Modification of Polyurethane/Graphene Oxide with Dielectric Barrier Plasma Treatment for Proper Coating Adhesion on Fiberglass
Abstract
1. Introduction
2. Materials and Methods
2.1. Fiberglass Substrates
2.2. Graphene Oxide
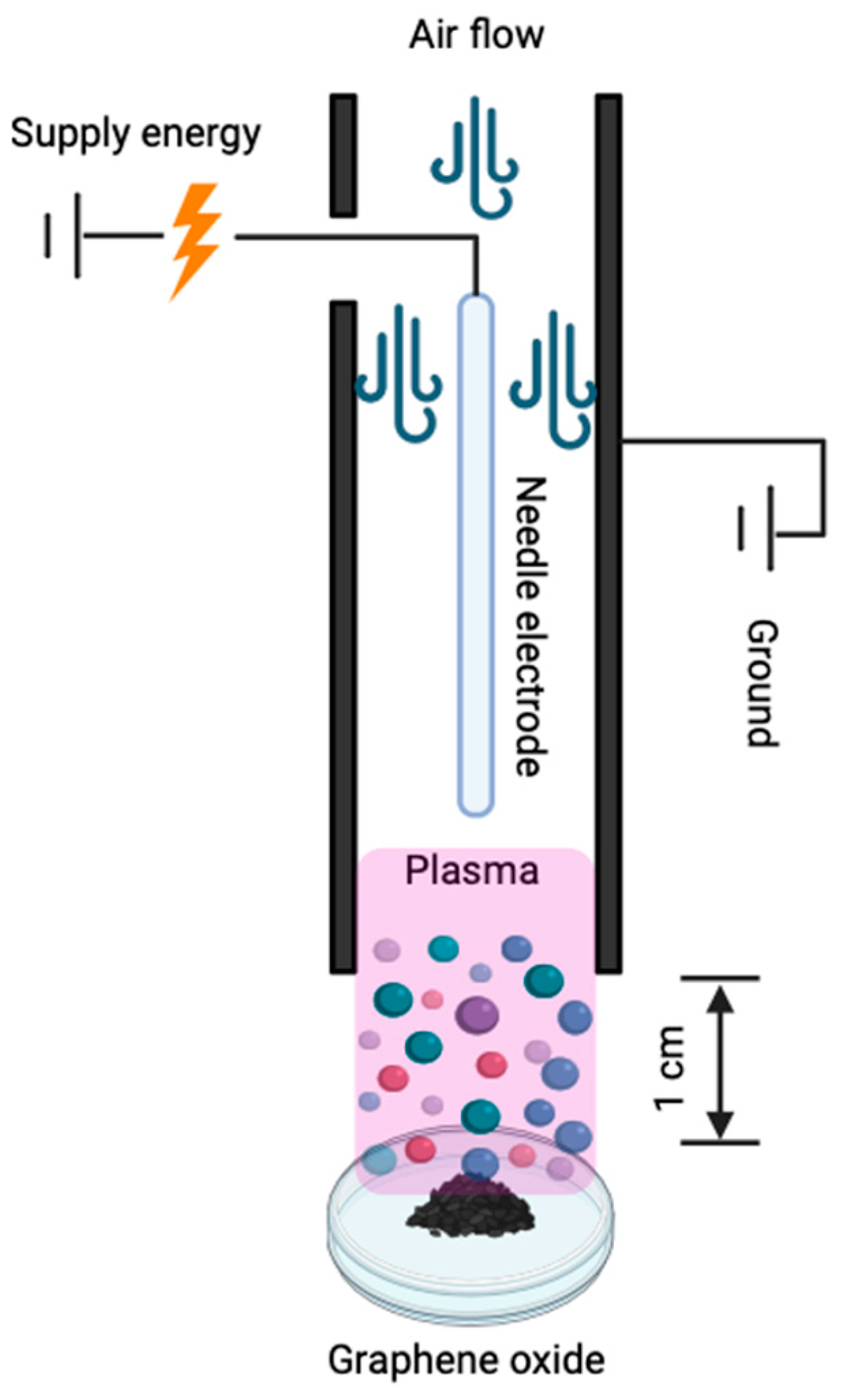
2.3. Dielectric Barrier Plasma Treatment
2.4. Application of the Coating
3. Results and Discussion
3.1. GF Substrates
3.1.1. Contact Angle and Surface Energy
3.1.2. Raman Spectroscopy for GF
3.2. Graphene Oxide Modification
3.2.1. Raman Spectroscopy for GO
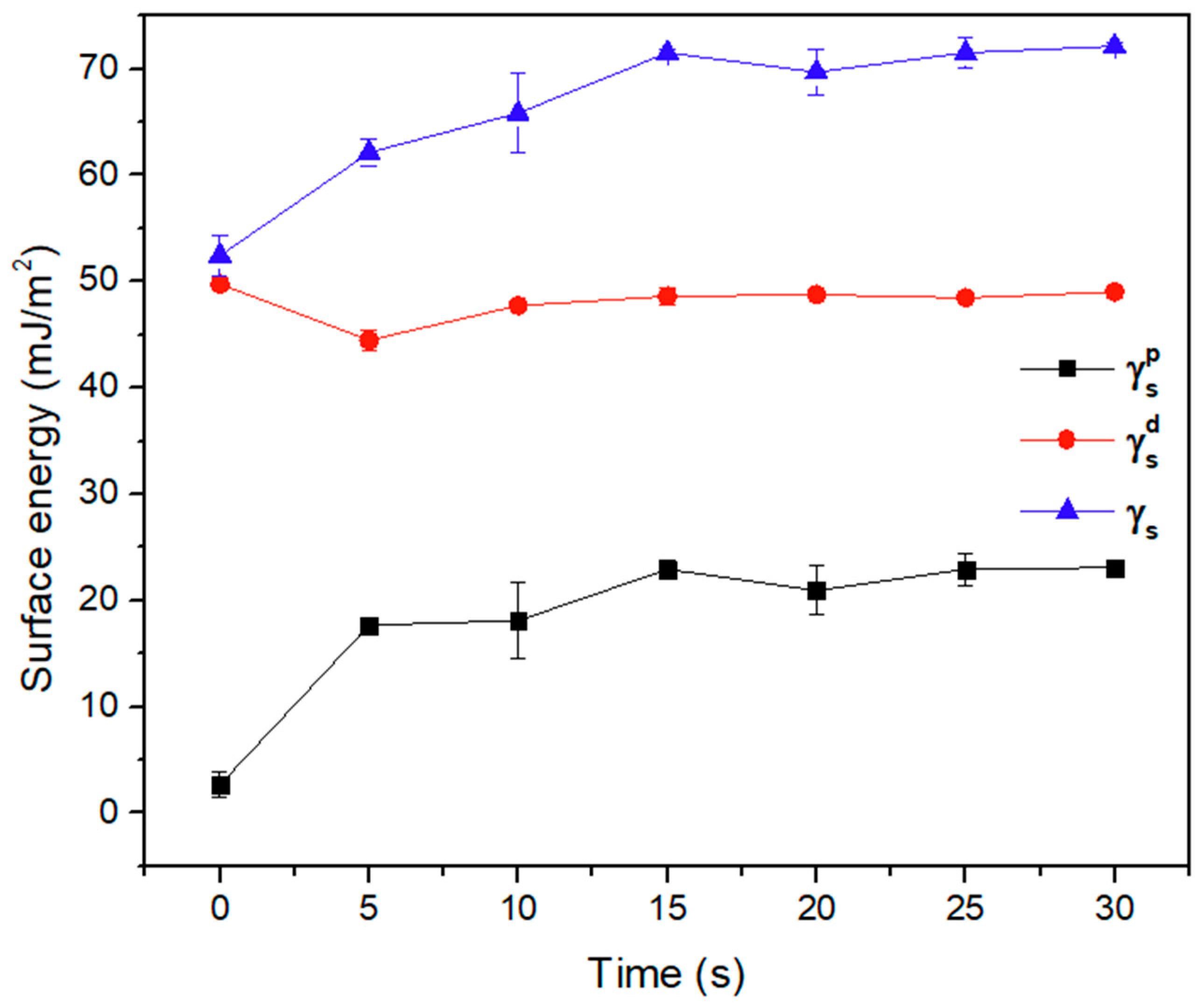
3.2.2. Surface Energy Test
3.3. Polyurethane/Graphene Oxide (PU/GO) Coatings
3.3.1. FTIR Spectroscopy for PU/GO
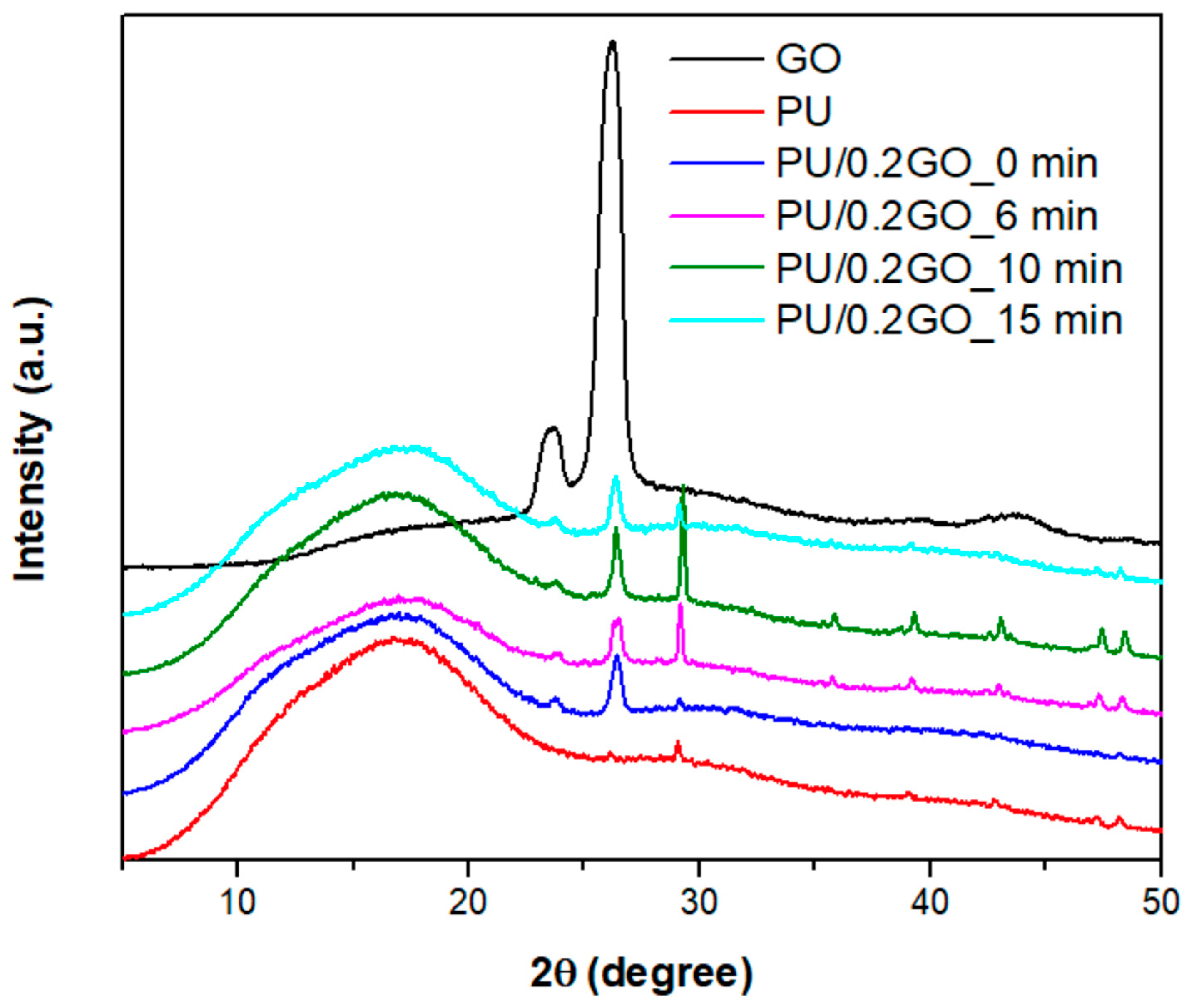
3.3.2. X-Ray Diffraction for PU/GO
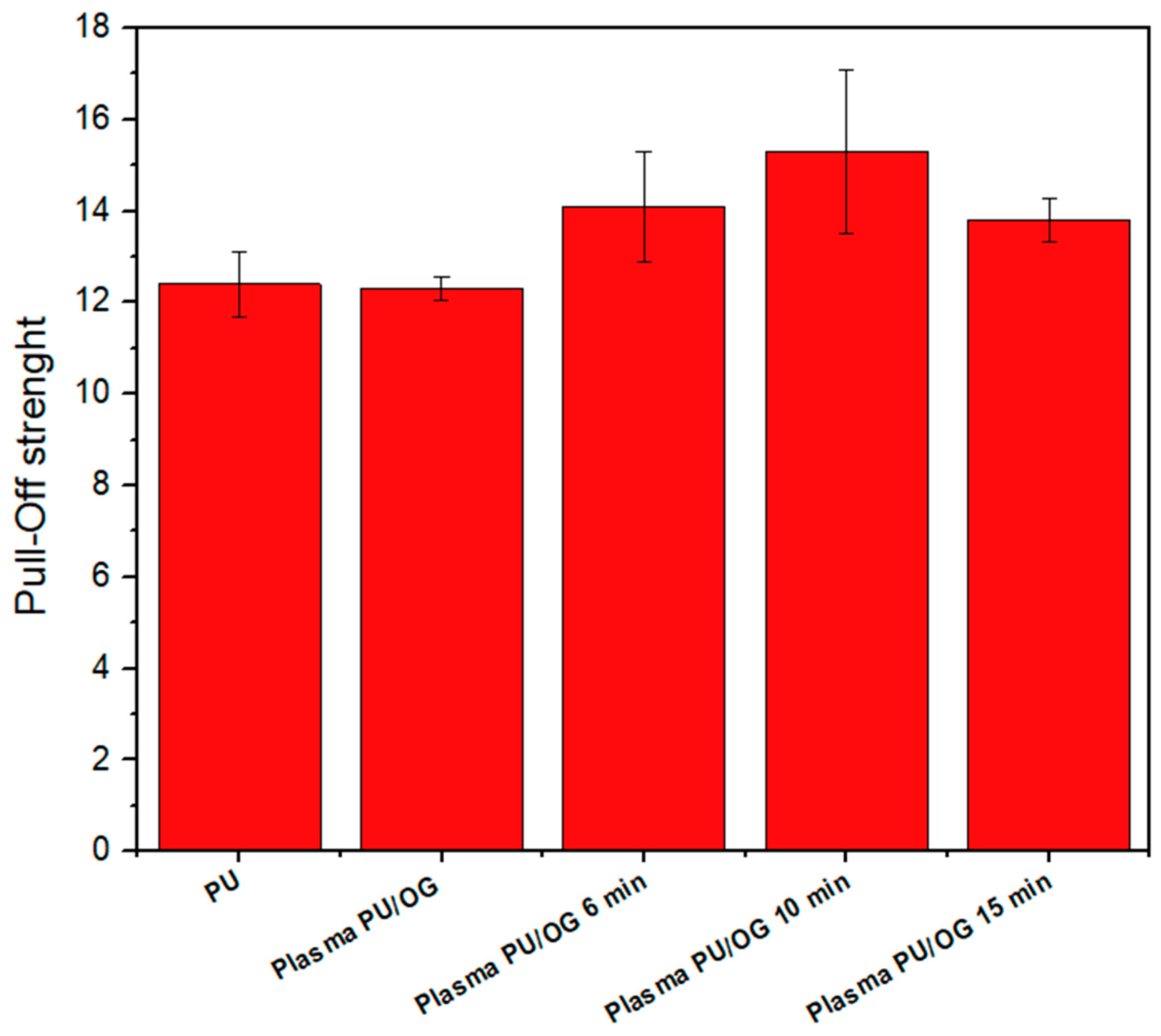
3.3.3. Adhesion Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walayat, K.; Haeri, S.; Ma, J.; Zhang, Y. PD–DEM hybrid modeling of leading-edge erosion in wind turbine blades under controlled impact scenarios. Comput. Part. Mech. 2024, 11, 1903–1921. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hwang, Y.M.; Lee, J.; Kim, N.; Ko, S. A Drone-driven X-ray Image-based Diagnosis of Wind Turbine Blades for Reliable Operation of Wind Turbine. IEEE Access 2024, 12, 56141–56158. [Google Scholar] [CrossRef]
- Lopez, J.C.; Kolios, A. An autonomous decision-making agent for offshore wind turbine blades under leading edge erosion. Renew. Energy 2024, 227, 120525. [Google Scholar] [CrossRef]
