Evaluation of Integral and Surface Hydrophobic Modification on Permeation Resistance of Foam Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PFC Density Modulation and Specimen Preparation
2.3. Specimen Preparation and Test Program
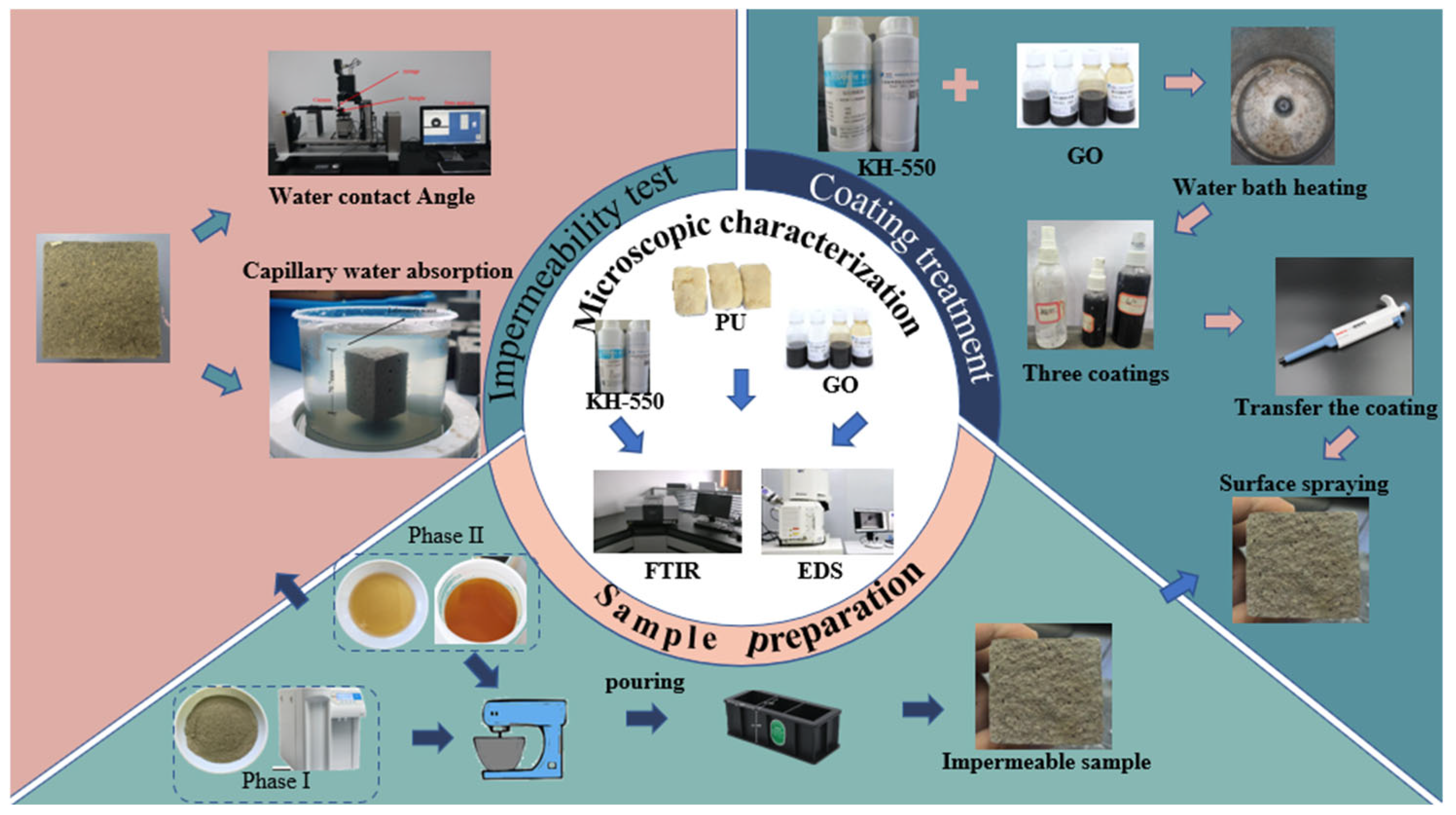
2.3.1. Preparation of Impermeable Specimens
2.3.2. Preparation of GO/KH-550 Composite Emulsion (G/S)
2.3.3. Test Scheme
2.4. Test Methods
2.4.1. Water Contact Angle Test Method
2.4.2. Water Absorption Test
2.4.3. Carbonization and Freeze–Thaw Test Methods
2.4.4. Concrete Surface Coating Method
3. Results and Analysis
3.1. Effect of PU on Seepage Resistance of Foam Concrete
3.2. Effect of Carbonation Environment on PFC Impermeability Properties
3.3. Effect of Freeze–Thaw Cycling on the Permeability of PFC
