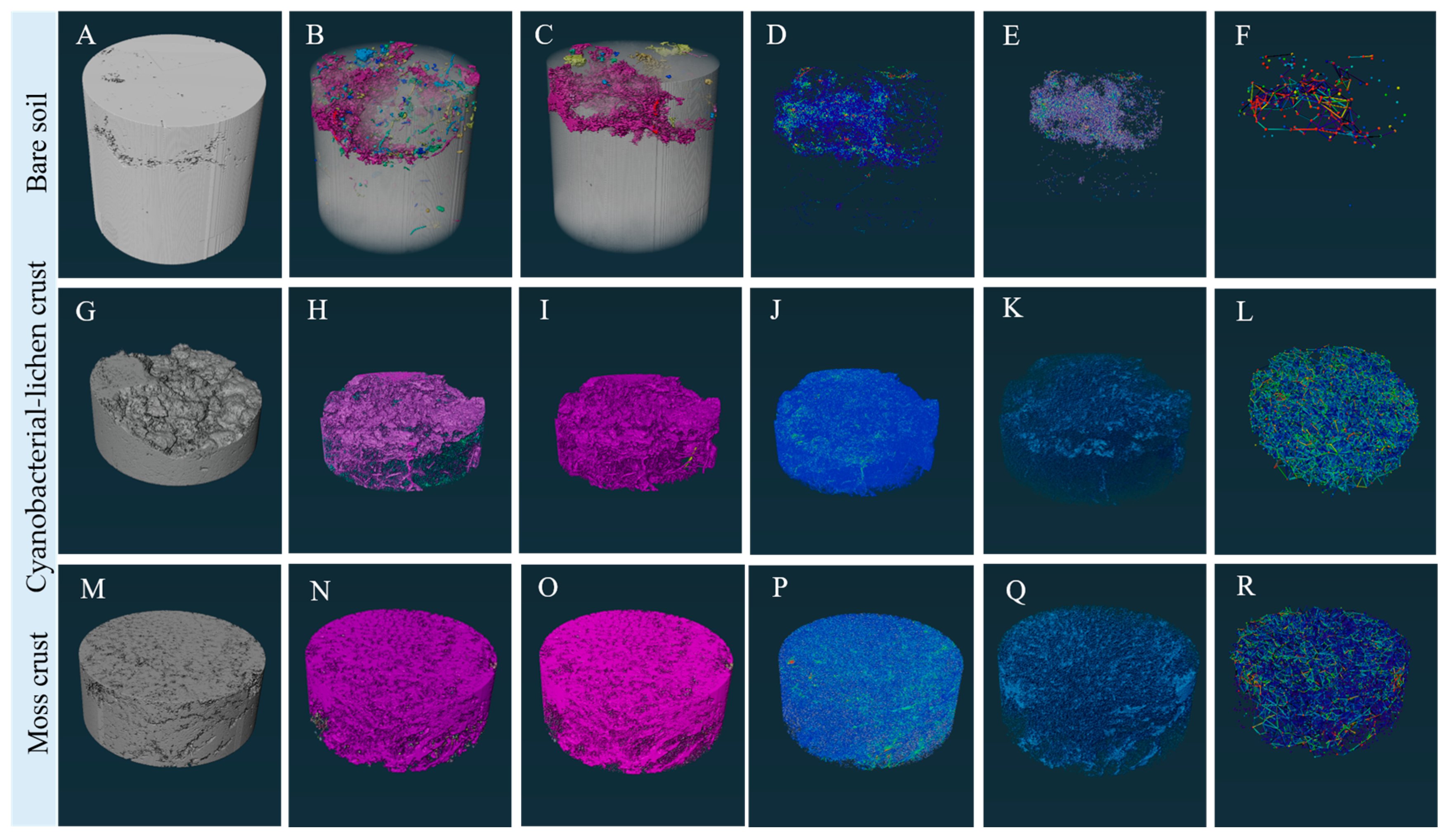
3.2. Microscopic Morphological Differences Between Biocrusts and Rammed Earth
3.3. Effect of Biocrusts on Soil Pore Characteristic Parameters and Spatial Network Structure
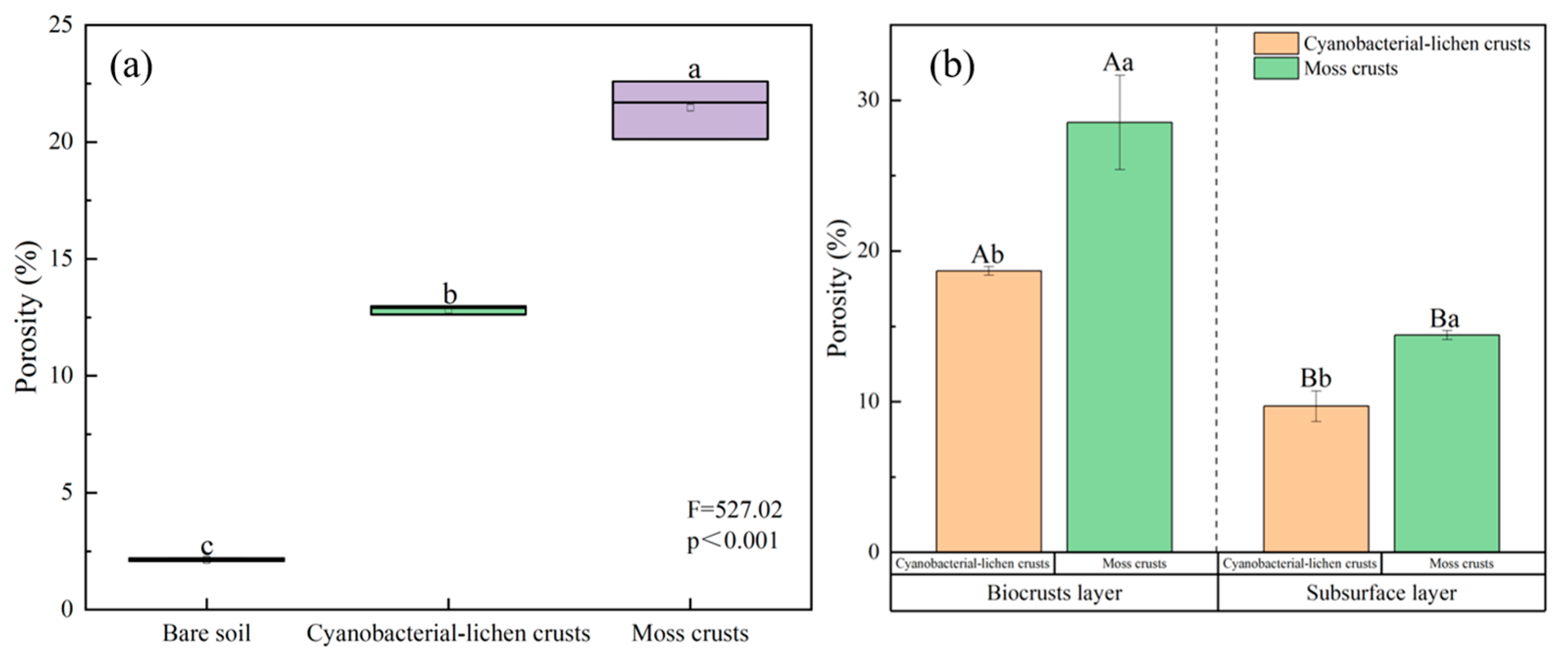
3.3.1. Effect of Biocrusts on Porosity and Pore Size
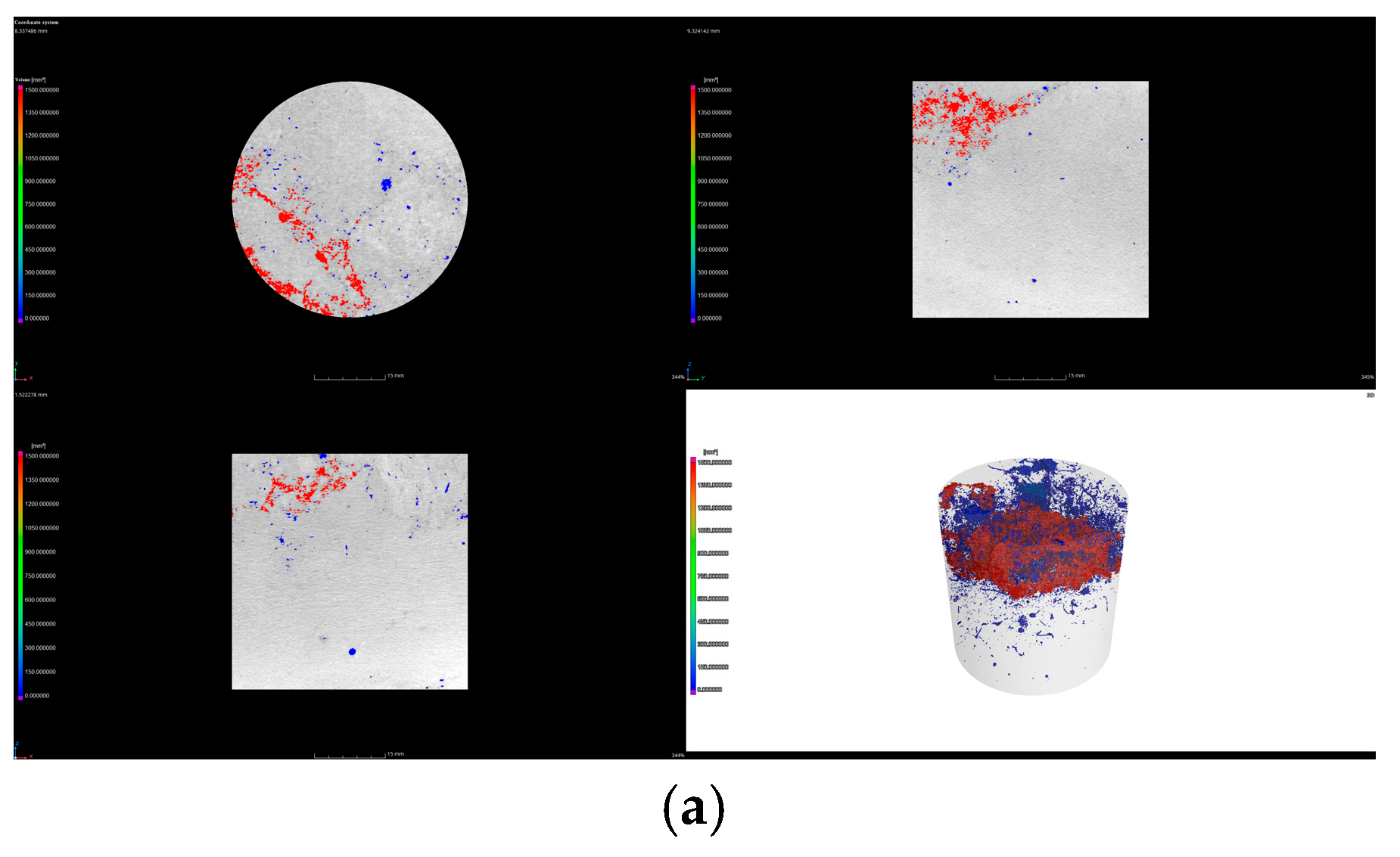
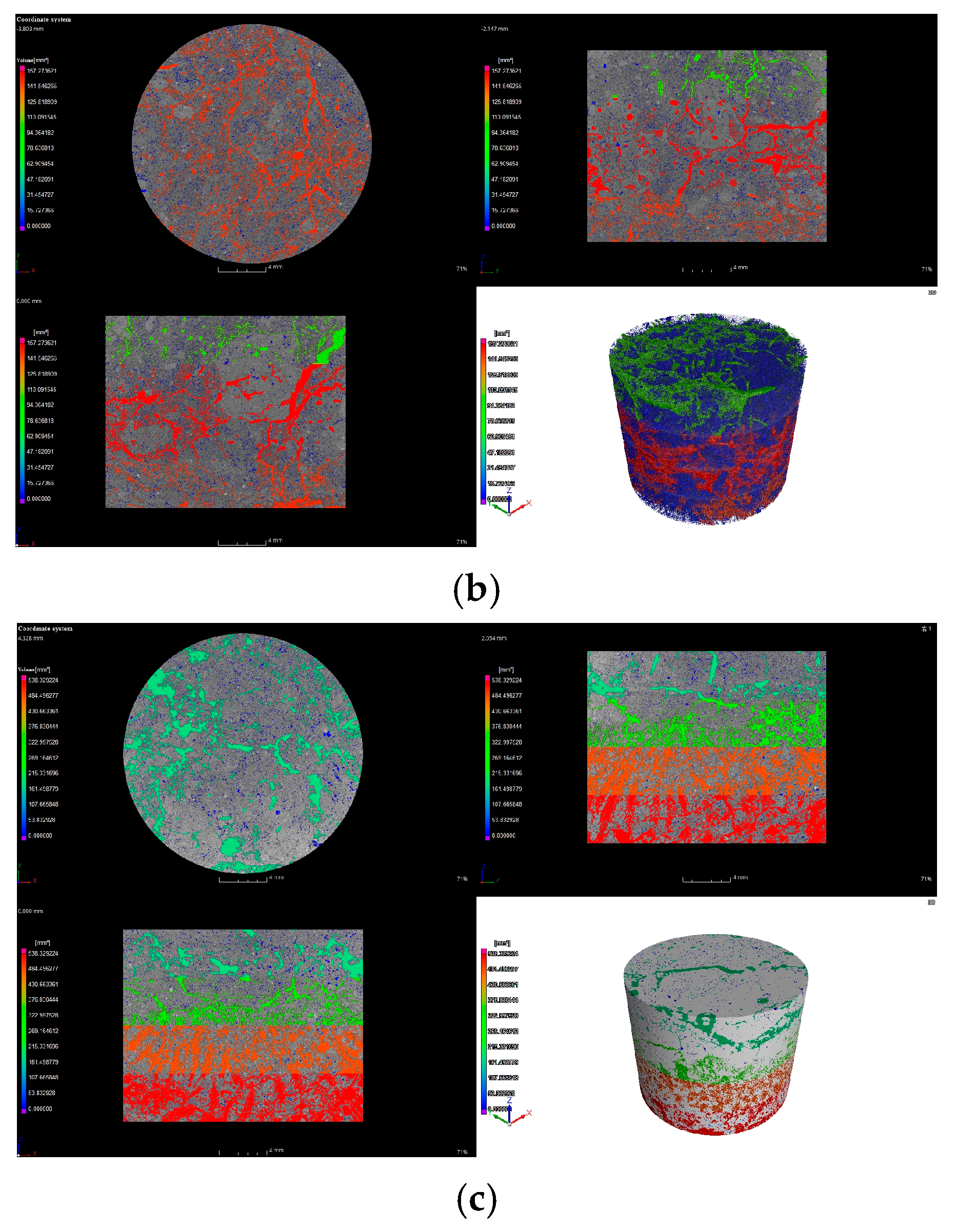
3.3.2. Effect of Biocrusts on Three-Dimensional Soil Pore Characteristics
3.3.3. Effect of Biocrusts on Characterisation Parameters of Porosity
3.3.4. Effect of Biocrusts on Modelling of Soil Pore Networks
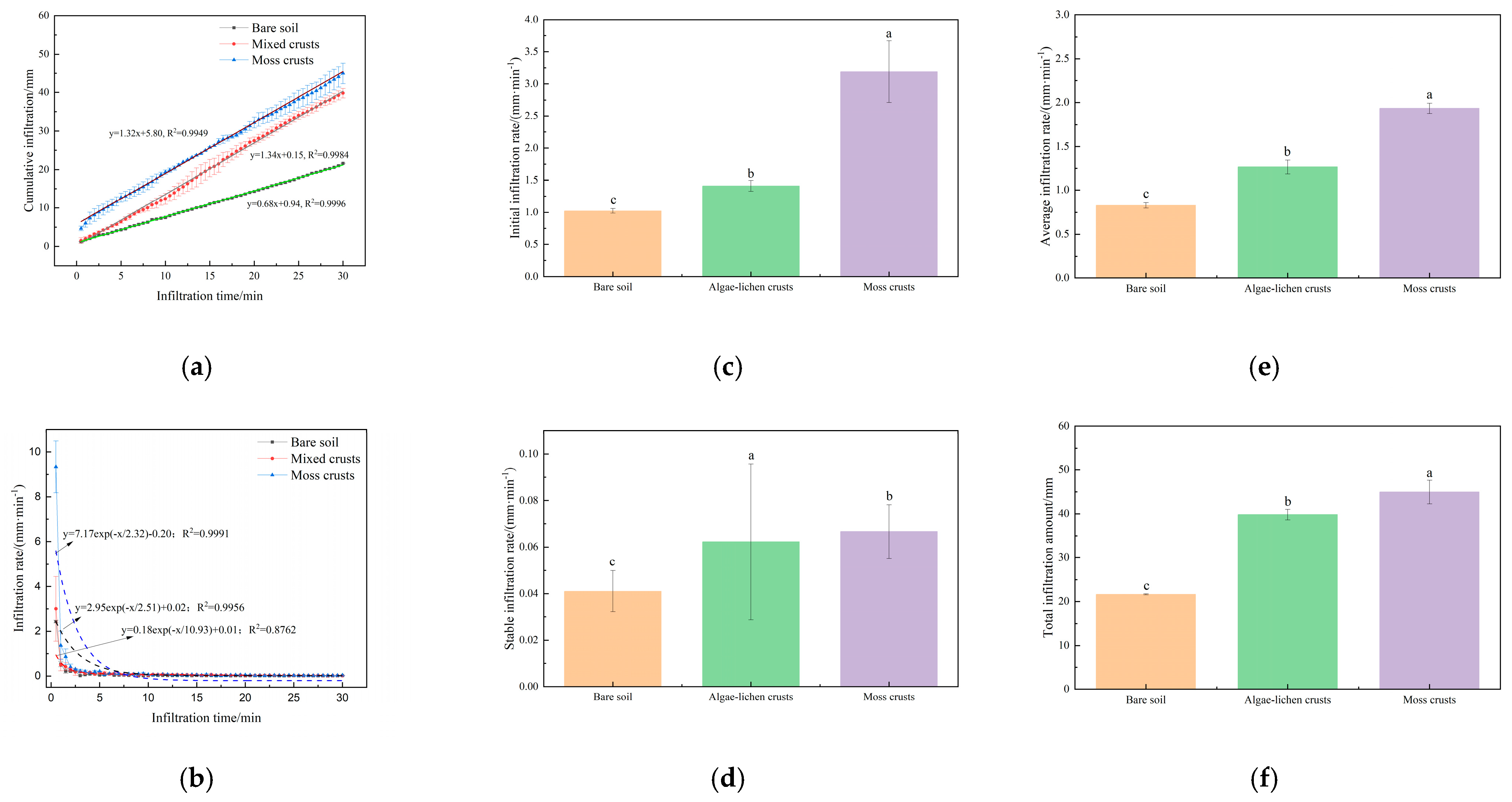
3.4. Impact of Biocrusts on Precipitation Infiltration
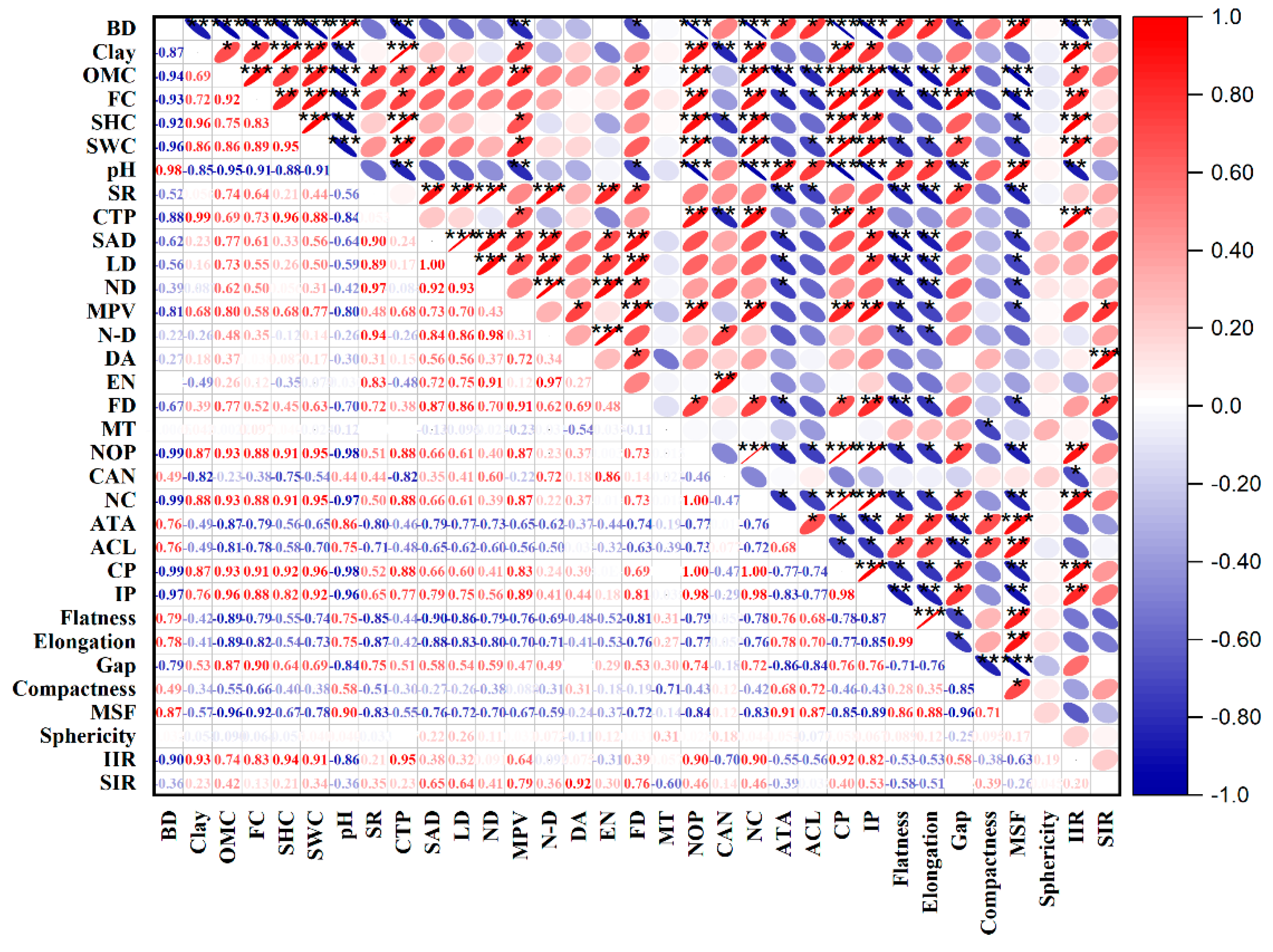
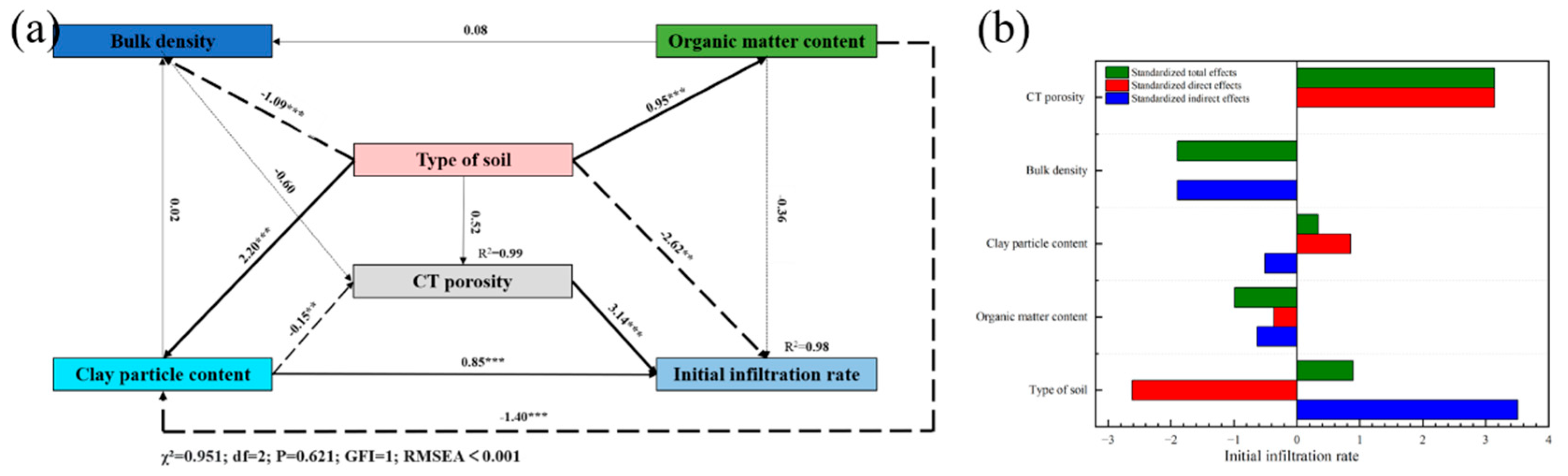
3.5. Influence of Soil Properties on Soil Pore Characteristics and Infiltration
4. Discussion
4.1. Biocrusts on the Artificially Compacted Soils Alter Physical Properties
4.2. Modification of Pore Structure of Artificially Compacted Soil by Biocrusts
4.3. Biocrusts Alters the Erosion Resistance of the Compacted Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Wang, X.; Sun, M.; Chen, W.; Guo, Q.; Zhang, H. Conservation of Jiaohe ancient earthen site in China. J. Rock Mech. Geotech. Eng. 2011, 3, 270–281. [Google Scholar] [CrossRef]
- Holtorf, C. Can less be more? Heritage in the age of terrorism. Public Archaeol. 2006, 5, 101–109. [Google Scholar] [CrossRef]
- Cao, Y.; Bowker, M.A.; Delgado-Baquerizo, M.; Xiao, B. Biocrusts protect the Great Wall of China from erosion. Sci. Adv. 2023, 9, eadk5892. [Google Scholar] [CrossRef]
- Chen, H.; Chen, W.; Li, X.; Jia, B.; Zhang, S. The effect of SH-lime composite material on capillary water rise resistance in the Great Wall. J. Build. Eng. 2024, 85, 108512. [Google Scholar] [CrossRef]
- Richards, J.; Guo, Q.; Viles, H.; Wang, Y.; Zhang, B.; Zhang, H. Moisture content and material density affects severity of frost damage in earthen heritage. Sci. Total Environ. 2022, 819, 153047. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, C.; Zhang, S.; Chen, W.; Qi, Q.; Wu, H. Characterization on Flaking of Rammed Earthen Sites Using SMO Algorithm and Surface Topography Analysis: A Case Study of Jiaohe Ruins. ACM J. Comput. Cult. Herit. 2024, 17, 1–20. [Google Scholar] [CrossRef]
- Quagliarini, E.; Lenci, S.; Iorio, M. Mechanical properties of adobe walls in a Roman Republican domus at Suasa. J. Cult. Herit. 2010, 11, 130–137. [Google Scholar] [CrossRef]