- Carraro, M.; De Vanna, F.; Zweiri, F.; Benini, E.; Heidari, A.; Hadavinia, H. CFD Modeling of Wind Turbine Blades with Eroded Leading Edge. Fluids 2022, 7, 302. [Google Scholar] [CrossRef]
- Law, H.; Koutsos, V. Leading edge erosion of wind turbines: Effect of solid airborne particles and rain on operational wind farms. Wind Energy 2020, 23, 1955–1965. [Google Scholar] [CrossRef]
- Pryor, S.C.; Barthelmie, R.; Cadence, J.; Dellwik, E.; Hasager, C.B.; Kral, S.T.; Reuder, J.; Rodgers, M.; Veraart, M. Atmospheric Drivers of Wind Turbine Blade Leading Edge Erosion: Review and Recommendations for Future Research. Energies 2022, 15, 8553. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Tempelis, A.; Kuthe, N.; Mahajan, P. Recent developments in the protection of wind turbine blades against leading edge erosion: Materials solutions and predictive modeling. Renew. Energy 2023, 215, 118966. [Google Scholar] [CrossRef]
- Wang, B.; Xing, G.; Zhang, J.; Sheng, M.; Wang, J.; Wang, Z. Relationship between wet coating thickness and nanoparticle loadings based on the performance of mixed matrix composite membranes. J. Membr. Sci. 2023, 667, 121167. [Google Scholar] [CrossRef]
- Liang, G.; Yao, F.; Qi, Y.; Gong, R.; Li, R.; Liu, B.; Zhao, Y.; Lian, C.; Li, L.; Dong, X.; et al. Improvement of Mechanical Properties and Solvent Resistance of Polyurethane Coating by Chemical Grafting of Graphene Oxide. Polymers 2023, 15, 882. [Google Scholar] [CrossRef]
