1. Introduction
One of the main reasons for the reduction in the service life of metal structures in construction is the low adhesion of protective coatings to metal and weak cohesive interaction in coatings, which prevents the formation of the required level of performance characteristics for reliable protection of metal structures.
Sudden changes in climate and weather conditions have a certain impact on the service life of metal structures and their protective coatings. Some regions of countries are characterized by a wide range of temperatures from −40 °C to +50 °C, sudden temperature changes, intense solar radiation, frequent transitions through 0 °C, various types of precipitation, and increased moisture and gas content in the air, which in turn leads to rapid destruction of protective paint coatings.
Various parameters characterizing the protective properties of water-dispersion paint and varnish compositions (vapor permeability, moisture absorption, resistance to atmospheric factors and aggressive environments) depend on the quality of dispersion of pigments and fillers [
1,
2,
3]. The process of penetration of various substances through the coating consists of sorption (dissolution), diffusion, and desorption from the back side of the film. When polymers are filled, certain factors unrelated to interfacial interactions contribute to their insulating properties [
4]. Based on model concepts of the penetration of gases, vapors, and liquids through environments containing impermeable inclusions, a number of mathematical expressions have been obtained and tested in practice that relate the barrier and diffusion characteristics of filled polymers to the volume fraction, shape, and packing density of dispersed phase particles [
5].
The protective effect of coatings may vary with changes in dispersion; in most cases, an increase in dispersion contributes to an enhancement of the protective performance of paint coatings. Adhesive strength plays a significant role in the protective and decorative function of coatings [
6,
7,
8,
9,
10,
11,
12]. Coatings made of monomeric or oligomeric film-forming agents, which are converted into a polymer (three-dimensional) state directly on the substrate, have the highest adhesive strength.
An increase in the polarity of the comonomer ensures an increase in the adhesive properties of the films. Thus, when 4.3% methacrylic acid is added to the acrylic copolymer, the adhesion to glass increases from 8.2 MPa to 12 MPa [
13], and an increase in the content of methacrylic acid first leads to increased adhesion, and then the coating begins to crack and peel off.
The incorporation of various unsaturated esters of silane polymers into acrylic polymers through copolymerization ensures a significant increase in the adhesive strength of coatings [
14,
15,
16].
An aqueous dispersion of an acrylic polymer copolymerized with a phosphate emulsifier is known, which provides the high protective ability of coatings [
17]. The properties of coatings based on copolymers of methyl methacrylate, butyl acrylate, and methacrylic acid with the addition of dimethylpolysiloxane, copolymers of methyl methacrylate, butyl acrylate, and pyrrolidone with the addition of dimethylpolysiloxane, and a mixture of the two types of polymers were studied. An improvement in the quality of coatings (tensile strength, heat resistance, water resistance, surface structure) based on a mixture of polymers has been shown [
18].
Among water-based paints and varnishes, the most common materials are those based on dispersions of acrylic copolymers (individual acrylates), acrylic copolymers (styrene acrylates), as well as homo- and copolymers of vinyl acetate (with ethylene, ethylene vinyl chloride, esters of acrylic or methacrylic acid) [
19].
In [
20,
21], it was shown that the introduction of 5%–7% methacrylic acid, methyl methacrylamide, and other hydrophilic monomers into alkyl acrylate copolymers significantly improves the frost resistance of the polymers, their resistance to repeated freezing and thawing, mechanical stress, and the incorporation of electrolytes.
Clumping of particles during film formation occurs with greater dehydration of the system and, consequently, with a denser packing of particles in the film-forming gel [
22,
23,
24]. The improvement of the water resistance of films formed from a copolymer containing methacrylamide is associated with the fibrillar orientation of the polymer in the film, which leads to the greatest realization of the interaction between the polar groups of the polymer [
25,
26,
27,
28,
29].
Hollow glass microspheres are widely used as fillers for various functional materials and coatings [
30,
31], which is due to their unique combination of physical properties: spherical shape, thin walls, low density, relatively high all-round compressive strength, and good thermal and acoustic insulation.
The production of acrylic copolymers by emulsifier-free emulsion polymerization is a more promising approach. The method of emulsifier-free emulsion polymerization (EFEP) makes it possible to obtain more durable coatings in comparison with coatings obtained by traditional emulsion polymerization, which is explained by the absence of an adsorbed layer of surface-active substances (SASs) on the surface of the dispersed phase particles, preventing full interaction between the dispersion particles during film formation [
32].