- Shao, M.; Li, L.; Wang, S.; Wang, E.; Li, Z. Deterioration mechanisms of building materials of Jiaohe ruins in China. J. Cult. Herit. 2013, 14, 38–44. [Google Scholar] [CrossRef]
- Du, Y.; Chen, W.; Cui, K.; Gong, S.; Pu, T.; Fu, X. A model characterizing deterioration at earthen sites of the Ming Great Wall in Qinghai Province, China. Soil Mech. Found. Eng. 2017, 53, 426–434. [Google Scholar] [CrossRef]
- Parracha, J.L.; Silva, A.S.; Cotrim, M.; Faria, P. Mineralogical and microstructural characterisation of rammed earth and earthen mortars from 12 th century Paderne Castle. J. Cult. Herit. 2020, 42, 226–239. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; He, C.; Xiao, L.; Wang, N.; Jiang, L.; Chen, J.; Liu, K.; Chen, Q.; Gu, Y. Diversity and composition of microbial communities in Jinsha earthen site under different degree of deterioration. Environ. Res. 2024, 242, 117675. [Google Scholar] [CrossRef]
- Hueck, H. The biodeterioration of materials as part of hylobiology. Mater. Und Org. 1965, 1, 5–34. [Google Scholar]
- Lim, A.B.; Matero, F.G.; Henry, M.C. Greening deterioration: Soft capping as preventive conservation for masonry ruin sites. APT Bull. J. Preserv. Technol. 2013, 44, 53–61. [Google Scholar]
- Hosseini, Z.; Caneva, G. Evaluating hazard conditions of plant colonization in Pasargadae World Heritage Site (Iran) as a tool of biodeterioration assessment. Int. Biodeterior. Biodegrad. 2021, 160, 105216. [Google Scholar] [CrossRef]
- Lee, Z.; Viles, H.A.; Wood, C.H. Soft Capping Historic Walls: A Better Way of Conserving Ruins? English Heritage: London, UK, 2009. [Google Scholar]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.-D. Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Bettina Weber, B.; Belnap, J.; Büdel, B. What is a biocrust? A refined, contemporary definition for a broadening research community. Biol. Rev. 2022, 97, 1768–1785. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Reed, S.; Travers, S.K.; Bowker, M.A.; Maestre, F.T.; Ding, J.; Havrilla, C.; Rodriguez-Caballero, E.; Barger, N.; Weber, B. The pervasive and multifaceted influence of biocrusts on water in the world’s drylands. Glob. Change Biol. 2020, 26, 6003–6014. [Google Scholar] [CrossRef]