- El-Shamy, O.A.A.; Deyab, M. Eco-friendly biosynthesis of silver nanoparticles and their improvement of anti-corrosion performance in epoxy coatings. J. Mol. Liq. 2023, 376, 121488. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Samsudin, N.A.; Mohammad, M.; Razali, H.; Soltani, S.; Amin, N.A. Application of graphene-based materials in developing sustainable infrastructure: An overview. Composites. Part B Eng. 2022, 245, 110188. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z. Preparation and characterization of a novel polyurethane/polyurethane modified graphene oxide composites. Colloid Polym. Sci. /Colloid Polym. Sci. 2021, 299, 1767–1776. [Google Scholar] [CrossRef]
- Ince, J.C.; Mudasir, P.; Mathews, L.D.; Pai, A.R.; Al-Qatatsheh, A.; Abbasi, S.; Yin, Y.; Hameed, N.; Duffy, A.R.; Lau, A.K.; et al. Overview of emerging hybrid and composite materials for space applications. Adv. Compos. Hybrid Mater. 2023, 6, 130. [Google Scholar] [CrossRef]
- Tounici, A.; Martín-MartíNez, J.M. Addition of Graphene Oxide in Different Stages of the Synthesis of Waterborne Polyurethane-Urea Adhesives and Its Influence on Their Structure, Thermal, Viscoelastic, and Adhesion Properties. Materials 2020, 13, 2899. [Google Scholar] [CrossRef]
- Wang, H.; Ye, M.; Wu, J.; Li, Y.; Guo, L.; Yang, D. Development of amine functionalized graphene oxide/fluorinated polyurethane topcoat with integrated anti-corrosion, anti-aging, and anti-bacterial performance. Prog. Org. Coat. 2024, 189, 108337. [Google Scholar] [CrossRef]