Thus, water-dispersion paint and varnish materials based on acrylic dispersion obtained by emulsifier-free emulsion polymerization, and finely dispersed fillers such as diatomite and hollow glass microspheres, remain insufficiently studied and are of current relevance.
It is known that one way to improve the properties of PCMs is to modify them with various fillers, in particular silicate fillers [
33]. One of the most widely used silicate fillers for modifying the structure of polymer materials is kaolin, and one of the most promising is wollastonite [
34].
The use of kaolin, in addition to its inertness to chemical reagents and low cost, is also due to its wide range of modification and activation possibilities [
35], which can further contribute to strengthening the structure and improving the properties of PCMs, as well as the manifestation of additive and synergistic effects [
36]. The filling of polymer materials with wollastonite is of interest due to the needle-like structure of the mineral, which allows for an increase in the strength characteristics of materials [
37].
Non-emulsifier emulsion copolymerization, unlike traditional copolymerization, is a promising industrial method for producing acrylate polymers, as it solves the problem of removing residual emulsifiers from polymers and prevents them from entering wastewater [
38].
We have previously demonstrated the possibility of obtaining colloidally stable latexes based on n-butyl acrylate (BA) and polar comonomers without using chemically saturated emulsifiers with a high degree of conversion in the synthesis formula [
39].
The use of diatomite as a filler in construction technology is diverse. Diatomite is used in the synthesis of liquid glass, in dry construction mixtures, and as an anti-caking additive in the production of paint and varnish films [
6,
7].
The increase in the consumption of high-quality water-based paint and varnish materials has led to stricter requirements for the decorative, physical, mechanical, and protective properties of coatings. Most manufacturers try to solve the problem of product quality by replacing equipment, which does not bring the expected effect but only increases production costs. The leap in the quality of coatings formed on the basis of water-dispersion film formers was also a consequence of developments in the field of polymer modification, which led to the creation of a new generation of film formers, stable low-molecular emulsifiers hydrolysis, forming coatings with high physical–mechanical, adhesive, and insulating properties at moderate temperatures.
The task of creating water-dispersion paint and varnish compositions with protective and decorative properties is quite complex, since water-dispersion film-forming systems have a number of features that make their use difficult. They have relatively low film-forming ability, which leads to defects in the coating structure and low insulating ability. The presence of a relatively large amount of hydrophilic additives in the coating—dispersants, thickeners, etc., which are necessary for the manufacture of paints—results in the low water and corrosion resistance of the coatings and their low adhesive strength, especially when wet.
The introduction of various modifiers into the formulation of water-dispersion paints makes it possible to improve the technological (storage stability, defect-free application) and operational (adhesive strength, light, heat, water, and abrasion resistance, scratch resistance) properties of coatings, intensify technological processes, reduce raw material consumption, shorten the duration of the technological process, reduce energy and labor consumption per unit of paint and varnish material produced, and increase the environmental friendliness of paints used in industry and construction, promoting the intensive replacement of traditional organic solvent-based paints and varnishes with these materials [
40,
41,
42].
Thus, the analysis of current trends in paint and coating materials indicates insufficient research into the use of mineral components such as diatomite as a fine filler in combination with hollow glass microspheres. At the same time, it highlights the potential for developing water-dispersion acrylic compositions based on modern technological solutions aimed at improving the long-term protective properties of coatings through the modification of aqueous polymer dispersions with multifunctional surfactants.
In this regard, the aim of the present research is to develop a scientifically grounded formulation and technological solution for producing a modified water-dispersion composition based on synthesized polymer dispersions obtained via surfactant-free copolymerization and fine fillers composed of diatomite and hollow glass microspheres, with the goal of enhancing protective and performance characteristics.
The scientific novelty of the study lies in the synthesized polymer dispersions and the developed formulations of water-dispersion coating compositions based on them, incorporating fine fillers (modified diatomite, hollow glass microspheres, and titanium dioxide). These compositions are recommended for use in various construction applications, including architectural and industrial coatings for wood, metal, and mineral surfaces.