3.4. Effect of Three Hydrophobic Coatings on the Impermeability of PFC
3.4.1. Functionalization Tests for G/S
3.4.2. Contact Angle and Water Absorption Curve for GO Groups
3.4.3. Contact Angle and Water Absorption Curves for KH-550 Groups
3.4.4. Contact Angle and Water Absorption Curves for G/S Groups
4. Conclusions
- 1.
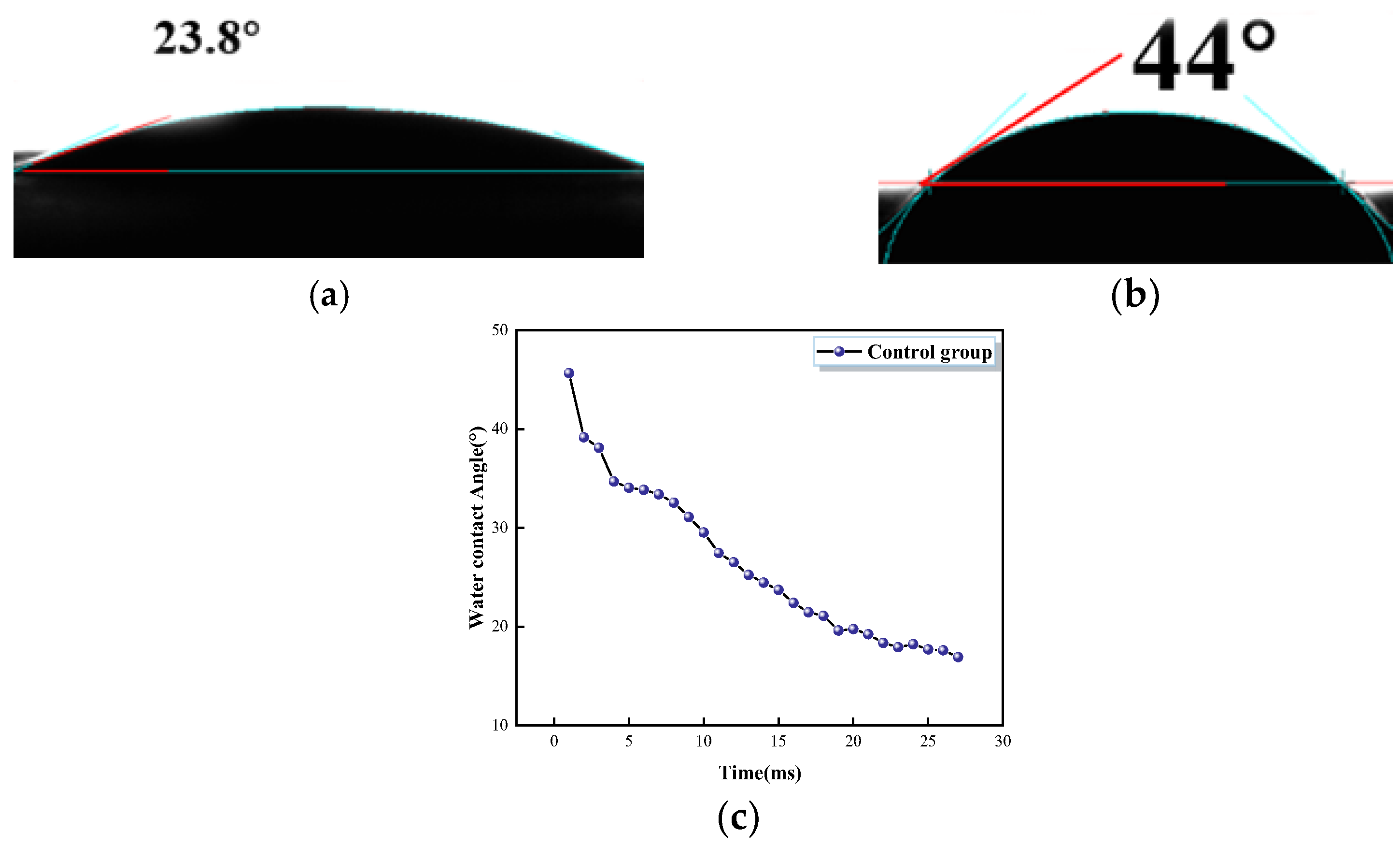
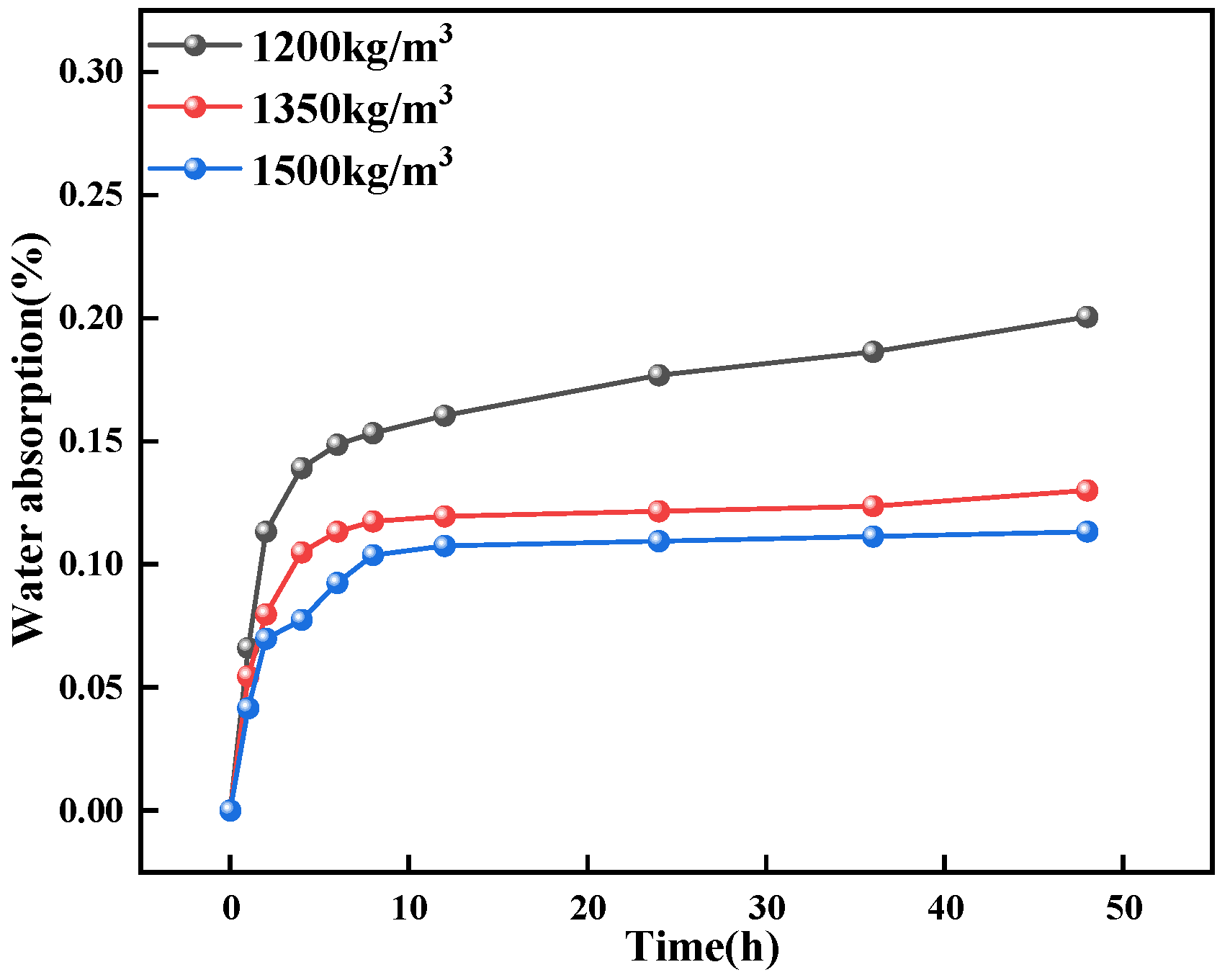
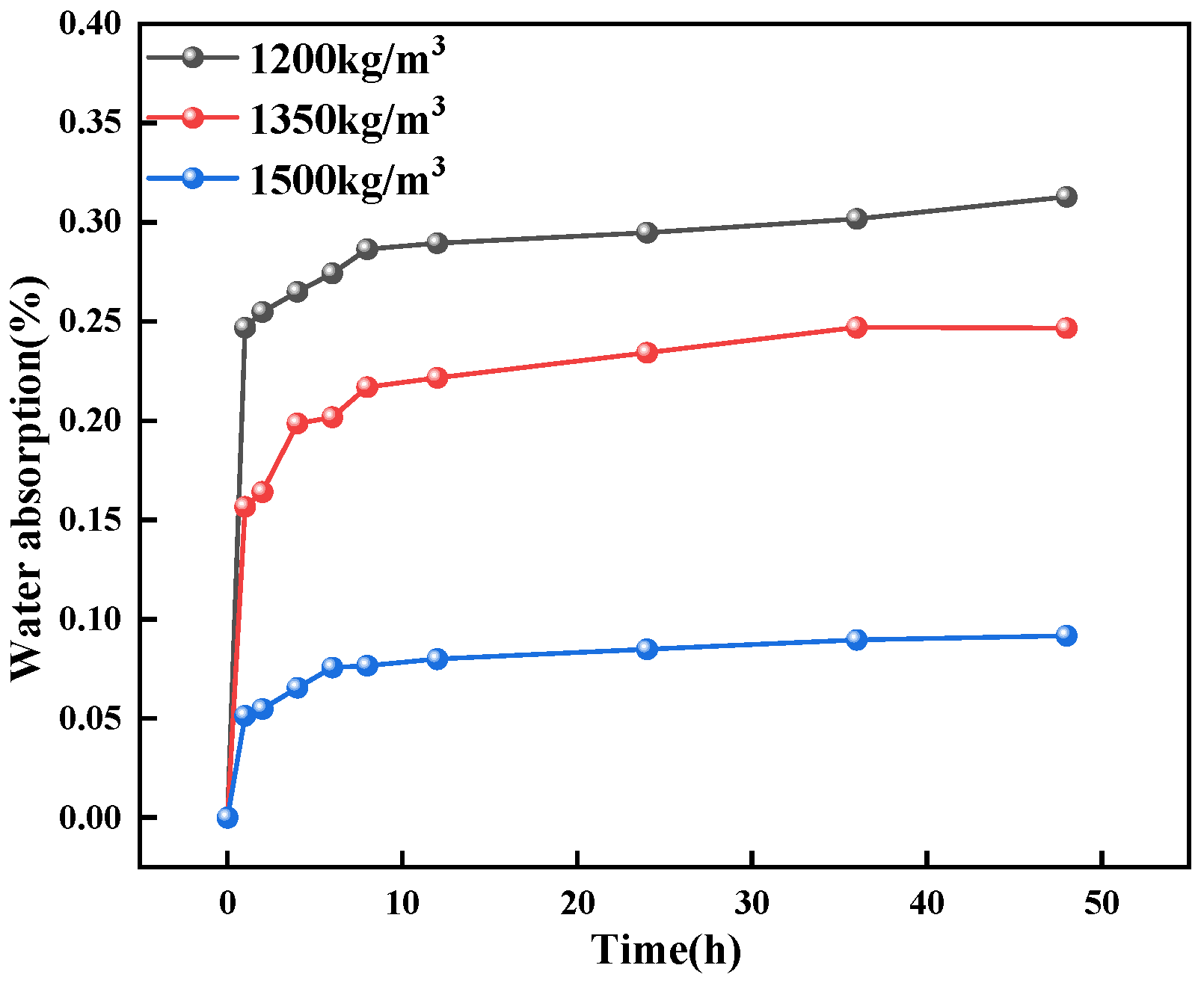
- PU has a strong hydrophobic effect on concrete. The water contact angle of ordinary concrete is 23.8°, while that of PFC is 44°, an increase of 9.4° compared to ordinary concrete. The water absorption of PFC specimens at 48 h is 0.28% for PU-1200, 0.13% for PU-1350, and 0.07% for PU-1500. This indicates that adding PU foam to concrete can effectively enhance its water resistance.
