Two-Step Plasma Electrolytic Oxidation of Advanced High-Strength Steel in Aluminate and Silicate Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PEO Experiments
2.3. Microstructural Analysis
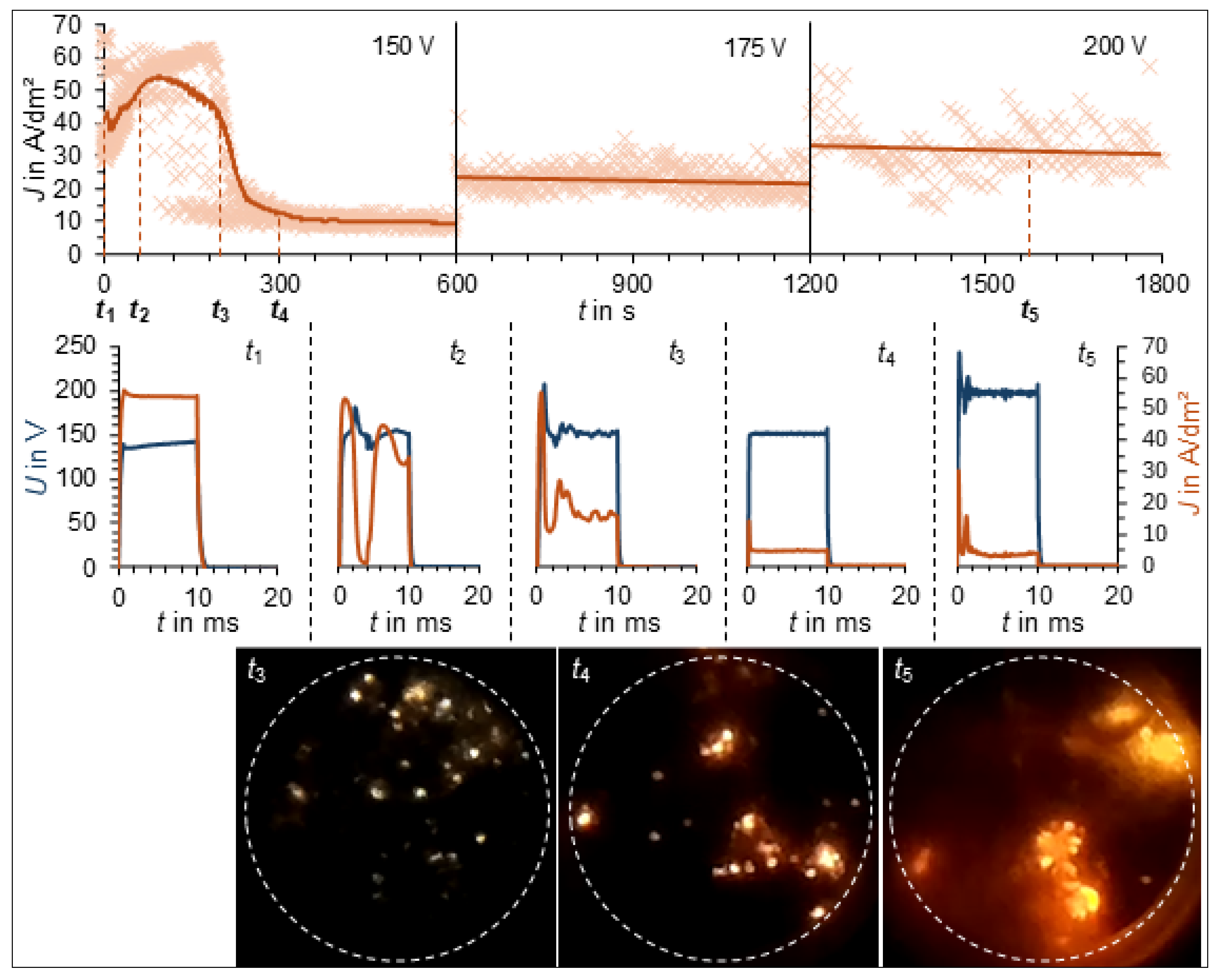
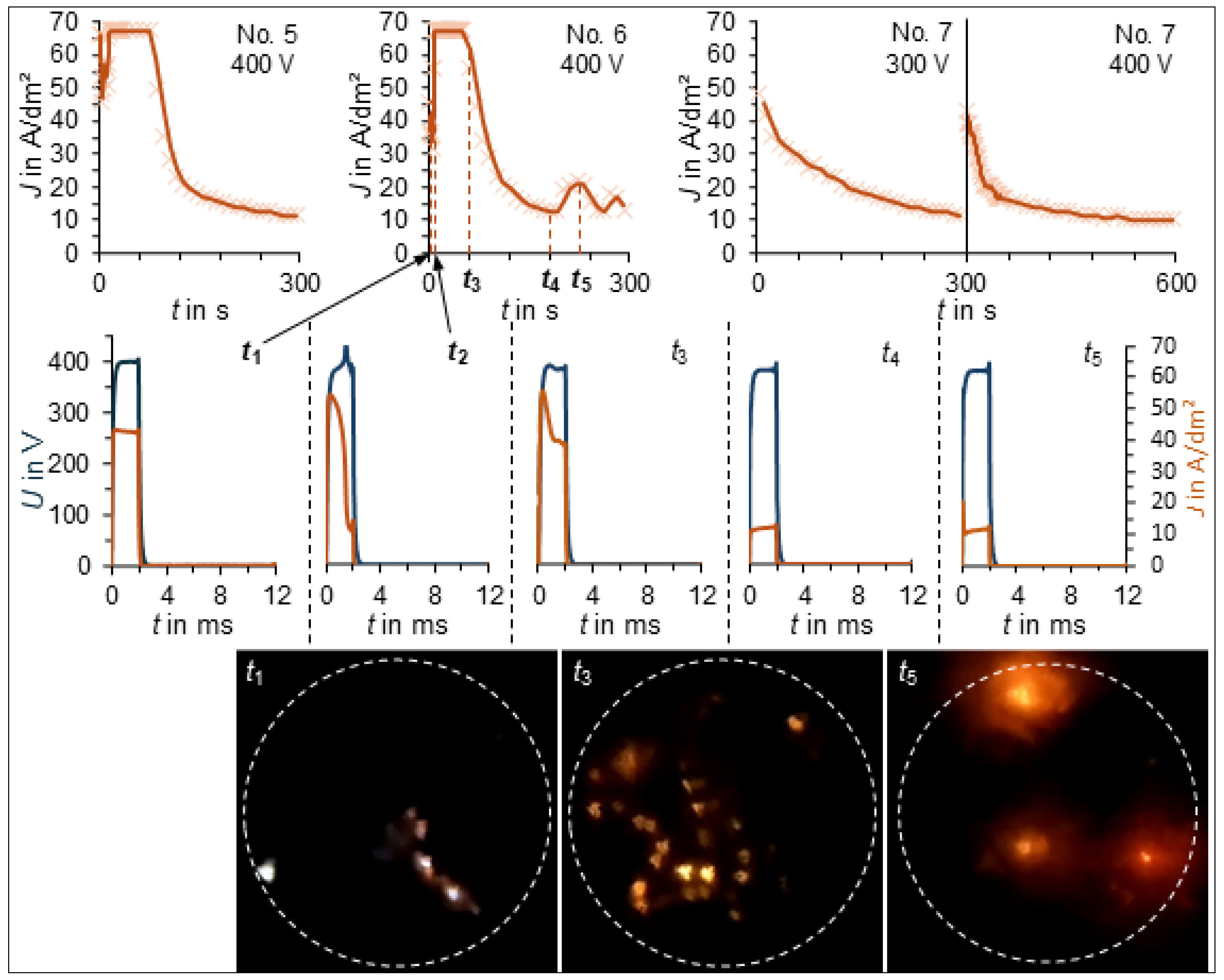
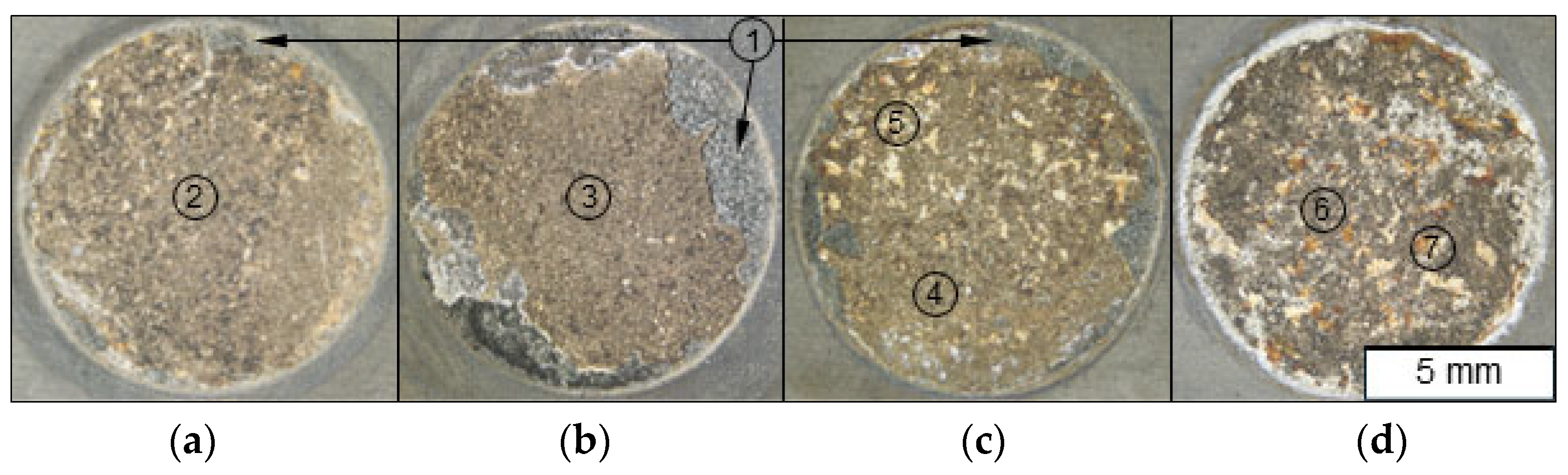
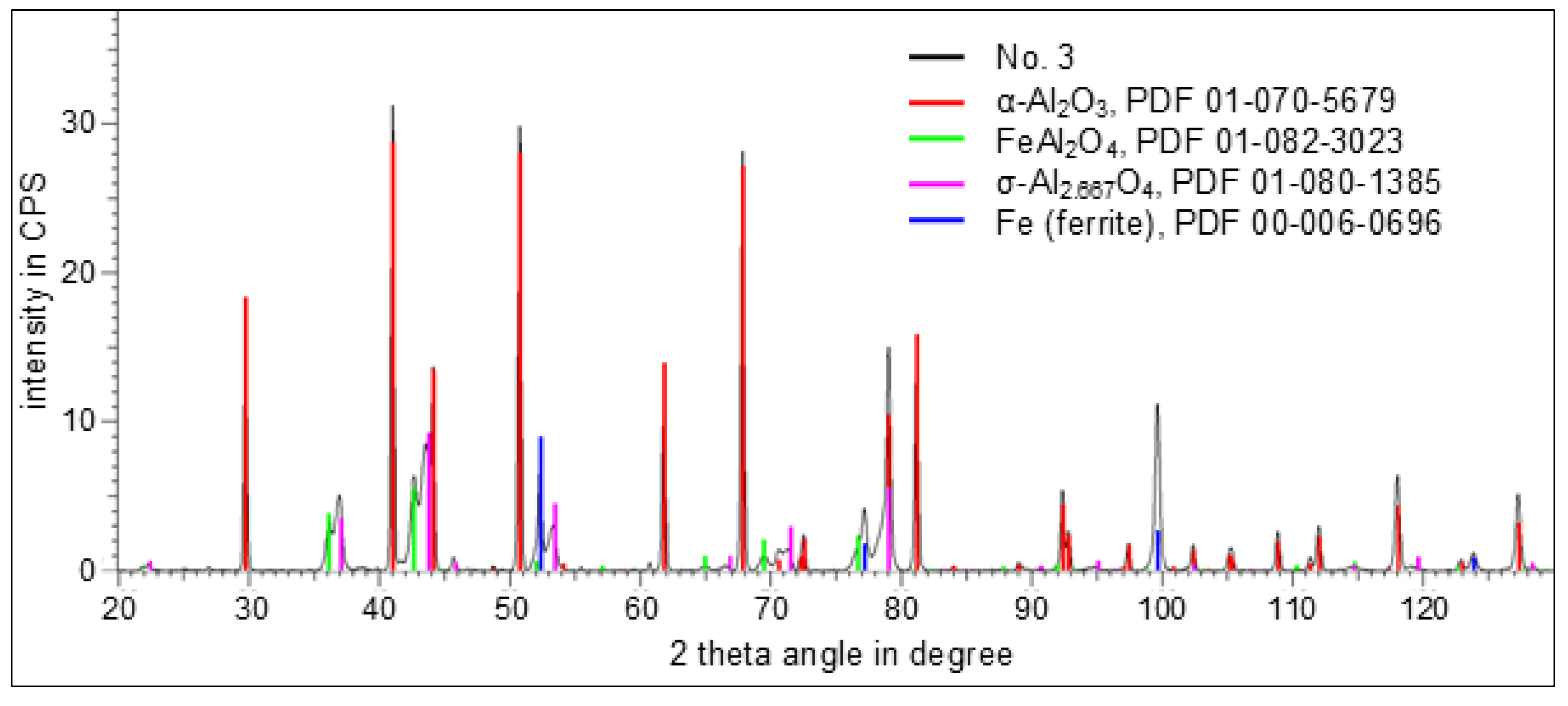
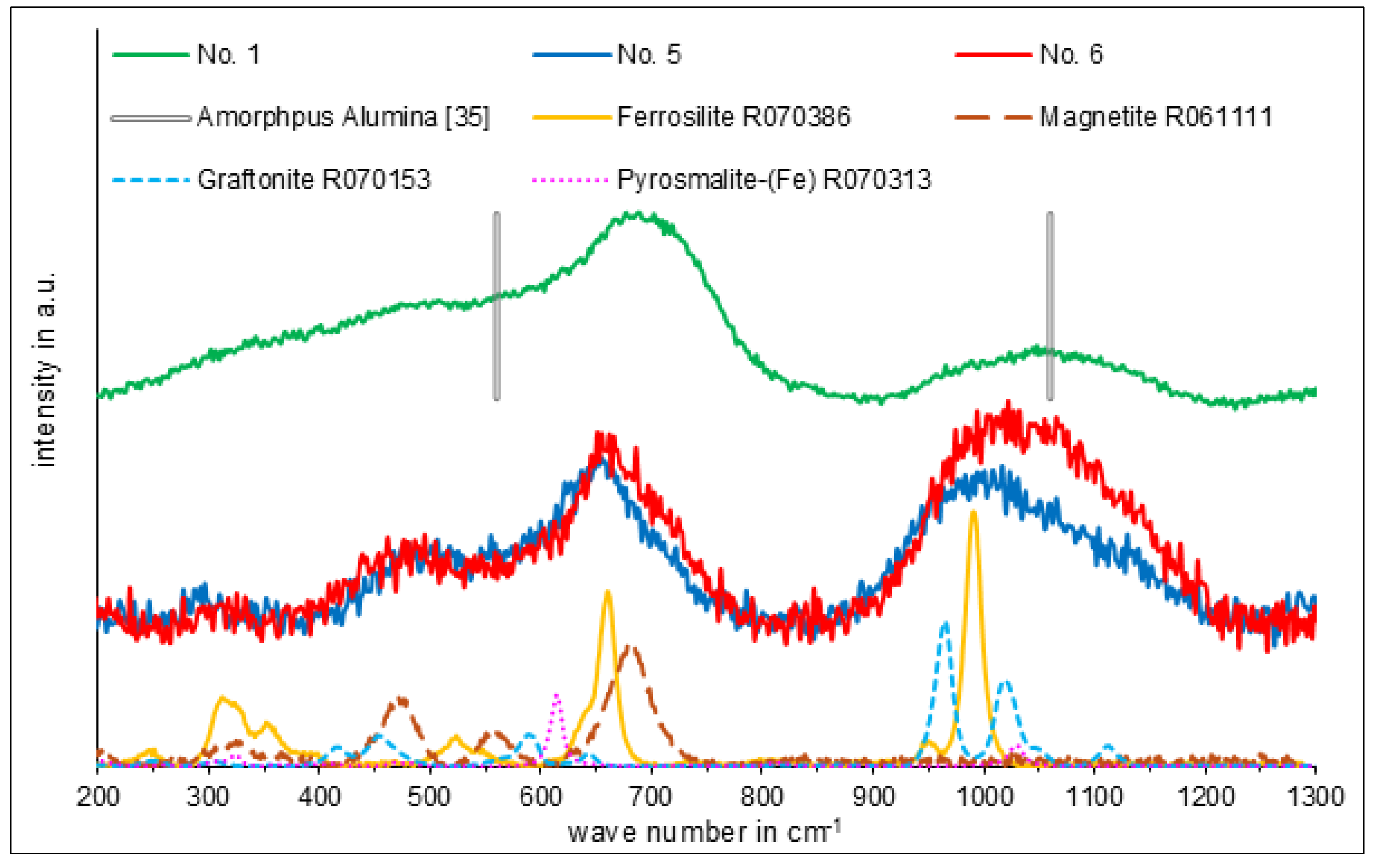
3. Results
3.1. PEO Process Characteristics
3.2. Macroscopical and Microstructural Features
3.3. Phase Analysis
4. Discussion
4.1. Layer Evolution in the Aluminate Electrolyte
4.2. Layer Evolution in the Silicate Electrolyte
4.3. Layer Evolution in the Silicate Electrolyte After PEO in the Aluminate Electrolyte
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieber, M.; Simchen, F.; Morgenstern, R.; Scharf, I.; Lampke, T. Plasma Electrolytic Oxidation of High-Strength Aluminium Alloys—Substrate Effect on Wear and Corrosion Performance. Metals 2018, 8, 356. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloy. Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Babaei, K.; Fattah-Alhosseini, A.; Chaharmahali, R. A review on plasma electrolytic oxidation (PEO) of niobium: Mechanism, properties and applications. Surfaces Interfaces 2020, 21, 100719. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Molaei, M.; Babaei, K.; Fattah-Alhosseini, A. New Promising Ceramic Coatings for Corrosion and Wear Protection of Steels: A Review. Surfaces Interfaces 2021, 23, 100997. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Lampke, T. Electrolyte influence on ignition of plasma electrolytic oxidation processes on light metals. Surf. Coat. Technol. 2017, 315, 205–213. [Google Scholar] [CrossRef]
- Cheng, B.; Lu, H.; Bai, K.; Wang, C.; Wang, W.; Guo, F. Formation of ceramic coatings on non-valve metal low carbon steel using micro-arc oxidation technology. Ceram. Int. 2024, 50, 23541–23549. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, Y.; Kang, S.-H.; Tu, W.; Cheng, Y. A re-understanding of the breakdown theory from the study of the plasma electrolytic oxidation of a carbon steel — A non-valve metal. Electrochim. Acta 2018, 284, 681–695. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Chu, C.-H.; Zhang, M.-X.; Wang, H.-B.; Xue, S.-X. Wear resistance of alumina-coated oil casing steel N80 via MAO with rare earth additive. Ceram. Int. 2017, 43, 6397–6402. [Google Scholar] [CrossRef]