2. Materials and Methods
2.1. Materials
In the course of the research, analytical work was carried out to study the properties of acrylic copolymers and monomers included in their composition. Acrylic acid, methyl methacrylate, acrylic acid nitrile, methyl methacrylate, butyl acrylate, and styrene were selected as the main monomers for the synthesis. Acrylic acid and acrylic acid nitrile act as stabilizers. The main supplier of chemical products for synthesis is “MMA GROUP” LLP, located in Almaty, Kazakhstan.
Butyl acrylate is the main monomer providing the elasticity of the copolymer. Methyl methacrylate and styrene stiffen the copolymer. Ammonium persulfate was used as the initiator, and tertbutyl hydroperoxide was introduced to increase the degree of conversion. Syntheses with various formulations were carried out, which are presented in
Table 1.
During the experimental studies, the amount of synthesized acrylate dispersion (dispersions A, B, and C) and pigment (titanium dioxide) varied, with lime selected as the base filler. A “composition-property” experimental design was used (
Table 2). The factors studied were the quantitative contents: x1—synthesized acrylate dispersion; x2—titanium dioxide (TiO
2), as well as the proportion of fillers in the mixture: lime (v1), diatomite (v2), and hollow microspheres (v3), where v1 + v2 + v3 = 1 and 0 ≤ vi ≤ 1. The content of other components of the mixture was assumed to be constant for all compositions. The levels of variation in the variable factors and the experimental design are shown in
Table 2 and
Table 3.
The domestic VD-AK-111 (GOST 28196-89) [
43] was taken as the reference for water-dispersion paints during comparative tests. This paint is intended for exterior and interior painting of buildings and structures on brick, concrete, plastered, and other porous surfaces. Coatings based on VD-AK-111 water-dispersion paint retain their protective properties for at least 5 years in a temperate climate.
The experimental research plan is presented in
Table 4.
Water-dispersion paint VD-AK-111 was considered as a prototype.
Table 5 shows the standardized indicators of the main characteristics of water-dispersion compositions.
Fillers:
Hollow glass microspheres—Micro-powder is obtained by grinding and sieving synthesized glass. The fundamental point of these methods is the creation of conditions for the preliminary dissolution of a certain amount of gases during the manufacture of intermediates and the release of gas during thermal dissociation. Elevated heating temperatures cause the glass powder to soften and release gases, which in turn promotes the formation of bubbles within the particles. This process leads to the development of hollow, spherical structures known as microspheres. Rapid cooling is then essential to stabilize and preserve the size and shape of these formed microspheres.
The chemical resistance of glass microspheres makes them compatible with most polymers.
Their characteristic diameters depend on specific applications and usually range from 10 to 200 microns, with a shell wall thickness of 0.5–2.0 µm [
38].
Diatomite—In its natural state, diatomites have a large evenly distributed porosity, reaching 80%–85%. Silica in diatomites is in an amorphous state and amounts to 78%–95%.
The modification of the diatomaceous filler was carried out by the thermal method and it was found that at a temperature of 650 °C it was the most effective. At a firing temperature of 650 °C, diatomite acquires a light shade, which allows it to be used as a filler in water-dispersion paints. From the point of view of energy consumption, heat treatment of diatomaceous filler at T = 650 °C is more economical.
Hydrated lime (slaked lime)—Slaked lime is a finely dispersed white powder obtained after ground lime is slaked with steam. It has a particle size of less than 0.2 mm, a hydrated water content of less than 1.5%, and is classified as grade 1–2.
TiO2 Pigment—Rutile Titanium dioxide R906 from Chemistry and Technology LLP, Almaty, Kazakhstan obtained by the chloride method was chosen as the pigment. Rutile titanium dioxide is characterized by increased whiteness due to surface treatment with aluminum oxides, silicon oxides, and zirconium compounds, as well as high resistance to photo-oxidative degradation. The higher refractive index provides the rutile modification pigment with greater opacity, which is its main advantage over anatase.