- 2.
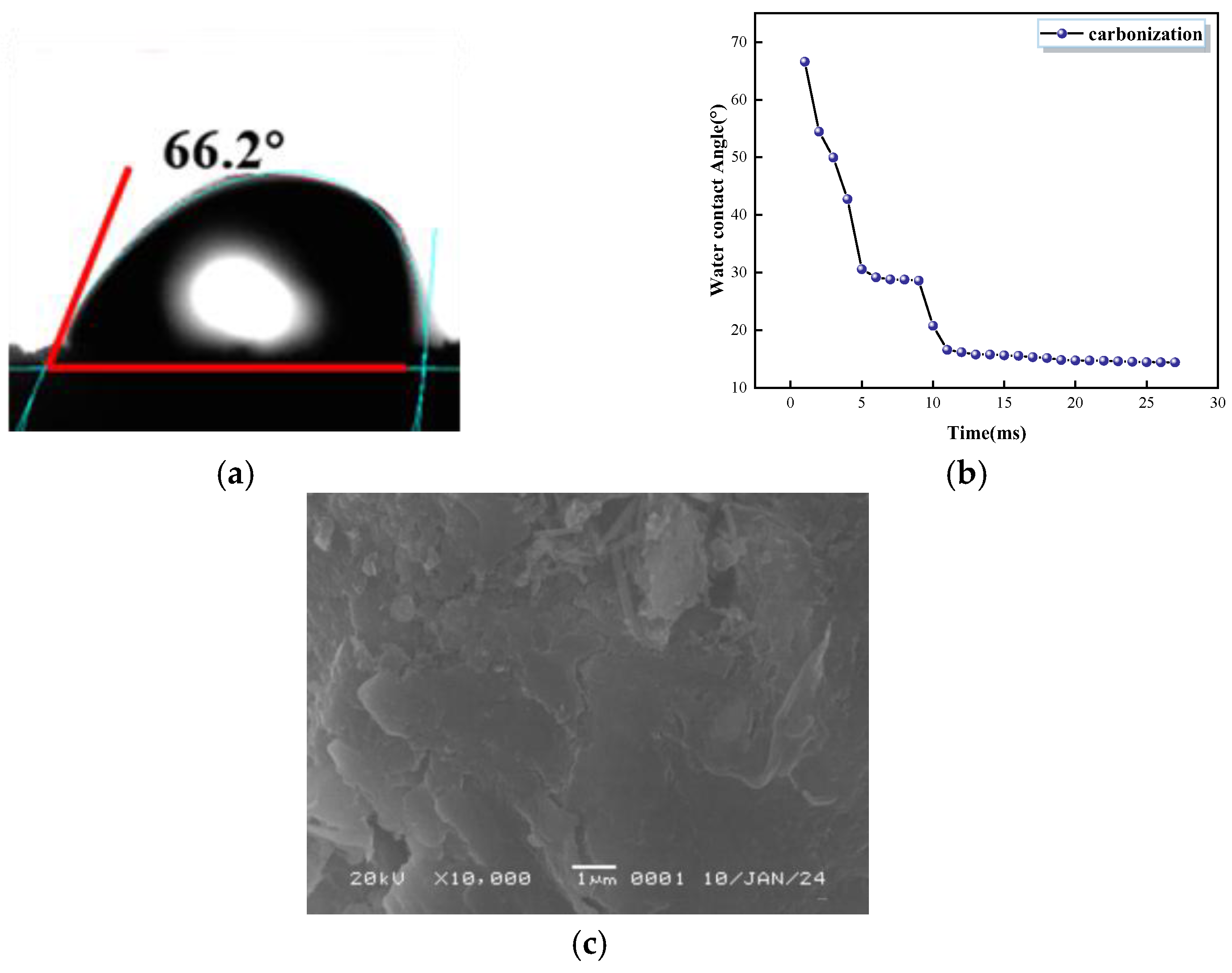
- Carbonization enhances hydrophobicity. The water contact angle of the PFC specimen with a carbonized layer was 66.2° initially, and it decreased to 14.4° at 27 ms. After carbonization, the PFC surface became covered with CaCO3 particles, leading to a gradual increase in the contact angle. The water absorption test, conducted 48 h after carbonization for the control group, showed that the water absorption rate was 0.2% for CG-1200, 0.13% for CG-1350, and 0.1% for CG-1500.
- 3.
- Freeze–thaw cycles degrade hydrophobic properties. As these cycles repeat, the internal structure of PFC deteriorates, causing micro-cracks to merge into larger cracks. This expansion of internal water storage space results in a reduced water contact angle. After freeze–thaw treatment, the water contact angle of the PFC specimen was 10.8°. The water absorption rates were 0.31% for FT-1200, 0.24% for FT-1350, and 0.1% for FT-1500.
- 4.