- Li, X.; Hui, R.; Tan, H.; Zhao, Y.; Liu, R.; Song, N. Biocrust research in China: Recent progress and application in land degradation control. Front. Plant Sci. 2021, 12, 751521. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, B.; Li, Y.; Yang, W.; Lei, Y.; Han, H.; He, J. Biological soil crust distribution in Artemisia ordosica communities along a grazing pressure gradient in Mu Us Sandy Land, Northern China. J. Arid Land 2013, 5, 172–179. [Google Scholar] [CrossRef]
- Gao, L.; Bowker, M.A.; Xu, M.; Sun, H.; Tuo, D.; Zhao, Y. Biological soil crusts decrease erodibility by modifying inherent soil properties on the Loess Plateau, China. Soil Biol. Biochem. 2017, 105, 49–58. [Google Scholar] [CrossRef]
- Xiao, B.; Hu, K. Moss-dominated biocrusts decrease soil moisture and result in the degradation of artificially planted shrubs under semiarid climate. Geoderma 2017, 291, 47–54. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Hui, R.; Xie, M. Recovery of microbial community structure of biological soil crusts in successional stages of Shapotou desert revegetation, northwest China. Soil Biol. Biochem. 2017, 107, 125–128. [Google Scholar] [CrossRef]
- Richards, J.; Mayaud, J.; Zhan, H.; Wu, F.; Bailey, R.; Viles, H. Modelling the risk of deterioration at earthen heritage sites in drylands. Earth Surf. Process. Landf. 2020, 45, 2401–2416. [Google Scholar] [CrossRef]
- Wu, F.; Ding, X.; Zhang, Y.; Gu, J.-D.; Liu, X.; Guo, Q.; Li, J.; Feng, H. Metagenomic and metaproteomic insights into the microbiome and the key geobiochemical potentials on the sandstone of rock-hewn Beishiku Temple in Northwest China. Sci. Total Environ. 2023, 893, 164616. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, J.; Wang, W.; Shi, Y.; Gao, Y.; Zhan, H.; Luo, Y.; Jia, R. Vascular plants and biocrusts ameliorate soil properties serving to increase the stability of the Great Wall of China. Sci. Total Environ. 2024, 951, 175506. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Q.; Pei, Q.; Chen, W.; Wang, Y.; Zhang, B.; Yu, J. Destruction or protection? Experimental studies on the mechanism of biological soil crusts on the surfaces of earthen sites. Catena 2023, 227, 107096. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, B.; Li, S.; Yu, X.; Kidron, G.J.; Heitman, J. Direct evidence and mechanism for biocrusts-induced improvements in pore structure of dryland soil and the hydrological implications. J. Hydrol. 2023, 623, 129846. [Google Scholar] [CrossRef]
- Grose, M.J.; Gilligan, C.A.; Spencer, D.; Goddard, B. Spatial heterogeneity of soil water around single roots: Use of CT-scanning to predict fungal growth in the rhizosphere. New Phytol. 1996, 133, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Peyton, R.; Gantzer, C. Evaluation of constructed and natural soil macropores using X-ray computed tomography. Geoderma 1990, 46, 13–29. [Google Scholar] [CrossRef]