2.2. Methods
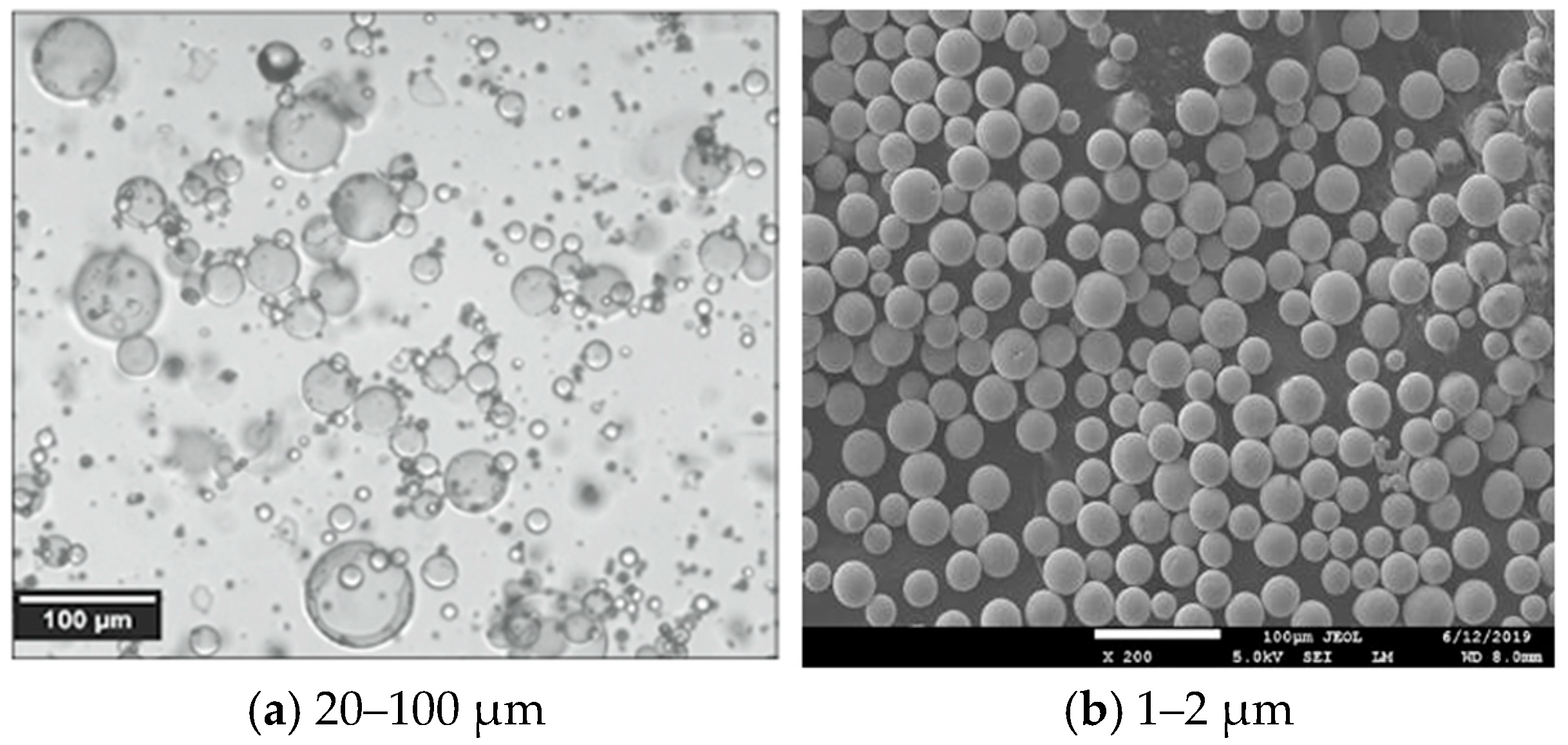
Hollow microspheres were separated from defective particles by flotation in water. Microspheres with sizes of 20–100 µm, an average diameter of 75 µm, and a wall thickness of 1–2 µm were obtained (
Figure 1). The bulk density of the dried microspheres was 0.107–0.252 g/cm
3, and the thermal conductivity coefficient was 0.05–0.07 W/(m–K). Hollow glass microspheres serve as a highly opaque and reflective pigment, making them suitable for use in the production of water-dispersion paint formulations.
The granulometric composition of the fine filler (diatomite) (
Figure 2) was determined using a Shimadzu SALD–3101 fine media particle size analyzer (Shimadzu Corporation, Kyoto, Japan) with a measurement range from 50 nm to 3 mm.
The morphologies and elemental distribution are provided by scanning electron microscopy (SEM, JSM–7800F, Tokyo, Japan), and transmission electron microscopy (TEM, JEOL2100F, Tokyo, Japan).
The effect of UV radiation duration on the coating properties of modified water-dispersion emulsions based on synthesized acrylic dispersions was investigated. The paint was applied evenly to steel DC01 (EN 10130) [
44]. Steel grade DC01 is a high-quality structural carbon steel. The chemical composition of the steel is presented in
Table 6.