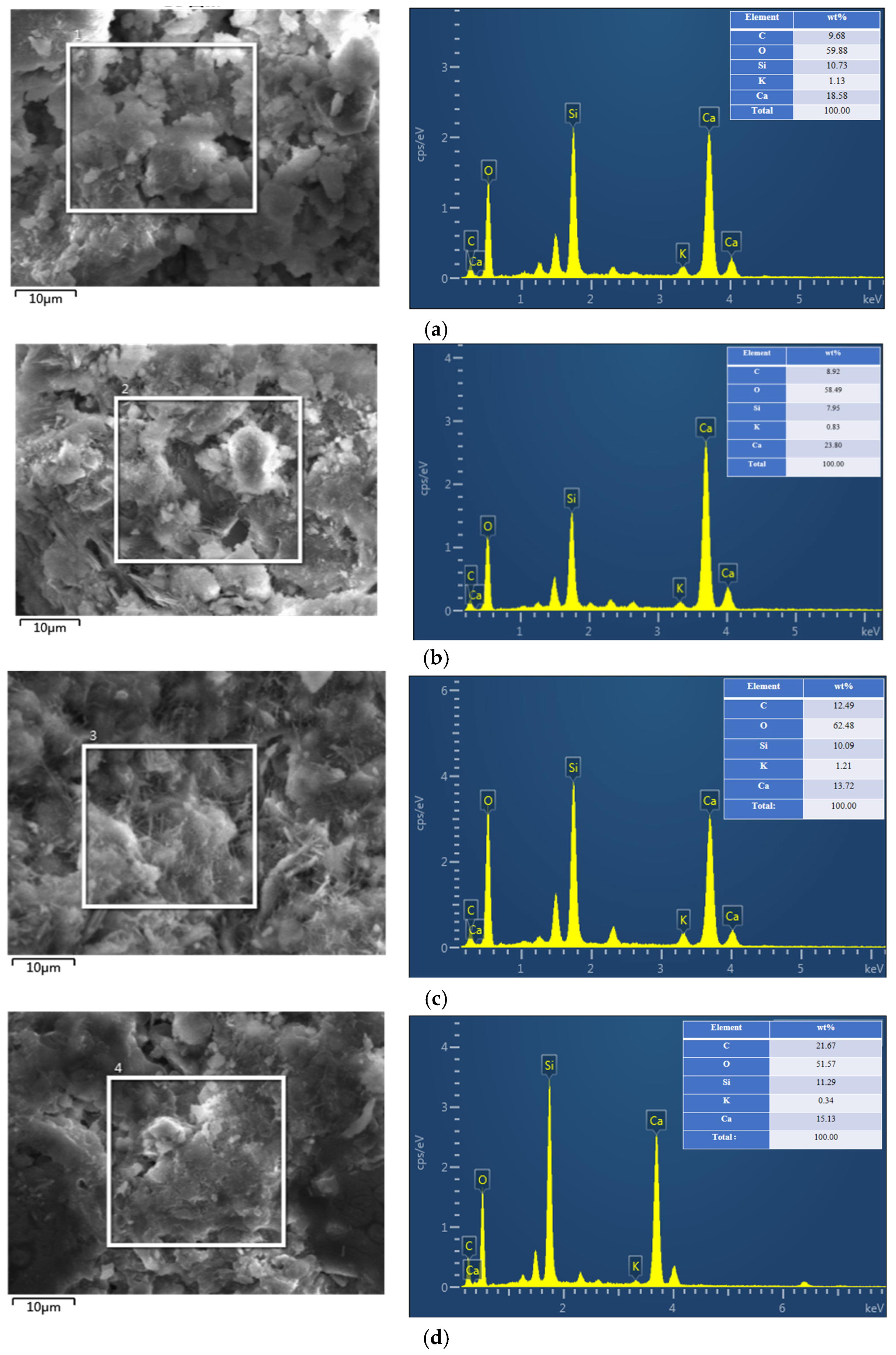
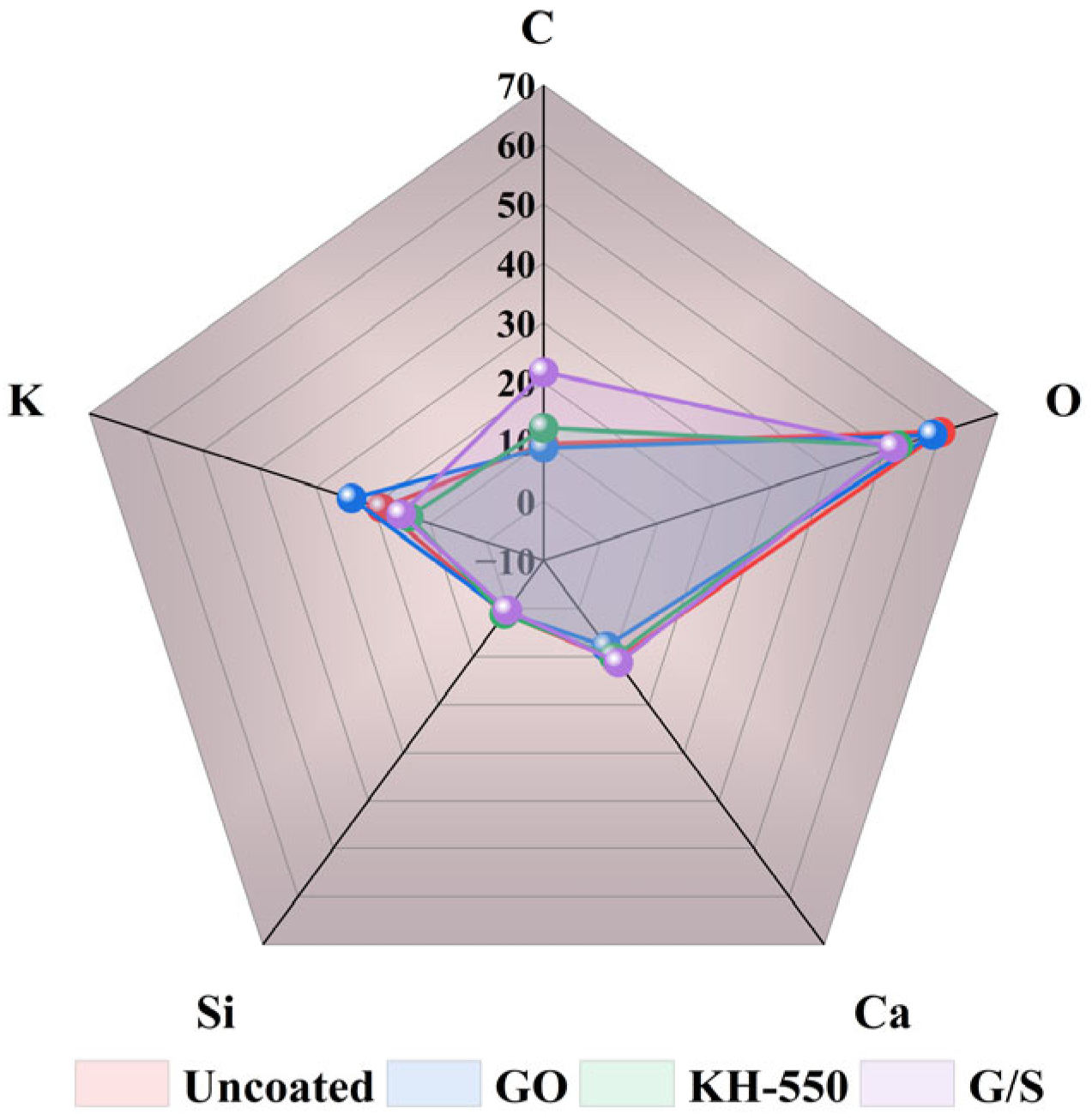
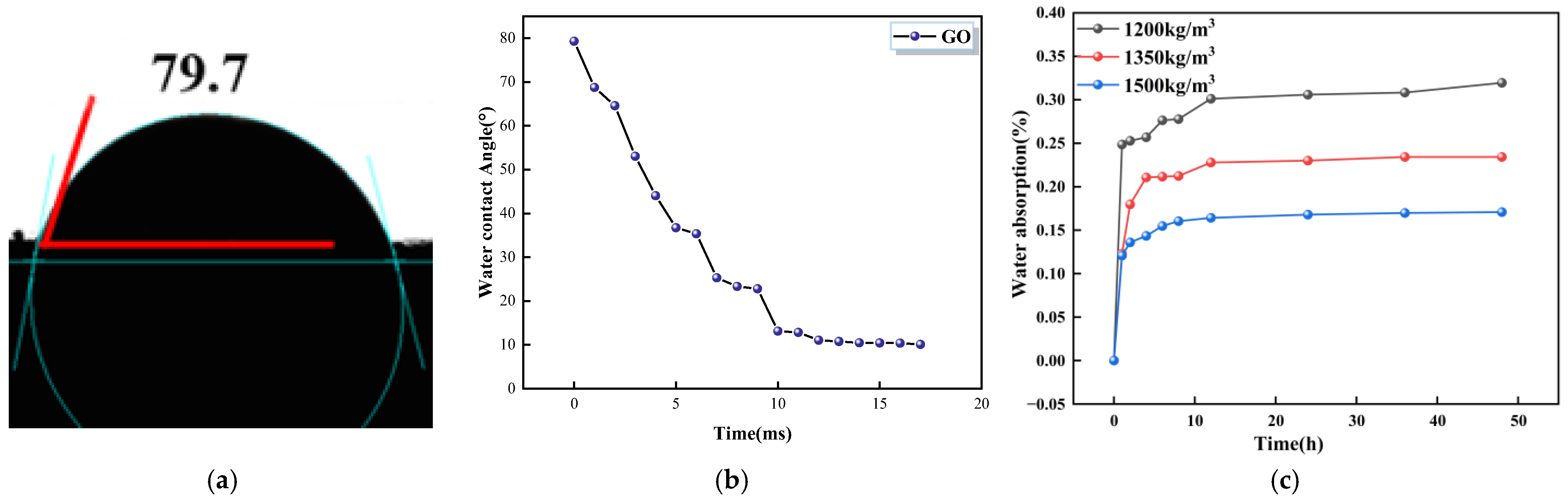
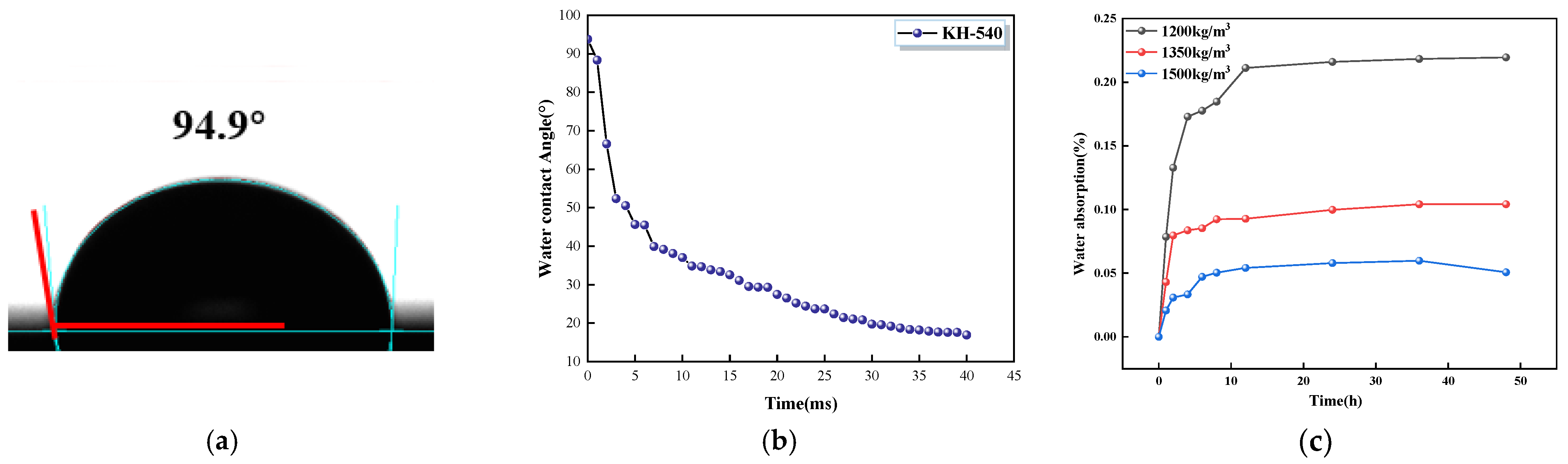
- GO, KH-550, and G/S all enhanced the impermeability of PFC, with G/S having the most significant effect on improving impermeability performance. Functionalization between GO and KH-550 was achieved, resulting in the appearance of new bands at 954 and 854 cm−1 in the FTIR spectra of G/S after the interaction between GO and KH-550. For the GO-coated PFC, the water contact angle of the specimen was 79.7°, which is an increase of 35.7° compared to the uncoated PFC. This suggests that GO alone did not significantly enhance the water repellency of the concrete surface. However, after treating the concrete surface with the silane emulsion KH-550, the water contact angle increased to 94.9°, changing the surface from hydrophilic to hydrophobic. After G/S treatment, the water contact angle further escalated to 120.2°, markedly surpassing the angle obtained with only the silane emulsion. The water absorption of concrete coated with GO dispersion was similar to that of uncoated PFC. However, when PFC was treated with KH-550, the water absorption of specimens coated at different densities was reduced to 22%, 23%, and 29%, respectively. After treatment with the G/S group, the water absorption of PFC specimens at different densities was further reduced to 41%, 46%, and 29%.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PU | Polyurethane |
| PFC | Polyurethane foam concrete |
| FTIR | Fourier transform infrared spectrum |
| EDS | Energy dispersive X-ray spectrometer |
| GO | Graphene oxide |
| G/S | Graphene oxide and silane coupling agent complex emulsion |
| MDI | Diphenylmethane diisocyanate |
| PP | Polyether polyols |
| BSP | Basalt stone powder |
| XRD | X-ray diffractometer |
| KH-550 | Silane coupling agent |
References
- Wu, H.; Zhang, Y.; Li, M.; Chen, X.; Wang, J.; Liu, S. Study on preparation and performance of advanced aerogel foamed concrete with ultra-light aerogel. Constr. Build. Mater. 2023, 366, 130166. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, L.; Zhang, H.; Liu, Y.; Chen, M.; Li, X. Integral hydrophobic concrete without using silane. Constr. Build. Mater. 2019, 227, 116678. [Google Scholar] [CrossRef]
- Yu, J.; Chen, S.; Wang, K.; Liu, H.; Zhang, M.; Li, Y. Insights on the capillary transport mechanism in the sustainable cement hydrate impregnated with graphene oxide and epoxy composite. Compos. Part B Eng. 2019, 173, 106907. [Google Scholar] [CrossRef]
- Huang, K.; Si, Y.; Hu, J. Fluid Unidirectional Transport Induced by Structure and Ambient Elements across Porous Materials: From Principles to Applications. Adv. Mater. 2024, 36, 2402527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, Z. Deformation-based monitoring of water migration in rocks using distributed fiber optic strain sensing: A laboratory study. Water Resour. Res. 2019, 55, 8368–8383. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhang, S.S.; Chen, E.; Li, W.G. A review on corrosion detection and protection of existing reinforced concrete (RC) structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Liu, M.; Chen, Y.; Li, H.; Zhang, K. Hydrophobic or superhydrophobic modification of cement-based materials: A systematic review. Compos. Part B Eng. 2022, 243, 110104. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Wang, H.; Liu, X.; Zhang, M.; Li, S. Research on the performance of foamed concrete based on superhydrophobic bulk modification. Constr. Build. Mater. 2024, 438, 137231. [Google Scholar] [CrossRef]