- Lipiec, J.; Hatano, R. Quantification of compaction effects on soil physical properties and crop growth. Geoderma 2003, 116, 107–136. [Google Scholar] [CrossRef]
- Perret, J.; Prasher, S.; Kantzas, A.; Langford, C. Three-dimensional quantification of macropore networks in undisturbed soil cores. Soil Sci. Soc. Am. J. 1999, 63, 1530–1543. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Mooney, S.J.; Peng, X. Effects of long-term inorganic and organic fertilizations on the soil micro and macro structures of rice paddies. Geoderma 2016, 266, 66–74. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Xiao, B.; Bowker, M.A. Moss-biocrusts strongly decrease soil surface albedo, altering land-surface energy balance in a dryland ecosystem. Sci. Total Environ. 2020, 741, 140425. [Google Scholar] [CrossRef]
- Jester, W.; Klik, A. Soil surface roughness measurement—Methods, applicability, and surface representation. Catena 2005, 64, 174–192. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Han, W.; Sun, M.; Wang, X. Research on the major diseases and protection of the Great Wall Site in Gansu Province. Cult. Relics Prot. Archaeol. Sci. 2007, 19, 28–32. (In Chinese) [Google Scholar] [CrossRef]
- Komaei, A.; Soroush, A.; Fattahi, S.M.; Ghanbari, H. Influence of environmental stresses on the durability of slag-based alkali-activated cement crusts for wind erosion control. Sci. Total Environ. 2023, 902, 166576. [Google Scholar] [CrossRef] [PubMed]
- Miralles, I.; Domingo, F.; Cantón, Y.; Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolase enzyme activities in a successional gradient of biological soil crusts in arid and semi-arid zones. Soil Biol. Biochem. 2012, 53, 124–132. [Google Scholar] [CrossRef]
- Aldughaishi, U.; Grattan, S.R.; Nicolas, F.; Peddinti, S.R.; Bonfil, C.; Ogunmokun, F.; Abou Najm, M.; Nocco, M.; Kisekka, I. Assessing the impact of recycled water reuse on infiltration and soil structure. Geoderma 2024, 452, 117103. [Google Scholar] [CrossRef]
- Pan, J.; Xu, N.; Tang, Y.; Cheng, M.; Zhang, L.; Wang, B.; Lan, J. Quantitative evaluation of plants on top surface of the Great Wall in Dazhuangke using the analytical hierarchy process. Herit. Sci. 2023, 11, 191. [Google Scholar] [CrossRef]