- Yang, W.; Peng, Z.; Liu, B.; Liu, W.; Liang, J. Influence of Silicate Concentration in Electrolyte on the Growth and Performance of Plasma Electrolytic Oxidation Coatings Prepared on Low Carbon Steel. J. Mater. Eng. Perform. 2018, 27, 2345–2353. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Mihalache, M.; Iosub, I. Preparation and characterization of coatings on carbon steel obtained by PEO in silicate/carbonate electrolyte. Surf. Coat. Technol. 2016, 296, 96–103. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z. In situ formation of low friction ceramic coatings on carbon steel by plasma electrolytic oxidation in two types of electrolytes. Appl. Surf. Sci. 2009, 255, 6240–6243. [Google Scholar] [CrossRef]
- Yang, W.; Li, Q.; Liu, W.; Liang, J.; Peng, Z.; Liu, B. Characterization and properties of plasma electrolytic oxidation coating on low carbon steel fabricated from aluminate electrolyte. Vacuum 2017, 144, 207–216. [Google Scholar] [CrossRef]
- Freire, L.; Nóvoa, X.; Montemor, M.; Carmezim, M. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Joiret, S.; Keddam, M.; Nóvoa, X.R.; Pérez, M.C.; Rangel, C.; Takenouti, H. Use of EIS, ring-disk electrode, EQCM and Raman spectroscopy to study the film of oxides formed on iron in 1M NaOH. Cem. Concr. Compos. 2002, 24, 7–15. [Google Scholar] [CrossRef]
- DorMohammadi, H.; Pang, Q.; Murkute, P.; Árnadóttir, L.; Isgor, O.B. Investigation of iron passivity in highly alkaline media using reactive-force field molecular dynamics. Corros. Sci. 2019, 157, 31–40. [Google Scholar] [CrossRef]
- Morgenstern, R.; Rojas, C.A.; Simchen, F.; Meinhold, V.; Mehner, T.; Lampke, T. Passivation and pH-Induced Precipitation during Anodic Polarization of Steel in Aluminate Electrolytes as a Precondition for Plasma Electrolytic Oxidation. Coatings 2023, 13, 656. [Google Scholar] [CrossRef]
- Eremin, N.I.; Mironov, Y.A.; Volokhov, V.E. Structure and Behaviour of Aluminate Ions in Solution. Russ. Chem. Rev. 1974, 43, 92–106. [Google Scholar] [CrossRef]
- Karpushenkov, S.A.; Shchukin, G.L.; Belanovich, A.L.; Savenko, V.P.; Kulak, A.I. Plasma electrolytic ceramic-like aluminum oxide coatings on iron. J. Appl. Electrochem. 2009, 40, 365–374. [Google Scholar] [CrossRef]