- Aktas, Y.D.; Zhu, H.; D’Ayala, D.; Weeks, C. Impact of surface waterproofing on the performance of brick masonry through the moisture exposure life-cycle. Build. Environ. 2021, 197, 107844. [Google Scholar] [CrossRef]
- Chen, X.; De Kock, T.; Cnudde, V.; Van Den Bossche, N. The influence of material characteristics and environmental circumstances on the efficiency of water repellent treatments of historic facades. Constr. Build. Mater. 2023, 386, 131610. [Google Scholar] [CrossRef]
- Weisheit, S.; Unterberger, S.H.; Bader, T.; Lackner, R. Assessment of test methods for characterizing the hydrophobic nature of surface-treated High Performance Concrete. Constr. Build. Mater. 2016, 110, 145–153. [Google Scholar] [CrossRef]
- Nguyen-Ha, T.M.; Kim, J.H.; Lee, S.Y.; Park, M.J.; Chen, X.; Wang, L. Novel high-performance sustainable polyurethane nanocomposite foams: Fire resistance, thermal stability, thermal conductivity, and mechanical properties. Chem. Eng. J. 2023, 474, 145585. [Google Scholar] [CrossRef]
- Geramipour, T.; Oveisi, H. Effects of foaming parameters on microstructure and compressive properties of aluminum foams produced by powder metallurgy method. Trans. Nonferrous Met. Soc. China 2017, 27, 1569–1579. [Google Scholar] [CrossRef]
- An, Y.; Zhang, H.; Liu, X.; Wang, M.; Chen, L.; Li, S. Foaming stabilization and mechanical properties of high-toughness aluminum foam fabricated using non-thickening foaming technology. J. Mater. Process. Technol. 2021, 296, 117212. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Poulesquen, A. Design of lightweight metakaolin based geopolymer foamed with hydrogen peroxide. Ceram. Int. 2019, 45, 1322–1330. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Wang, X.; Liu, M.; Zhang, K.; Li, H. Green synthesis of high porosity waste gangue microsphere/geopolymer composite foams via hydrogen peroxide modification. J. Clean. Prod. 2019, 227, 483–494. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, L.; Zhang, H.; Liu, Y.; Chen, M.; Li, X. Self-healing polyurethane-elastomer with mechanical tunability for multiple biomedical applications in vivo. Nat. Commun. 2021, 12, 4395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.; Wang, K.; Zhang, M.; Li, Y.; Liu, H. Chemical upcycling of commodity thermoset polyurethane foams towards high-performance 3D photo-printing resins. Nat. Chem. 2023, 15, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Sui, T.; Baimpas, N.; Dolbnya, I.P.; Prisacariu, C.; Korsunsky, A.M. Multiple-length-scale deformation analysis in a thermoplastic polyurethane. Nat. Commun. 2015, 6, 6583. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.; So, H. TPU-assisted adhesive PDMS film for dry or underwater environments. NPG Asia Mater. 2024, 16, 26. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Q.; Wen, Y.; Wang, Z.; Xie, H. Improved thermal stability and flame retardancy of soybean oil-based polyol rigid polyurethane foams modified with magnesium borate hydroxide and ammonium polyphosphate. Sci. Rep. 2024, 14, 17340. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.H.; Lee, D.S.; Seo, W.J.; Kim, W.N. Properties of Rigid Polyurethane Foams with Blowing Agents and Catalysts. Polym. J. 2004, 36, 368–373. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Wang, K.; Liu, H.; Zhang, M.; Li, Y. Durability of waste concrete powder-based geopolymer reclaimed concrete under carbonization and freeze–thaw cycles. Constr. Build. Mater. 2023, 403, 133155. [Google Scholar] [CrossRef]
- Chen, J.; Chen, B.; Qiang, S.; Chen, S. Study on strength deterioration mechanism of foamed concrete under freeze-thaw cycles: Experiment and numerical simulation. Constr. Build. Mater. 2024, 438, 137083. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Zhang, H.; Liu, Y.; Chen, M.; Liu, K. Application of silane protective materials in the concrete durability improvement in recent years: A review. Eng. Fail. Anal. 2024, 160, 108140. [Google Scholar] [CrossRef]
- Wu, C.; Hou, D.; Yin, B.; Chen, B.; Li, Z. A polystyrene acrylate-polysiloxane multicomponent protective coating with interpenetrating polymer network structure for cementitious materials. Prog. Org. Coat. 2024, 195, 108640. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Wang, H.; Liu, X.; Chen, M.; Li, S. Silane treatment for sisal fibers to improve the degradation resistance and interface with cement matrix. Constr. Build. Mater. 2024, 429, 136435. [Google Scholar] [CrossRef]
- Yu, H.; Chen, L.; Wang, K.; Zhang, M.; Liu, S.; Li, Y. The roles of oxygen-containing functional groups in modulating water purification performance of graphene oxide-based membrane. Chem. Eng. J. 2020, 389, 124375. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, F.; Wang, W.; Li, N.; Yu, S. The absorption effect and mechanism of graphene oxide removal from aqueous solution by basalt stone power. Arab. J. Chem. 2024, 17, 105805. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, F.; Li, N.; Wang, W.; Wang, B.; Yu, P. Polyurethane foam lightweight concrete: Preparation, CO2 fixation properties and mechanism. J. CO2 Util. 2025, 97, 103109. [Google Scholar] [CrossRef]
- Parsons, L.A.; Nwaubani, S.O. Abrasion-wear resistance of precarbonated and water-cured concrete made using ABS plastic derived from waste electrical and electronic equipment. Cem. Concr. Res. 2024, 179, 107470. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, Z.; Tang, X.-Z.; Yue, C.Y.; Yang, J. Enhanced interphase between epoxy matrix and carbon fiber with carbon nanotube-modified silane coating. Compos. Sci. Technol. 2014, 99, 131–140. [Google Scholar] [CrossRef]
- Chen, K.; Wang, L.; Zhang, H.; Liu, Y.; Chen, M.; Li, S. Effect of silane coupling agent on compatibility interface and properties of wheat straw/polylactic acid composites. Int. J. Biol. Macromol. 2021, 182, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tang, W.; Liu, W.; Dong, Z.; Xing, F. Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Constr. Build. Mater. 2015, 93, 522–527. [Google Scholar] [CrossRef]
- Code, C. Model Code for Service Life Design; Federation Internationale du Beton: Lausanne, Switzerland, 2006. [Google Scholar]
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2009.