The curing was carried out by irradiating the coating surface with UV radiation. The UV radiation source was an excimer lamp based on a krypton bromine (KrBr) gas mixture, which produces ultraviolet radiation with a wavelength of 207 nm. The coatings were confirmed within 15, 45, and 60 min.
The corrosion resistance of water-dispersion coatings was investigated by accelerated testing by obtaining polarographic curves in a saturated sodium chloride solution. The curves were recorded using a PU–1 polarograph coupled to a Graphite–2 interface unit at a scan rate of 10 mV/s [
39].
The main properties of protective coatings were determined [
45,
46,
47,
48,
49,
50] according to Government Standards: opacity (ST RK 8784–75) [
51], washability (ST RK 52020–2003) [
52], water and moisture absorption (ST RK 21513–76) [
53], cohesive strength (ST RK 27890–88) [
54]. The adhesion of protective coatings was measured on a PSO–5MG4S adhesive meter (50 × 50 mm fungus) and on various substrates: concrete and steel.
The hardness of the coatings was measured on a WILSON VH 3100 (Buehler, Lake Bluff, IL, USA) hardness tester equipped with a Knoop indenter designed to measure the hardness of polymer materials [
55,
56,
57,
58]. The Knoop method is often used to measure the hardness of thin materials, coatings, and brittle samples using micro- and macro-hardness testers. The hardness of coatings is determined by the decay time of the pendulum oscillations on the test surface. Principle of operation: the pendulum oscillates on the sample, and the decay time of the oscillations is compared with the “glass number” (the decay time on a reference glass). A pyramid-shaped diamond tip is used to press into the sample.
Substrate preparation methods
Concrete substrate: the test sample consists of a concrete substrate, a protective coating, and an adhesive tear-off element (disc, mushroom) attached to it. For testing, cube samples measuring 70 × 70 × 70 mm are made from concrete mix; substrates in the form of slabs measuring 100 × 100 mm and at least 50 mm thick are also used. When determining the adhesion of paint coatings, it is recommended to use concrete with a compressive strength class of B30. For 28 days, the concrete samples harden at a temperature of (20 ± 5) °C and relative humidity of (65 ± 5)%. Before applying protective coatings, the surface of concrete samples must be smooth, free of cement milk, dust-free, and comply with category A3 according to Government Standard (GOST 13015) [
59]. The surface of metal discs (mushrooms) intended for gluing must be smooth and free of rust, thermal oxides, oils, etc. A paint coating is applied to the surface of the concrete samples, and after the exposure period, metal discs are glued to the paint coatings of the samples. Excess glue is removed before it hardens. After the glue has hardened, the paint coatings are cut to the base around the perimeter of the metal discs.
Metal substrate (steel): Before applying protective coatings to the surface of steel samples (steel plates measuring 150 × 70 × 2 mm), clean them with a mechanical abrasive tool and solvents. During abrasive cleaning, condensation must be prevented on the treated surface. After cleaning, the metal surface should be dusted, degreased, primed, and painted. If the time interval between cleaning and priming exceeds the specified time, a temporary protective coating should be applied to the surface.
The density of the water-dispersion paint was determined using a method based on determining the mass of the paint placed in a pycnometer of known volume at a certain temperature. The density of the paint was determined as the ratio of the mass of the paint to its volume. The pycnometer was filled with the water-dispersion paint under investigation up to a certain mark, avoiding the formation of air bubbles. The pycnometer with the paint is kept at the temperature at which the measurement is taken, usually room temperature. After the temperature has been established, the pycnometer with the paint is weighed. The following formula is used to calculate the density:
where ρ is the density of the paint, m
k is the mass of the pycnometer with paint, m
p is the mass of the empty pycnometer, V is the volume of the pycnometer.
Methods of mathematical processing of experimental results
The experimental results were processed using Microsoft Excel and Advanced Grapher 2.2 software.
Determination of average values
The arithmetic mean was determined using the AVERAGE function in Microsoft Excel. The mean linear deviation ±Δ was determined using the STANDARD DEVIATION function in Microsoft Excel. The limits were determined as the minimum and maximum values obtained when measuring a given quantity. The number of values obtained N was indicated for each quantity determined.