- Gregory, P.J. RUSSELL REVIEW Are plant roots only “in” soil or are they “of” it? Roots, soil formation and function. Eur. J. Soil Sci. 2022, 73, e13219. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Guo, Q.; Pei, Q. Discussion on the environmental adaptability of weather-resistant measures for earthen sites in China. Built Herit. 2022, 6, 19. [Google Scholar] [CrossRef]
- Dhaliwal, J.K.; Kumar, S. 3 D-visualization and quantification of soil porous structure using X-ray micro-tomography scanning under native pasture and crop-livestock systems. Soil Tillage Res. 2022, 218, 105305. [Google Scholar] [CrossRef]
- Dorau, K.; Uteau, D.; Hövels, M.P.; Peth, S.; Mansfeldt, T. Soil aeration and redox potential as function of pore connectivity unravelled by X-ray microtomography imaging. Eur. J. Soil Sci. 2022, 73, e13165. [Google Scholar] [CrossRef]
- Wildenschild, D.; Vaz, C.; Rivers, M.; Rikard, D.; Christensen, B. Using X-ray computed tomography in hydrology: Systems, resolutions, and limitations. J. Hydrol. 2002, 267, 285–297. [Google Scholar] [CrossRef]
- Dal Ferro, N.; Charrier, P.; Morari, F. Dual-scale micro-CT assessment of soil structure in a long-term fertilization experiment. Geoderma 2013, 204, 84–93. [Google Scholar] [CrossRef]
- Katuwal, S.; Norgaard, T.; Moldrup, P.; Lamandé, M.; Wildenschild, D.; de Jonge, L.W. Linking air and water transport in intact soils to macropore characteristics inferred from X-ray computed tomography. Geoderma 2015, 237, 9–20. [Google Scholar] [CrossRef]
- Du, Y.; Dong, W.; Cui, K.; Chen, W.; Yang, W. Research progress on the development mechanism and exploratory protection of the scaling off on earthen sites in NW China. Sci. China Technol. Sci. 2023, 66, 2183–2196. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Jia, B.; Bi, J.; Li, X. Study of salt migration on the upper part of the Great Wall under the rainfall-radiation cycle. Bull. Eng. Geol. Environ. 2022, 81, 419. [Google Scholar] [CrossRef]
- Belnap, J. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol. Process. Int. J. 2006, 20, 3159–3178. [Google Scholar] [CrossRef]
- Li, S.; Bowker, M.A.; Xiao, B. Biocrust impacts on dryland soil water balance: A path toward the whole picture. Glob. Change Biol. 2022, 28, 6462–6481. [Google Scholar] [CrossRef]
- Pande, S.; Kost, C. Bacterial unculturability and the formation of intercellular metabolic networks. Trends Microbiol. 2017, 25, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Baumgartl, T. Field evaluation of three modified infiltration models for the simulation of rainfall sequences. Soil Sci. 2016, 181, 45–56. [Google Scholar] [CrossRef]