- Wei, G. Three-dimensional porous graphene-polymer frameworks for electromagnetic interference shielding. In Elsevier EBooks; Elsevier: Amsterdam, The Netherlands, 2024; pp. 221–244. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Liu, W.Q.; Pan, J.; Huang, S.Q.; Mou, Z.G. Preparation and Properties of Modified Graphene Oxide/Polyurethane Composites. Integr. Ferroelectr. 2020, 206, 1–9. [Google Scholar] [CrossRef]
- Hamam, K.A.; Gaabour, L.H. Verify the changes in the structural and physical properties of PU/PEO embedded with graphene oxide. Results Phys. 2017, 7, 2427–2431. [Google Scholar] [CrossRef]
- George, J.S.; Poornima, V.P.; Paduvilan, J.K.; Salim, N.V.; Sunarso, J.; Kalarikkal, N.; Hameed, N.; Thomas, S. Advances and future outlook in epoxy/graphene composites for anticorrosive applications. Prog. Org. Coat. 2022, 162, 106571. [Google Scholar] [CrossRef]
- Acharya, S.; Swain, L.M.; Samal, R.R.; Parashar, S.K.S.; Sahoo, B.P. Fabrication of Multiwalled Carbon Nanotubes (MWCNT) and Reduced Graphene Oxide (rGO)-based thermoplastic polyurethane and polypyrrole nanocomposites for electromagnetic wave absorption application with a low reflection. Polym. Compos. 2023, 45, 3769–3784. [Google Scholar] [CrossRef]
- Olivieri, F.; Rollo, G.; De Falco, F.; Avolio, R.; Bonadies, I.; Castaldo, R.; Cocca, M.; Errico, M.E.; Lavorgna, M.; Gentile, G. Reduced graphene oxide/polyurethane coatings for wash-durable wearable piezoresistive sensors. Cellulose 2023, 30, 2667–2686. [Google Scholar] [CrossRef]