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Liu, X.; Wang, K.; Li, Y.; Zhang, H. Influence of damage degree on the degradation of concrete under freezing-thawing cycles. Constr. Build. Mater. 2020, 260, 119903. [Google Scholar] [CrossRef]
- Wei, B.; Wang, X.; Chen, L.; Liu, M.; Zhang, K.; Li, H. Surface modification of filter medium particles with silane coupling agent KH550. Colloids Surf. A 2013, 434, 276–280. [Google Scholar] [CrossRef]
- Zhong, J.; Li, H.; Yu, J.; Tan, T. Effects of natural fiber surface modification on mechanical properties of poly (lactic acid)(PLA)/sweet sorghum fiber composites. Polym. Plast. Technol. Eng. 2011, 50, 1583–1589. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.; Zhang, H.; Liu, Y.; Chen, M.; Li, S. Carbonaceous nanofiber membrane functionalized by beta-cyclodextrins for molecular filtration. ACS Nano 2011, 5, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, X.; Chen, S.; Wang, K.; Li, Y.; Zhang, H. Magnetic iron oxide/graphene oxide nanocomposites: Formation and interaction mechanism for efficient removal of methylene blue and p-tert-butylphenol from aqueous solution. Mater. Chem. Phys. 2018, 205, 240–252. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Wang, G.-S.; Cao, W.-Q.; Wei, Y.-Z.; Liang, J.-F.; Guo, L.; Cao, M.-S. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, J.; Song, M. An investigation of the mechanism of graphene toughening epoxy. Compos. Sci. Technol. 2012, 72, 737–743. [Google Scholar] [CrossRef]
- Kou, L.; Gao, C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Bittolo Bon, S.; Valentini, L.; Verdejo, R.; Garcia, M.A.; Epifani, M.; Hernández-Vélez, M.; López-Manchado, M.A. Deposition of amino-functionalized polyhedral oligomeric silsesquioxanes on graphene oxide sheets immobilized onto an amino-silane modified silicon surface. J. Mater. Chem. 2012, 22, 6213–6217. [Google Scholar]

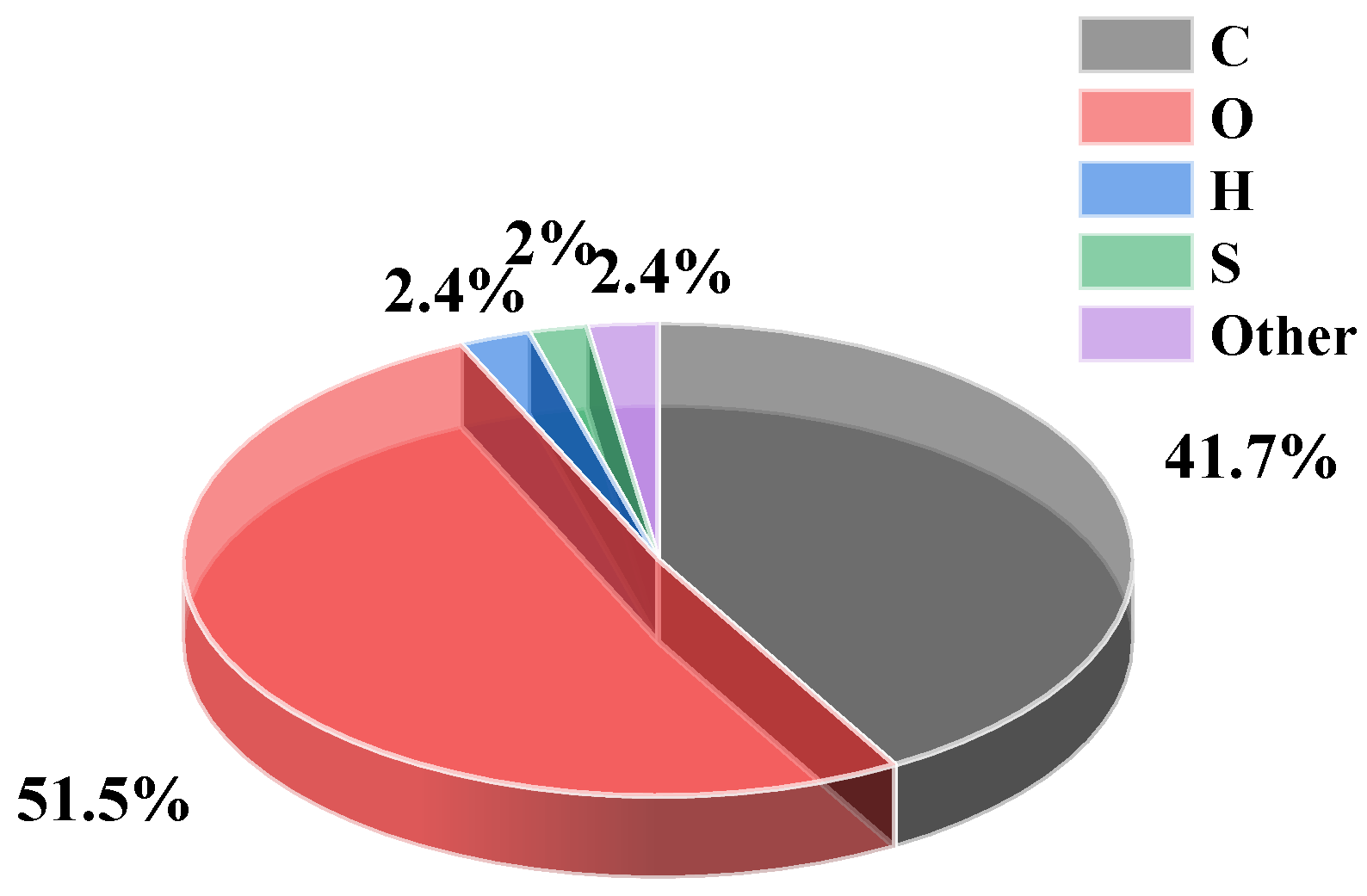
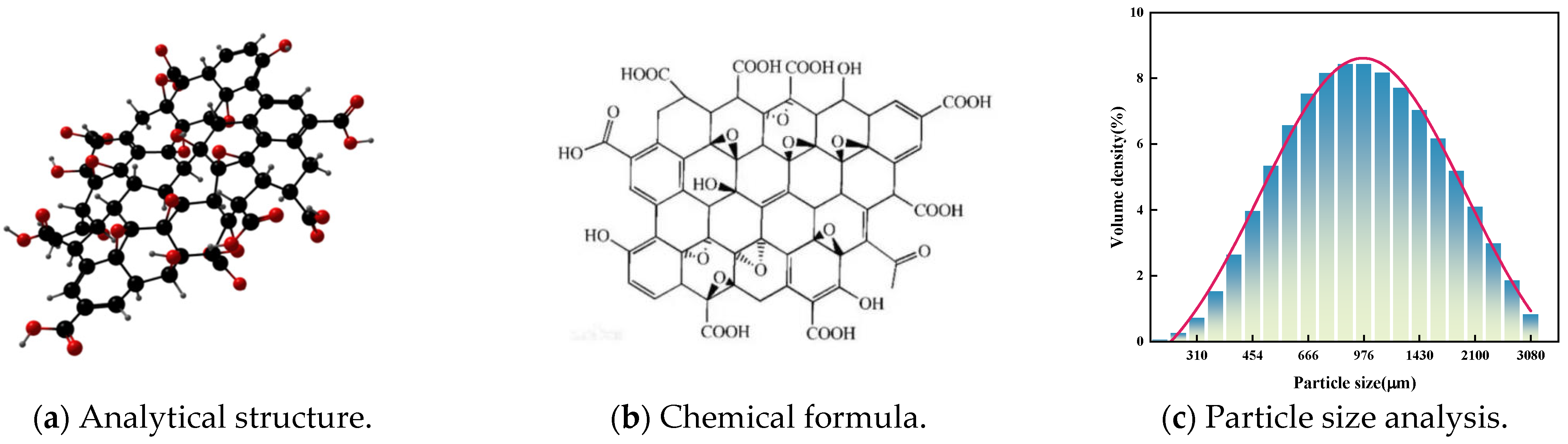
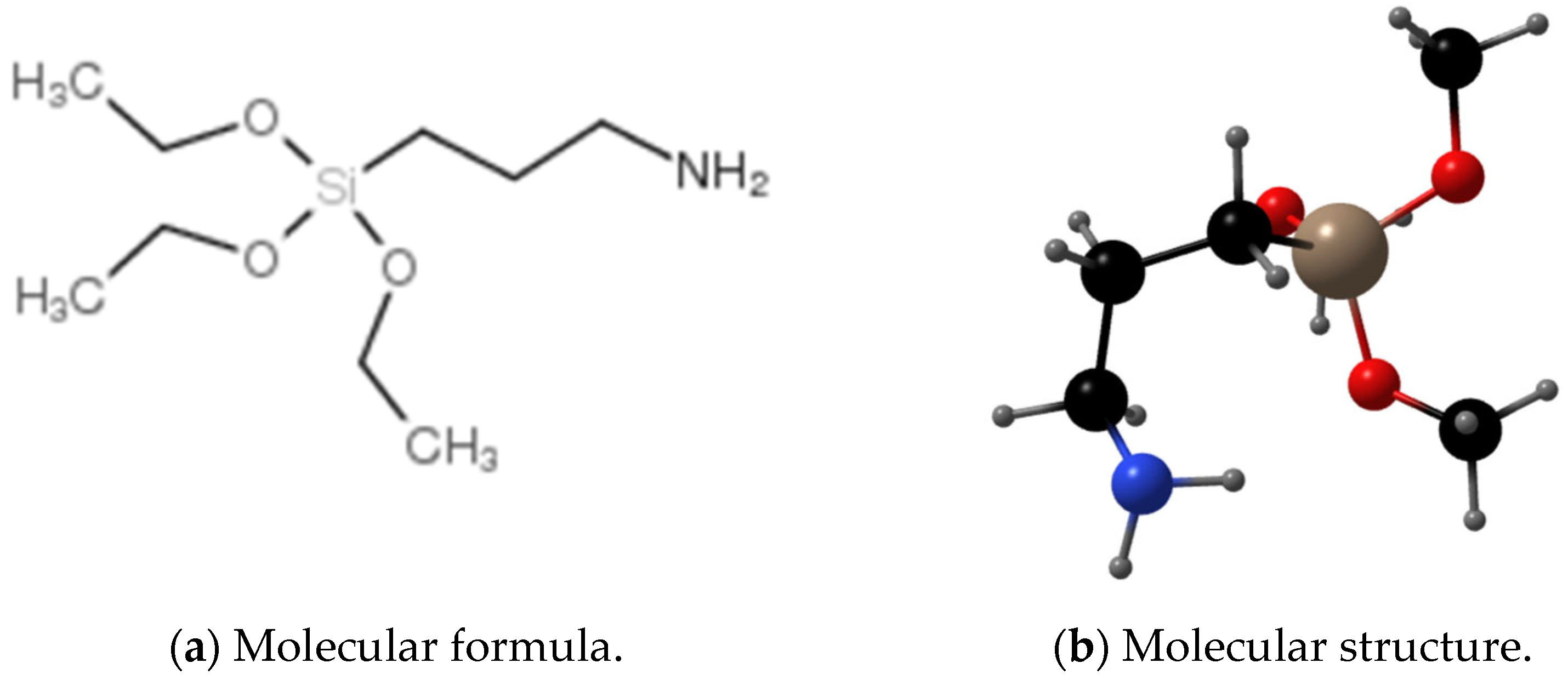
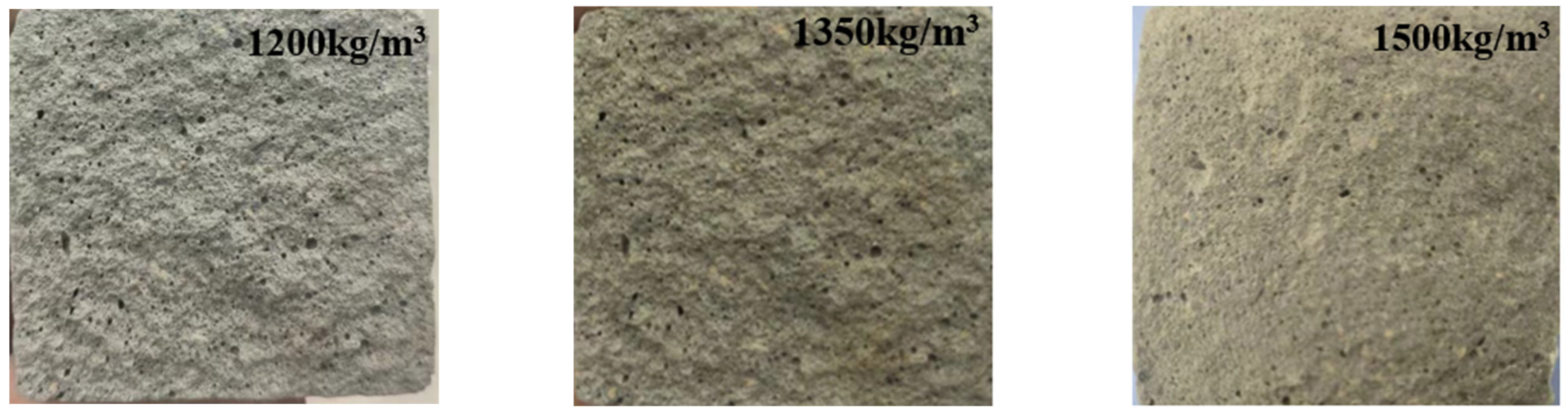







| Chemical Composition | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | FeO | Na2O | K2O | P2O5 | TiO2 | MnO | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 48.79 | 13.06 | 8.6 | 3.5 | 5.6 | 8.93 | 2.64 | 1.28 | 0.33 | 3.05 | 0.17 | 4.05 |