- Bodner, G.; Leitner, D.; Kaul, H.-P. Soil, Coarse and fine root plants affect pore size distributions differently. Plant 2014, 380, 133–151. [Google Scholar] [CrossRef]
- Tripathi, I.M.; Mahto, S.S.; Kushwaha, A.P.; Kumar, R.; Tiwari, A.D.; Sahu, B.K.; Jain, V.; Mohapatra, P.K. Dominance of soil moisture over aridity in explaining vegetation greenness across global drylands. Sci. Total Environ. 2024, 917, 170482. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Ren, J.; Gao, Y.; Wu, F.; Shi, Y.; Zhan, H.; Chi, C.; Jia, R. Microtopography-induced hydrological heterogeneity promotes the co-assembly of vascular plant and biocrust communities, providing synergistic protective functions for the Great Wall. Sci. Total Environ. 2025, 963, 178513. [Google Scholar] [CrossRef] [PubMed]

| Measurement Indicators | Bare Soil | Cyanobacterial-Lichen | Moss Crusts | F |
|---|---|---|---|---|
| Crust thickness(mm) | - | 7.99 ± 0.30 b | 14.47 ± 0.65 a | 412.52 |
| Total biomass (g·cm−2) | - | 0.08 ± 0.02 b | 0.11 ± 0.02 a | 8.377 |
| Chlorophyll a content (μg·g−1) | - | 0.15 ± 0.04 b | 2.29 ± 0.21 a | 412.51 |
| pH | 8.48 ± 0.08 a | 8.10 ± 0.03 b | 7.74 ± 0.01 c | 255.7 |
| Conductivity | 97.2 ± 0.27 b | 90.68 ± 0.68 c | 131.92 ± 5.19 a | 269.89 |
| Bulk density (g·cm−3) | 1.75 ± 0.01 a | 1.54 ± 0.01 b | 1.34 ± 0.01 c | 1081.73 |
| Total porosity (%) | 33.89% ± 0.56 c | 41.89% ± 0.60 b | 49.58% ± 0.43 a | 1081.73 |
| Percentage of clay (<2 μm, %) | 3.71 ± 0.04 c | 3.76 ± 0.09 b | 4.29 ± 0.03 a | 141.57 |
| Percentage of silt (2–20 μm, %) | 27.73 ± 0.17 b | 26.95 ± 0.27 c | 30.66 ± 0.06 a | 558.03 |
| Percentage of sand (20–2000 μm, %) | 68.56 ± 0.21 b | 69.29 ± 0.33 a | 65.05 ± 0.01 c | 506.89 |
| Organic matter content (g·kg−1) | 6.45 ± 0.26 c | 26.01 ± 3.43 b | 39.97 ± 6.52 a | 78.30 |
| Surface roughness | 2.01 ± 0.03 c | 9.54 ± 0.14 a | 6.08 ± 0.10 b | 7005.53 |
| Field capacity (cm3·cm−3) | 0.02 ± 0.02 c | 0.19 ± 0.07 b | 0.28 ± 0.05 a | 8.377 |
| Saturated water content (cm3 cm−3) | 0.11 ± 0.07 c | 0.21 ± 0.08 b | 0.42 ± 0.11 a | 15.59 |
| Saturated hydraulic conductivity (cm·min−1) | 0.03 ± 0.02 c | 0.08 ± 0.03 b | 0.29 ± 0.10 a | 15.59 |
| Soil Depth | Crust Types | Surface Area Density (mm2 mm−3) | Length Density (mm mm−3) | Network Density (Number mm−3) | Mean Pore Volume (×10−2) (mm3) | Node Density (Number mm−3) |