- Kurze, P. Herstellung, Charakterisierung und Anwendung von Al2O3-Schichten insbesondere auf Aluminium- und Eisenwerkstoffen. Ph.D. Thesis, Technische Hochschule Karl-Marx-Stadt, Karl-Marx-Stadt, Germany, March 1982. [Google Scholar]
- Dao Huu, N. Untersuchungen zur Anodischen Abscheidung von Schutzschichten aus Wäßrigen Aluminatlösungen auf Eisenwerkstoffen. Ph.D. Thesis, Technische Hochschule Karl-Marx-Stadt, Karl-Marx-Stadt, Germany, September 1979. [Google Scholar]
- Simchen, F.; Sieber, M.; Mehner, T.; Lampke, T. Characterisation Method of the Passivation Mechanisms during the pre-discharge Stage of Plasma Electrolytic Oxidation Indicating the Mode of Action of Fluorides in PEO of Magnesium. Coatings 2020, 10, 965. [Google Scholar] [CrossRef]
- Simchen, F.; Masoud-Nia, N.; Mehner, T.; Lampke, T. Formation of corundum-rich alumina coatings on low-carbon steel by plasma electrolytic oxidation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1147, 012007. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Z.; Yang, M.; Wang, Y.; Xia, Q.; Jiang, Z. A Fe3O4/FeAl2O4 composite coating via plasma electrolytic oxidation on Q235 carbon steel for Fenton-like degradation of phenol. Environ. Sci. Pollut. Res. 2016, 23, 14927–14936. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Li, W.; Wang, Q.; Wang, Y.; You, C. Preparation, characteristics and corrosion properties of α-Al2O3 coatings on 10B21 carbon steel by micro-arc oxidation. Surf. Coat. Technol. 2019, 358, 637–645. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Moga, S.; Negrea, D. Preparation and characterization of anticorrosive layers deposited by micro-arc oxidation on low carbon steel. Surf. Coat. Technol. 2014, 253, 194–198. [Google Scholar] [CrossRef]
- Amaral, S.; Müller, I. A RRDE study of the electrochemical behavior of iron in solutions containing silicate and sulphate at pH 10–13. Corros. Sci. 1999, 41, 759–771. [Google Scholar] [CrossRef]
- Lian, T.; Jones, D. Electrochemical Corrosion Behavior of Low Carbon Steel in Concentrated Geologic Vadose Waters. Corrosion 1999, 55, 1012–1019. [Google Scholar] [CrossRef]