| Materials | Smell | Boiling Point (°C) | Fusing Point (°C) | Flash Point (°C) | Solubility (mg/L) | Density | Log Kow(est) |
|---|---|---|---|---|---|---|---|
| MDI | Odorless | 314 | 37 | 396 | 1.51 | 1.2 | 5.22 |
| Dry Density (kg/m3) | Water–Binder Ratio | Filler Ratio | Cement (%) | Stone Powder (%) | Blowing Agent (%) |
|---|---|---|---|---|---|
| 1500 | 0.54 | 0.5 | 42.47 | 22.47 | 4.22 |
| 1350 | 0.54 | 0.5 | 43.22 | 21.72 | 4.58 |
| 1200 | 0.54 | 0.5 | 44.77 | 20.17 | 8.43 |
| Sample Density and Type | Blowing Agent | Environmental Treatment | Coating Treatment | ||
|---|---|---|---|---|---|
| 1500 kg/m3 | 1350 kg/m3 | 1200 kg/m3 | |||
| PU-1500 | PU-1350 | PU-1200 | PU | - | - |
| CG-1500 | CG-1350 | CG-1200 | Carbonization environment | - | |
| FT-1500 | FT-1350 | FT-1200 | Freeze–thaw environment | - | |
| GO-1500 | GO-1350 | GO-1200 | - | GO | |
| SCA-1500 | SCA-1350 | SCA-1200 | - | KH-550 | |
| C/S-1500 | C/S-1350 | C/S-1200 | - | C/S | |
| Reagents | GO | KH-550 | G/S |
|---|---|---|---|
| Coated amount (g/m2) | 1200 | 1200 | 1200 |
| Coated amount in one time (g/m2) | 600 | 600 | 600 |
| Interval between coating (/h) | 6 | 6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, L.; Yu, P.; Wang, F.; Jiang, P. Evaluation of Integral and Surface Hydrophobic Modification on Permeation Resistance of Foam Concrete. Coatings 2025, 15, 854. https://doi.org/10.3390/coatings15070854
Ying L, Yu P, Wang F, Jiang P. Evaluation of Integral and Surface Hydrophobic Modification on Permeation Resistance of Foam Concrete. Coatings. 2025; 15(7):854. https://doi.org/10.3390/coatings15070854
Chicago/Turabian StyleYing, Liangbo, Pengfei Yu, Fuping Wang, and Ping Jiang. 2025. "Evaluation of Integral and Surface Hydrophobic Modification on Permeation Resistance of Foam Concrete" Coatings 15, no. 7: 854. https://doi.org/10.3390/coatings15070854
APA StyleYing, L., Yu, P., Wang, F., & Jiang, P. (2025). Evaluation of Integral and Surface Hydrophobic Modification on Permeation Resistance of Foam Concrete. Coatings, 15(7), 854. https://doi.org/10.3390/coatings15070854