- Hamzaj, A.K.; Dona, E.D.; Santhosh, N.; Shvalya, V.; Košiček, M.; Cvelbar, U. Plasma-Modification of graphene oxide for advanced ammonia sensing. Appl. Surf. Sci. 2024, 660, 160006. [Google Scholar] [CrossRef]
- El-Hossary, F.M.; Ghitas, A.; El-Rahman, A.M.A.; Ebnalwaled, A.A.; Shahat, M.A.; Fawey, M.H. Cold RF oxygen plasma treatment of graphene oxide films. J. Mater. Science. Mater. Electron. 2021, 32, 15718–15731. [Google Scholar] [CrossRef]
- Kumar, P.; Šilhavík, M.; Zafar, Z.A.; Červenka, J. Universal Strategy for Reversing Aging and Defects in Graphene Oxide for Highly Conductive Graphene Aerogels. J. Phys. Chem. C 2023, 127, 10599–10608. [Google Scholar] [CrossRef]
- Kong, W.; Ge, X.; Zhang, Q.; Zhang, F.; Kong, D.; Zhu, X.F.; Zhang, M.; Feng, Y. Ultrafast air-plasma reduction-exfoliation of graphene oxide aerogel at room temperature for capacitive deionization. Carbon 2023, 215, 118501. [Google Scholar] [CrossRef]
- Pathak, S.M.; Kumar, V.P.; Bonu, V.; Latha, S.; Mishnaevsky, L.; Lakshmi, R.; Bera, P.; Barshilia, H.C. Solid particle erosion studies of ceramic oxides reinforced water-based PU nanocomposite coatings for wind turbine blade protection. Ceram. Int. 2022, 48, 35788–35798. [Google Scholar] [CrossRef]
- ASTM D4541-22; Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers. ASTM International: West Conshohocken, PA, USA, 2022.