Coefficient of variation CV
This indicator represents the ratio of the standard deviation s to the arithmetic mean
. The result is expressed as a percentage:
Standard deviation s is a measure of how much the values in a sample deviate from their mean.
It is calculated using Formula (1):
Determining correlation coefficients
To perform correlation analysis, we used the correlation coefficient
R, which characterizes the existence of a linear relationship between two variables
x and
y. The correlation coefficient
R was calculated using Formula (2):
where
n is the number of experiments;
p is the number of input (independent) parameters;
i is the ordinal number of the experiment;
yi is the actual value of the output parameter in the
i-th experiment;
y1 is the calculated value of the output parameter, calculated using a multifactorial mathematical model, for the conditions (values of input parameters) of the
i-th experiment;
ȳ is the average value of the actual value of the output parameter for all n experiments (general average).
3. Results
Theoretical values of the glass transition temperature were calculated for the synthesized polymers and experimental values were obtained, which are presented in
Table 7.
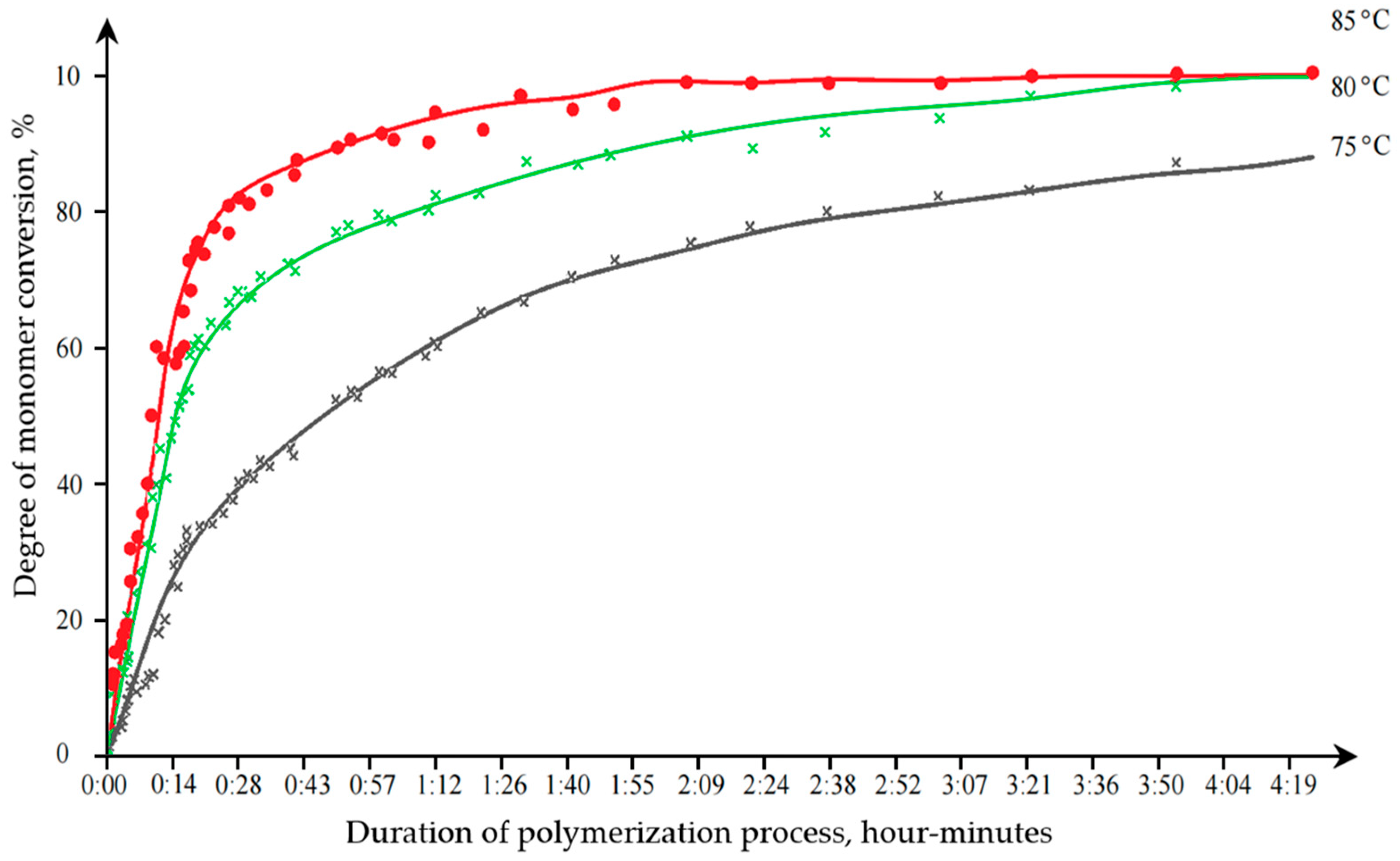
Kinetic studies were conducted to study the effect of synthesis conditions on the degree of monomer conversion and the duration of the emulsion polymerization process. The research results are reflected in
Figure 3.