|---|---|---|---|---|---|---|
| Entire Soil Column | Bare Soil | 9.06 ± 2.03 c | 0.31 ± 0.11 c | 13.92 ± 2.14 c | 0.03 ± 0.01 c | 13.14 ± 2.65 c |
| Cyanobacterial-LichenCyanobacterial-lichen Crust | 59.27 ± 19.03 a | 37.95 ± 15.84 a | 1463.06 ± 211.23 a | 0.07 ± 0.04 b | 3990.57 ± 538.35 a | |
| Moss Crust | 45.64 ± 0.35 b | 25.42 ± 0.12 b | 622.10 ± 49.12 b | 0.11 ± 0.03 a | 1039.79 ± 25.14 b | |
| Biocrust Layer | Cyanobacterial-Lichen Crust | 51.64 ± 8.59 a | 39.04 ± 10.17 a | 741.88 ± 90.04 a | 0.06 ± 0.04 b | 2938.97 ± 139.21 a |
| Moss Crust | 43.23 ± 2.9 b | 18.27 ± 5.0 b | 329.24 ± 40.09 b | 0.13 ± 0.08 a | 1363.56 ± 60.12 b | |
| Subsurface Layer | Cyanobacterial-Lichen Crust | 72.54 ± 20.25 a | 143.41 ± 21.52 a | 4086.82 ± 991.99 a | 0.02 ± 0.01 a | 4563.61 ± 553.51 a |
| Moss Crust | 42.79 ± 1.91 b | 61.66 ± 0.51 b | 916.17 ± 70.4 b | 0.02 ± 0.01 a | 2018.51 ± 74.75 b |
| Soil Depth | Crust Types | Degree of Anisotropy | Fractal Dimension | Euler Number (×105) | Mean Tortuosity |
|---|---|---|---|---|---|
| Entire Soil Column | Bare Soil | 0.22 ± 0.04 a | 2.30 ± 0.03 a | −0.12 ± 0.01 c | 1.14 ± 0.26 a |
| Cyanobacterial-Lichen Crust | 0.26 ± 0.07 a | 2.73 ± 0.22 a | 36.64 ± 7.59 a | 1.15 ± 0.07 a | |
| Moss Crust | 0.16 ± 0.03 a | 2.67 ± 0.22 a | 0.24 ± 0.06 b | 1.11 ± 0.11 a | |
| Biocrust Layer | Cyanobacterial-Lichen Crust | 0.34 ± 0.11 a | 2.67 ± 0.22 a | 36.64 ± 0.32 a | 1.14 ± 0.09 a |
| Moss Crust | 0.2 ± 0.03 a | 2.67 ± 0.27 a | −5.99 ± 0.30 b | 1.12 ± 0.17 a | |
| Subsurface Layer | Cyanobacterial-Lichen Crust | 0.35 ± 0.07 a | 2.74 ± 0.18 a | 9.84 ± 0.64 a | 1.16 ± 0.08 a |
| Moss Crust | 0.23 ± 0.06 a | 2.67 ± 0.22 a | 3.76 ± 0.36 b | 1.16 ± 0.08 a |
| Soil Depth | Crust Types | Mean Shape Factor | Sphericity | Flatness | Elongation |
|---|---|---|---|---|---|
| Entire Soil Column | Bare Soil | 6.47 ± 0.49 a | 0.55 ± 0.15 a | 0.45 ± 0.19 a | 0.31 ± 0.1 a |
| Cyanobacterial-Lichen Crust | 2.1 ± 0.95 b | 0.54 ± 0.25 a | −0.12 ± 0.06 b | −0.01 ± 0.01 c | |
| Moss Crust | 1.67 ± 0.17 c | 0.54 ± 0.22 a | −0.09 ± 0.04 c | 0.01 ± 0.01 b | |
| Biocrust Layer | Cyanobacterial-Lichen Crust | 1.45 ± 0.31 a | 0.54 ± 0.25 a | −0.18 ± 0.07 a | −0.07 ± 0.03 a |
| Moss Crust | 1.45 ± 0.04 a | 0.55 ± 0.05 a | −0.18 ± 0.07 a | −0.07 ± 0.02 a | |
| Subsurface Layer | Cyanobacterial-Lichen Crust | 1.27 ± 0.38 a | 0.54 ± 0.25 a | −0.13 ± 0.07 a | −0.02 ± 0.01 a |
| Moss Crust | 1.09 ± 0.01 b | 0.54 ± 0.11 a | −0.11 ± 0.02 a | −0.003 ± 0.001 b |