- Schmitt, W.; Flügel, W.; Schwenk, W. Fast pitting corrosion in a steel/water system as a result of unusual stabilization of anodes. Mater. Corros. 1992, 43, 39–47. [Google Scholar] [CrossRef]
- Palmer, A.N.; Palmer, M.V. Geochemistry of capillary seepage in Mammoth Cave. Acta Carsologica 2003, 30, 14–32. [Google Scholar]
- Yang, W.; Li, Q.; Liu, C.; Liang, J.; Peng, Z.; Liu, B. A comparative study of characterisation of plasma electrolytic oxidation coatings on carbon steel prepared from aluminate and silicate electrolytes. Surf. Eng. 2017, 34, 54–62. [Google Scholar] [CrossRef]
- Yang, W.; Li, Q.; Xiao, Q.; Liang, J. Improvement of corrosion protective performance of organic coating on low carbon steel by PEO pretreatment. Prog. Org. Coat. 2015, 89, 260–266. [Google Scholar] [CrossRef]
- Ma, C.; Liu, J.; Zhu, X.; Xue, W.; Yan, Z.; Cheng, D.; Fu, J.; Ma, S. Anticorrosive non-crystalline coating prepared by plasma electrolytic oxidation for ship low carbon steel pipes. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Database of Raman Spectroscopy, X-Ray Diffraction and Chemistry Data for Minerals. Available online: https://rruff.info (accessed on 29 January 2023).