- Pugh, K.; Nash, J.; Reaburn, G.; Stack, M. On analytical tools for assessing the raindrop erosion of wind turbine blades. Renew. Sustain. Energy Rev. 2020, 137, 110611. [Google Scholar] [CrossRef]
- Nagae, S.; Nakamae, K. Characterization of glass fiber/nylon-6 interface by laser Raman spectroscopy. Int. J. Adhes. Adhes. 2002, 22, 139–142. [Google Scholar] [CrossRef]
- Alosaimi, F.K.; Tùng, T.T.; Dao, V.; Huyen, N.K.; Nine, J.; Hassan, K.; Ma, J.; Lošić, D. Graphene-based multifunctional surface and structure gradients engineered by atmospheric plasma. Appl. Mater. Today 2022, 27, 101486. [Google Scholar] [CrossRef]
- Wróblewska, A.; Dużyńska, A.; Judek, J.; Stobiński, L.; Żerańska-Chudek, K.; Gertych, A.P.; Zdrojek, M. Statistical analysis of the reduction process of graphene oxide probed by Raman spectroscopy mapping. J. Phys. Condens. Matter 2017, 29, 475201. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M.H. Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste. Ceramics 2020, 3, 384–420. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, P.; Cao, Y.; Yu, P.; Chen, M.; Zhou, X. Evaluation of fiber surface modification via air plasma on the interfacial behavior of glass fiber reinforced laminated veneer lumber composites. Constr. Build. Mater. 2019, 233, 117315. [Google Scholar] [CrossRef]
- Arsenin, A.V.; Volkov, V.S.; Novikov, S.M. Comparison of CVD-grown and exfoliated graphene for biosensing applications. AIP Conf. Proc. 2021, 2359, 020035. [Google Scholar] [CrossRef]
- Kumar, S.; Goswami, M.; Singh, N.; Siddiqui, H.; Sathish, N.; Ashiq, M.; Kumar, S. Temperature-controlled exfoliation of graphite oxide: Studies of defects and transport properties of graphene oxide. Mater. Res. Express 2022, 9, 054001. [Google Scholar] [CrossRef]
- Huang, W.; Ptasińska, S. Functionalization of graphene by atmospheric pressure plasma jet in air or H2O2 environments. Appl. Surf. Sci. 2016, 367, 160–166. [Google Scholar] [CrossRef]
- Obaid, A.N.; Al-Bermany, E. Impact of graphene nanosheets on adhesion and corrosion performance of reinforced polyurethane coating for aerospace aluminium alloy 2024-T3. Int. J. Adhes. Adhes. 2024, 132, 103695. [Google Scholar] [CrossRef]
- Owji, E.; Ostovari, F.; Keshavarz, A. Electro-thermal properties and characterization of the flexible polyurethane-graphene nanocomposite films. Phys. Scr. 2022, 97, 105704. [Google Scholar] [CrossRef]
- Bagheri, F.; Saudi, A.; Bidram, E.; Asefnejad, A.; Sanati, A.; Zarrabi, A.; Rafienia, M. Fabrication of 3D porous polyurethane-graphene oxide scaffolds by a sequential two-step processing for non-load bearing bone defects. Phys. Scr. 2024, 99, 045023. [Google Scholar] [CrossRef]
- Kumar, S.H.B.V.; Muydinov, R.; Szyszka, B. Plasma Assisted Reduction of Graphene Oxide Films. Nanomaterials 2021, 11, 382. [Google Scholar] [CrossRef]
- Ott, A.K.; Ferrari, A.C. Raman spectroscopy of graphene and related materials. In Elsevier Ebooks; Elsevier: Amsterdam, The Netherlands, 2023; pp. 233–247. [Google Scholar] [CrossRef]