| Soil Depth | Crust Types | Connected Porosity (%) | Isolated Porosity (%) | Number of Node Pores | Average Coordination Number | Number of Channels | Average Throat Area (mm2) | Average Channel Length (mm) |
|---|---|---|---|---|---|---|---|---|
| Entire Soil Column | Bare Soil | 1.72 ± 0.22 c | 0.40 ± 0.14 c | 197 ± 23.07 c | 4.05 ± 0.32 b | 410 ± 20.22 c | 1 ± 0.46 a | 4.25 ± 1.96 a |
| Cyanobacterial-Lichen Crust | 2.29 ± 0.26 b | 10.62 ± 2.41 b | 3571 ± 606.09 b | 6.64 ± 1.29 a | 3570 ± 671.51 b | 0.01 ± 0.01 b | 1.33 ± 0.57 b | |
| Moss Crust | 4.73 ± 0.31 a | 16.74 ± 0.28 a | 7305 ± 96 a | 1.55 ± 0.26 c | 7232.33 ± 32.50 a | 0.01 ± 0.01 b | 1.01 ± 0.02 c | |
| Biocrust Layer | Cyanobacterial-Lichen Crust | 0.67 ± 0.31 b | 18.10 ± 2.98 b | 1580.33 ± 426.39 a | 7.16 ± 1.56 a | 7232 ± 309.71 a | 0.01 ± 0.01 a | 1.14 ± 0.21 a |
| Moss Crust | 27.39 ± 0.2 a | 1.14 ± 0.12 a | 1580 ± 19.52 a | 7.15 ± 0.1 a | 7235 ± 32.79 a | 0.01 ± 0.01 a | 1.04 ± 0.12 a | |
| Subsurface Layer | Cyanobacterial-Lichen Crust | 0.62 ± 0.18 b | 9.06 ± 1.03 b | 1232.33 ± 109.50 a | 4.45 ± 0.24 a | 3346 ± 267.25 b | 0.11 ± 0.06 a | 1.34 ± 0.34 a |
| Moss Crust | 2.5 ± 0.4 a | 12.21 ± 11.91 a | 2987 ± 116.89 b | 3.4 ± 0.26 b | 5077 ± 51.68 a | 0.01 ± 0.01 a | 1.31 ± 0.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wu, F.; Li, L.; Shang, R.; Li, D.; Xu, L.; Cui, J.; Zhao, X. Biocrusts Alter the Pore Structure and Water Infiltration in the Top Layer of Rammed Soils at Weiyuan Section of the Great Wall in China. Coatings 2025, 15, 908. https://doi.org/10.3390/coatings15080908
Yang X, Wu F, Li L, Shang R, Li D, Xu L, Cui J, Zhao X. Biocrusts Alter the Pore Structure and Water Infiltration in the Top Layer of Rammed Soils at Weiyuan Section of the Great Wall in China. Coatings. 2025; 15(8):908. https://doi.org/10.3390/coatings15080908
Chicago/Turabian StyleYang, Xiaoju, Fasi Wu, Long Li, Ruihua Shang, Dandan Li, Lina Xu, Jing Cui, and Xueyong Zhao. 2025. "Biocrusts Alter the Pore Structure and Water Infiltration in the Top Layer of Rammed Soils at Weiyuan Section of the Great Wall in China" Coatings 15, no. 8: 908. https://doi.org/10.3390/coatings15080908
APA StyleYang, X., Wu, F., Li, L., Shang, R., Li, D., Xu, L., Cui, J., & Zhao, X. (2025). Biocrusts Alter the Pore Structure and Water Infiltration in the Top Layer of Rammed Soils at Weiyuan Section of the Great Wall in China. Coatings, 15(8), 908. https://doi.org/10.3390/coatings15080908