- Sudare, T.; Zenzai, A.; Tamura, S.; Kiyama, M.; Hayashi, F.; Teshima, K. Hierarchical spheres of Mg–Al LDH for the removal of phosphate ions: Effect of alumina polymorph as precursor. CrystEngComm 2019, 21, 7211–7216. [Google Scholar] [CrossRef]
- Tuschel, D. Why Are the Raman Spectra of Crystalline and Amorphous Solids Different? Spectroscopy 2017, 32, 26–33. [Google Scholar]
- Jaspard-Mécuson, F.; Czerwiec, T.; Henrion, G.; Belmonte, T.; Dujardin, L.; Viola, A.; Beauvir, J. Tailored aluminium oxide layers by bipolar current adjustment in the Plasma Electrolytic Oxidation (PEO) process. Surf. Coat. Technol. 2007, 201, 8677–8682. [Google Scholar] [CrossRef]
- Rogov, A.B.; Yerokhin, A.; Matthews, A. The role of cathodic current in plasma electrolytic oxidation of aluminium: Current density ‘scanning waves’ on complex-shape substrates. J. Phys. D Appl. Phys. 2018, 51, 405303. [Google Scholar] [CrossRef]
- Rogov, A.B.; Matthews, A.; Yerokhin, A. Role of cathodic current in plasma electrolytic oxidation of Al: A quantitative approach to in-situ evaluation of cathodically induced effects. Electrochimica Acta 2019, 317, 221–231. [Google Scholar] [CrossRef]
- Durães, L.; Costa, B.F.; Santos, R.; Correia, A.; Campos, J.; Portugal, A. Fe2O3/aluminum thermite reaction intermediate and final products characterization. Mater. Sci. Eng. A 2007, 465, 199–210. [Google Scholar] [CrossRef]




| Number | Electrolyte Steps | Voltage Amplitude Levels in V | ||||
|---|---|---|---|---|---|---|
| 150 | 175 | 200 | 300 | 400 | ||
| 1 | Single | A | ||||
| 2 | Single | A | A | |||
| 3 | Single | A | A | A | ||
| 4 | Single | S | S | S | S | S |
| 5 | Two-Step | A | A | S | ||
| 6 | Two-Step | A | A | A | S | |
| 7 | Two-Step | A | A | A | S | S |
| Point | Molar Fraction in % | ||||
|---|---|---|---|---|---|
| Al | Fe | P | O | Na | |
| 1 | 30.5 | 6.0 | 5.5 | 56.6 | 1.3 |
| 2 | 47.5 | 4.1 | 0.4 | 47.6 | 0.4 |
| 3 | 39.7 | 3.7 | 2.8 | 52.3 | 1.5 |
| 4 | 34.1 | 12.0 | 0.5 | 52.7 | 0.8 |
| 5 | 47.7 | 1.7 | 0.4 | 49.8 | 0.5 |
| Point | Molar Fraction in % | ||||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Si | P | O | Na | K | |
| 1 | 0.5 | 0.8 | 37.1 | 1.7 | 59.9 | - | - |
| 2 | 0.3 | 13.0 | 6.2 | 18.3 | 61.4 | 0.5 | 0.2 |
| 3 * | - | 34.1 | 0.7 | 2.6 | 61.7 | - | - |
| 4 | 0.2 | 1.4 | 32.6 | 4.9 | 59.8 | 0.7 | 0.5 |
| 5 | 0.5 | 12.6 | 2.9 | 26.0 | 57.6 | - | 0.4 |
| 6 | 0.4 | 0.5 | 43.0 | 0.8 | 55.2 | - | - |
| 7 ** | 0.3 | 19.8 | 6.2 | 13.0 | 57.4 | 2.6 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgenstern, R.; Mehner, T.; Lampke, T. Two-Step Plasma Electrolytic Oxidation of Advanced High-Strength Steel in Aluminate and Silicate Solutions. Coatings 2025, 15, 850. https://doi.org/10.3390/coatings15070850
Morgenstern R, Mehner T, Lampke T. Two-Step Plasma Electrolytic Oxidation of Advanced High-Strength Steel in Aluminate and Silicate Solutions. Coatings. 2025; 15(7):850. https://doi.org/10.3390/coatings15070850
Chicago/Turabian StyleMorgenstern, Roy, Thomas Mehner, and Thomas Lampke. 2025. "Two-Step Plasma Electrolytic Oxidation of Advanced High-Strength Steel in Aluminate and Silicate Solutions" Coatings 15, no. 7: 850. https://doi.org/10.3390/coatings15070850
APA StyleMorgenstern, R., Mehner, T., & Lampke, T. (2025). Two-Step Plasma Electrolytic Oxidation of Advanced High-Strength Steel in Aluminate and Silicate Solutions. Coatings, 15(7), 850. https://doi.org/10.3390/coatings15070850