- Sharma, M.; Rani, S.; Pathak, D.K.; Bhatia, R.; Kumar, R.; Sameera, I. Temperature-dependent Raman modes of reduced graphene oxide: Effect of anharmonicity, crystallite size, and defects. Carbon 2021, 184, 437–444. [Google Scholar] [CrossRef]
- Alam, K.; Jo, Y.Y.; Park, C.; Cho, H. Synthesis of Graphene Oxide Using Atmospheric Plasma for Prospective Biological Applications. Int. J. Nanomed. 2020, 15, 5813–5824. [Google Scholar] [CrossRef]
- Jafari, A.; Mortaheb, H.R.; Gallucci, F. Plasma treatment for enhanced functionalization of graphene nanosheets by octadecyl amine. Chem. Eng. Res. Des. 2022, 187, 251–260. [Google Scholar] [CrossRef]
- El-Hossary, F.M.; Ghitas, A.; El-Rahman, A.M.A.; Shahat, M.A.; Fawey, M.H. The effective reduction of graphene oxide films using RF oxygen plasma treatment. Vacuum 2021, 188, 110158. [Google Scholar] [CrossRef]
- Yakin, F.S.M.; Abdullah, M.F.; Badaruddin, S.A.M.; Syono, M.I.; Soriadi, N. Surface modification and properties modulation of rGO film by short duration H2 and NH3 plasma treatment. Mater. Today Proc. 2021, 42, 2996–3001. [Google Scholar] [CrossRef]
- Ramamoorthy, H.; Buapan, K.; Chiawchan, T.; Thamkrongart, K.; Somphonsane, R. Exploration of the temperature-dependent correlations present in the structural, morphological, and electrical properties of thermally reduced free-standing graphene oxide papers. J. Mater. Sci. 2021, 56, 15134–15150. [Google Scholar] [CrossRef]
- Chadha, N.; Sharma, R.; Saini, P. A new insight into the structural modulation of graphene oxide upon chemical reduction is probed by Raman spectroscopy and X-ray diffraction. Carbon Lett. 2021, 31, 1125–1131. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, L.; Meng, Q.; Wang, W.; Li, Z.; Chang, M.; Liu, M.; Jin, Z.; Zhao, K. Polyurethane foam-derived nitrogen-enriched porous carbon/reduced graphene oxide composite with sandwich-like nanoarchitectures for supercapacitors. J. Mater. Science. Mater. Electron. 2018, 29, 9942–9953. [Google Scholar] [CrossRef]
- Sabet, M.; Soleimani, H.; Mohammadian, E.; Hosseini, S. The effect of graphene oxide on polyurethane’s mechanical, thermal characteristics and flame retardancy. Plast. Rubber Compos. 2020, 50, 61–70. [Google Scholar] [CrossRef]
- Sharma, R.; Chadha, N.; Saini, P. Determination of defect density, crystallite size, and a number of graphene layers in graphene analogs using X-ray diffraction and Raman spectroscopy. Indian J. Pure Appl. Phys. 2017, 55, 625–629. [Google Scholar]
- Ponnamma, D.; Sadasivuni, K.K.; Strankowski, M.; Moldenaers, P.; Thomas, S.; Grohens, Y. Interrelated shape memory and Payne effect in polyurethane/graphene oxide nanocomposites. RSC Adv. 2013, 3, 16068. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Kim, H.; Kim, G. Various defects in graphene: A review. RSC Adv. 2022, 12, 21520–21547. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, J.; Singh, R.C. Tailor-made exfoliated reduced graphene oxide nanosheets based on an oxidative-exfoliation approach. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, H.; Eslami-Farsani, R. An experimental investigation on the sulfur and nitrogen co-doping and oxidation of prepared graphene by electrochemical exfoliation of pencil graphite rods. Ceram. Int. 2020, 46, 28860–28869. [Google Scholar] [CrossRef]
- Colom, X.; Ávila, F.J.C.; Arias, M.J.L.; Sanjuan, G.; Galí, I.G. Análisis estructural de Óxidos de Grafeno (GO) y Óxidos de Grafeno reducidos (rGO). Afinidad 2020, 77, 167–174. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=7762528 (accessed on 17 May 2024).




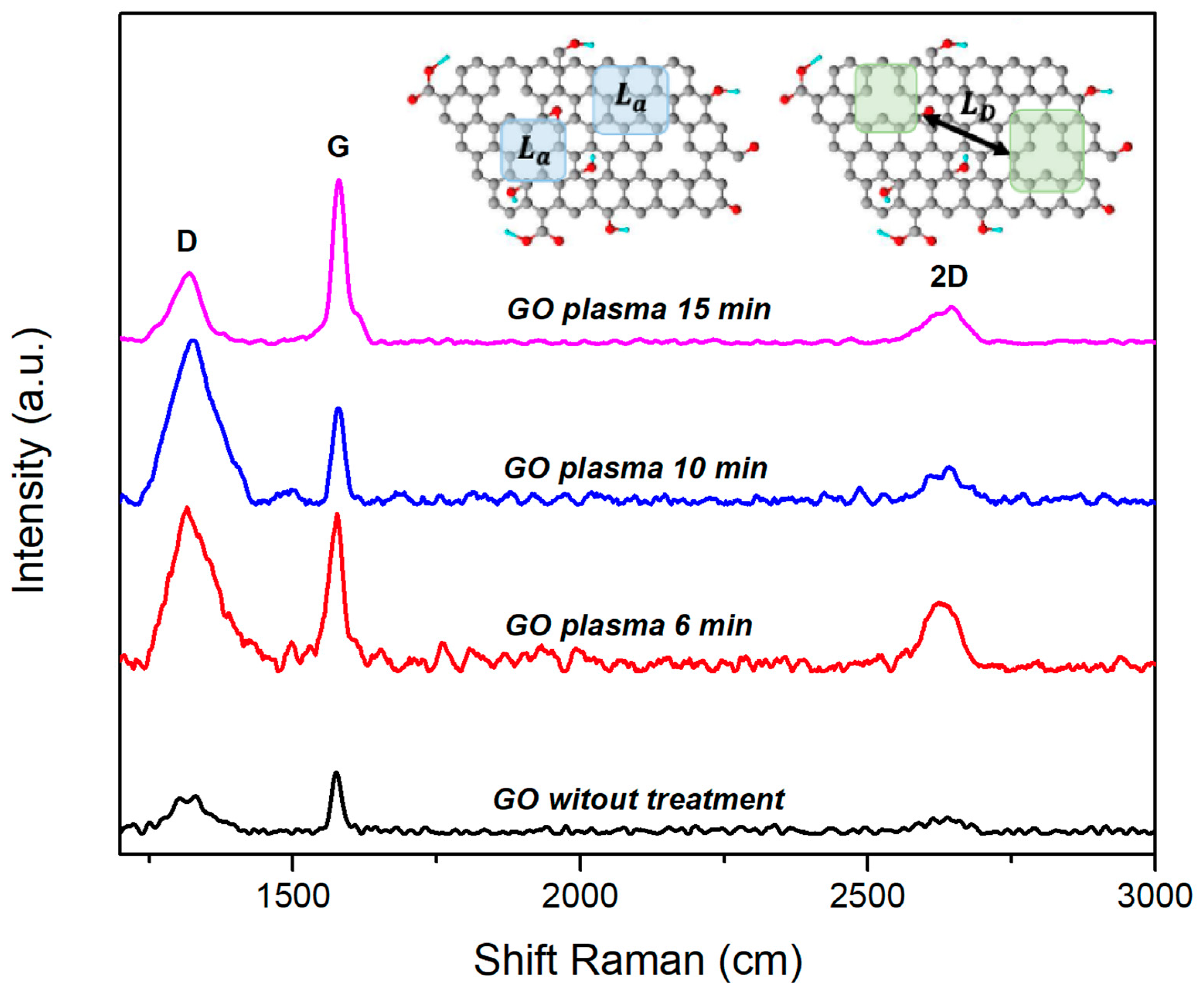



| Sample | |||||
|---|---|---|---|---|---|
| GO without treatment | 0.39 | 0.70 | 0.14 | 0.55 | 0.20 |
| GO plasma 6 min | 1.72 | 1.66 | 0.75 | 1.04 | 0.45 |
| GO plasma 10 min | 1.85 | 1.13 | 0.34 | 1.63 | 0.30 |
| GO plasma 15 min | 0.79 | 1.90 | 0.39 | 0.42 | 0.21 |
| Sample | La [nm] | Ld [nm2] | nd [nm−2] |
|---|---|---|---|
| GO without treatment | 166 | 40 | 3.33 × 1010 |
| GO plasma 6 min | 88 | 29 | 6.30 × 1010 |
| GO plasma 10 min | 56 | 23 | 9.87 × 1010 |
| GO plasma 15 min | 217 | 46 | 2.54 × 1010 |
| Sample | 2θ [°] | d [nm] | D [nm] |
Average [D] |
|---|---|---|---|---|
| PU/0.2GO_0 min | 26.40 | 0.339 | 16.86 | 8.67 |
| 16.77 | 0.53 | 0.499 | ||
| PU/0.2GO_6 min | 26.41 | 0.338 | 17.11 | 8.81 |
| 17.36 | 0.512 | 0.512 | ||
| PU/0.2GO_10 min | 26.40 | 0.339 | 20.44 | 10.50 |
| 16.99 | 1.638 | 0.565 | ||
| PU/0.2GO_15 min | 26.35 | 0.339 | 17.11 | 8.80 |
| 17.20 | 0.517 | 0.497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xosocotla, O.; Campillo, B.; Martínez, H.; Rodríguez-Rojas, M.d.P.; Campos, R.; Bustos-Terrones, V. Modification of Polyurethane/Graphene Oxide with Dielectric Barrier Plasma Treatment for Proper Coating Adhesion on Fiberglass. Coatings 2025, 15, 411. https://doi.org/10.3390/coatings15040411
Xosocotla O, Campillo B, Martínez H, Rodríguez-Rojas MdP, Campos R, Bustos-Terrones V. Modification of Polyurethane/Graphene Oxide with Dielectric Barrier Plasma Treatment for Proper Coating Adhesion on Fiberglass. Coatings. 2025; 15(4):411. https://doi.org/10.3390/coatings15040411
Chicago/Turabian StyleXosocotla, Oscar, Bernardo Campillo, Horacio Martínez, María del Pilar Rodríguez-Rojas, Rafael Campos, and Victoria Bustos-Terrones. 2025. "Modification of Polyurethane/Graphene Oxide with Dielectric Barrier Plasma Treatment for Proper Coating Adhesion on Fiberglass" Coatings 15, no. 4: 411. https://doi.org/10.3390/coatings15040411
APA StyleXosocotla, O., Campillo, B., Martínez, H., Rodríguez-Rojas, M. d. P., Campos, R., & Bustos-Terrones, V. (2025). Modification of Polyurethane/Graphene Oxide with Dielectric Barrier Plasma Treatment for Proper Coating Adhesion on Fiberglass. Coatings, 15(4), 411. https://doi.org/10.3390/coatings15040411