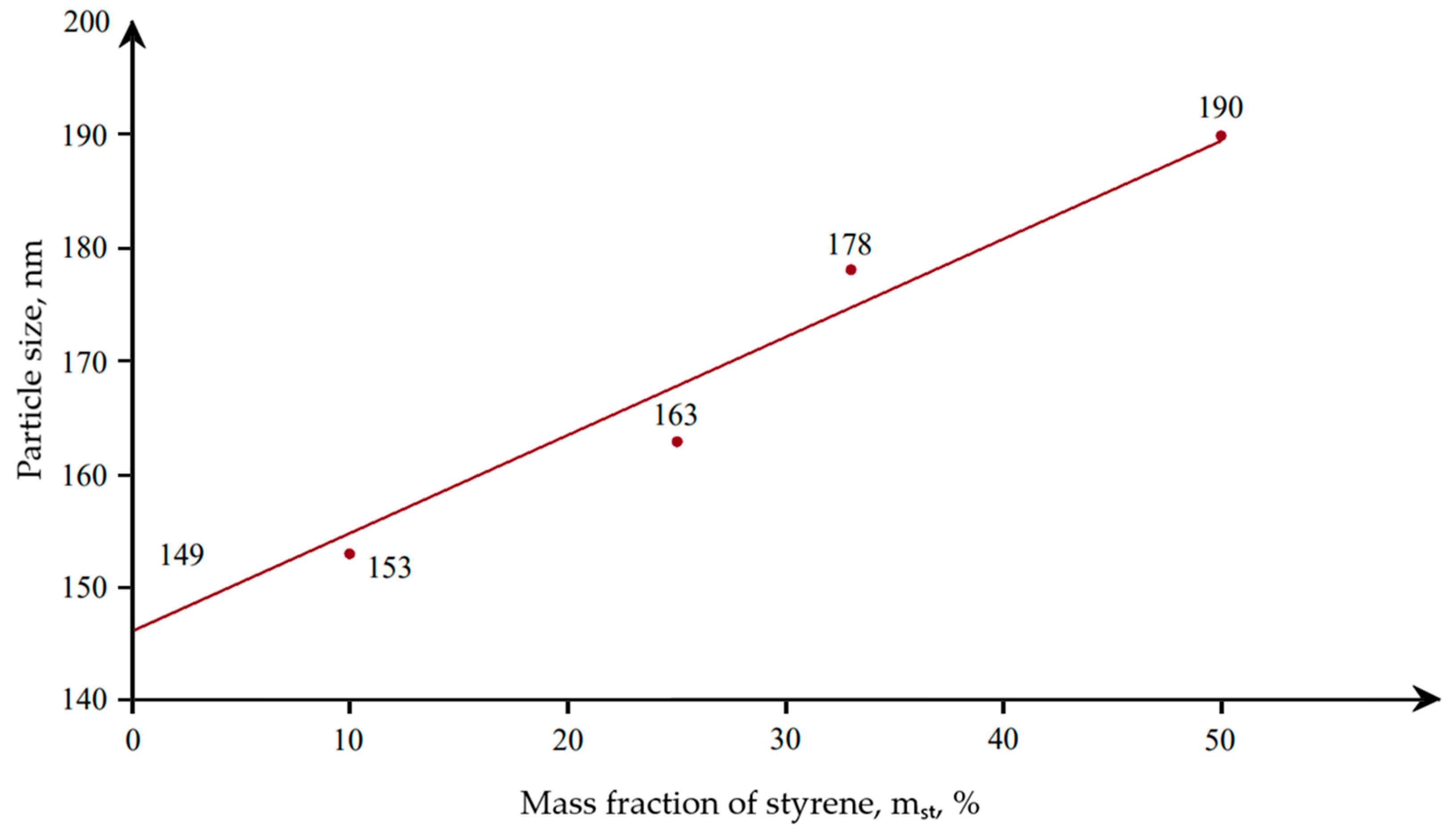
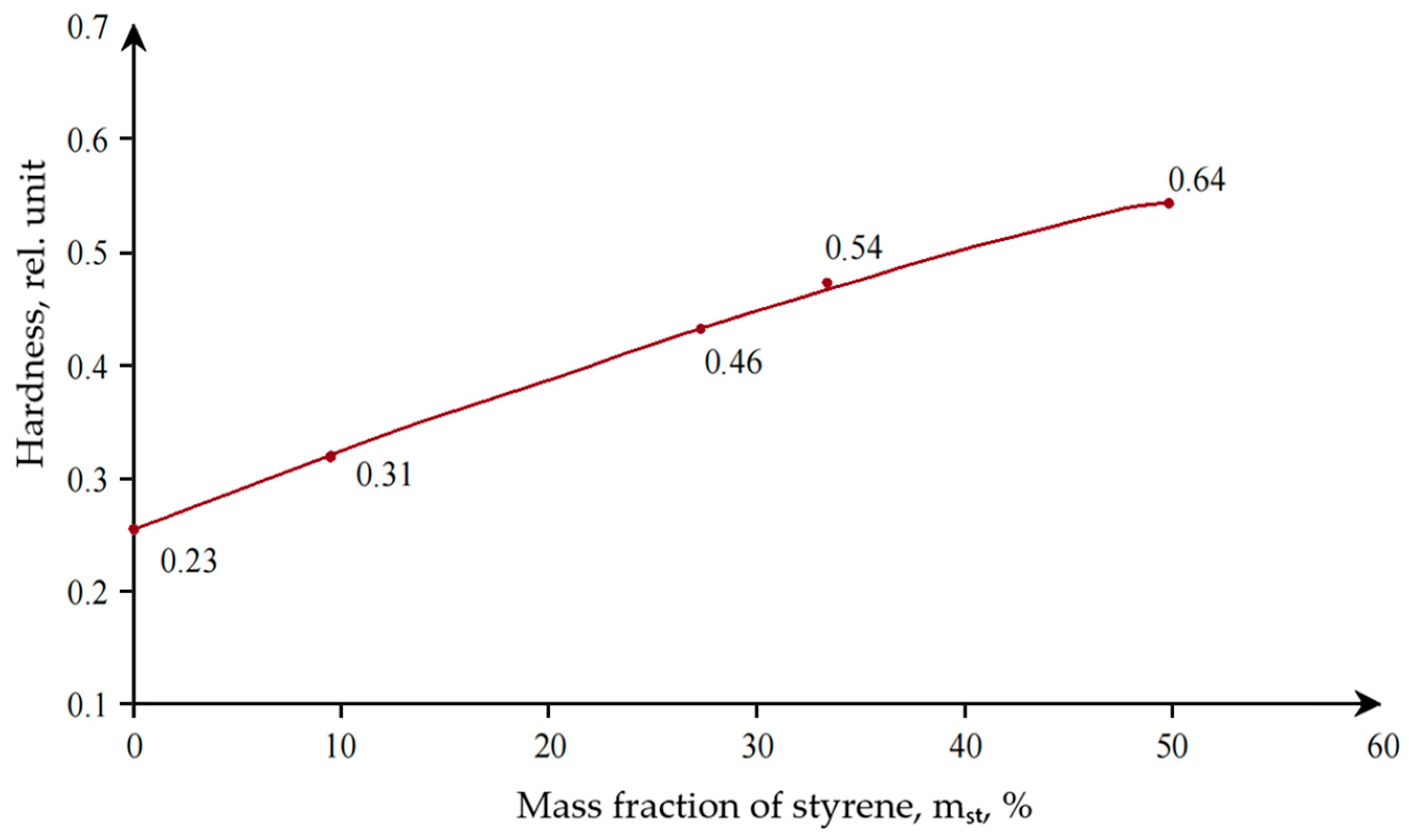
The optimal time for emulsion polymerization was 2 h at a temperature of 85 °C, at which the highest degree of conversion was achieved. In the course of the research, the effect of styrene content on the properties of the obtained dispersions was established (
Figure 4 and
Figure 5).
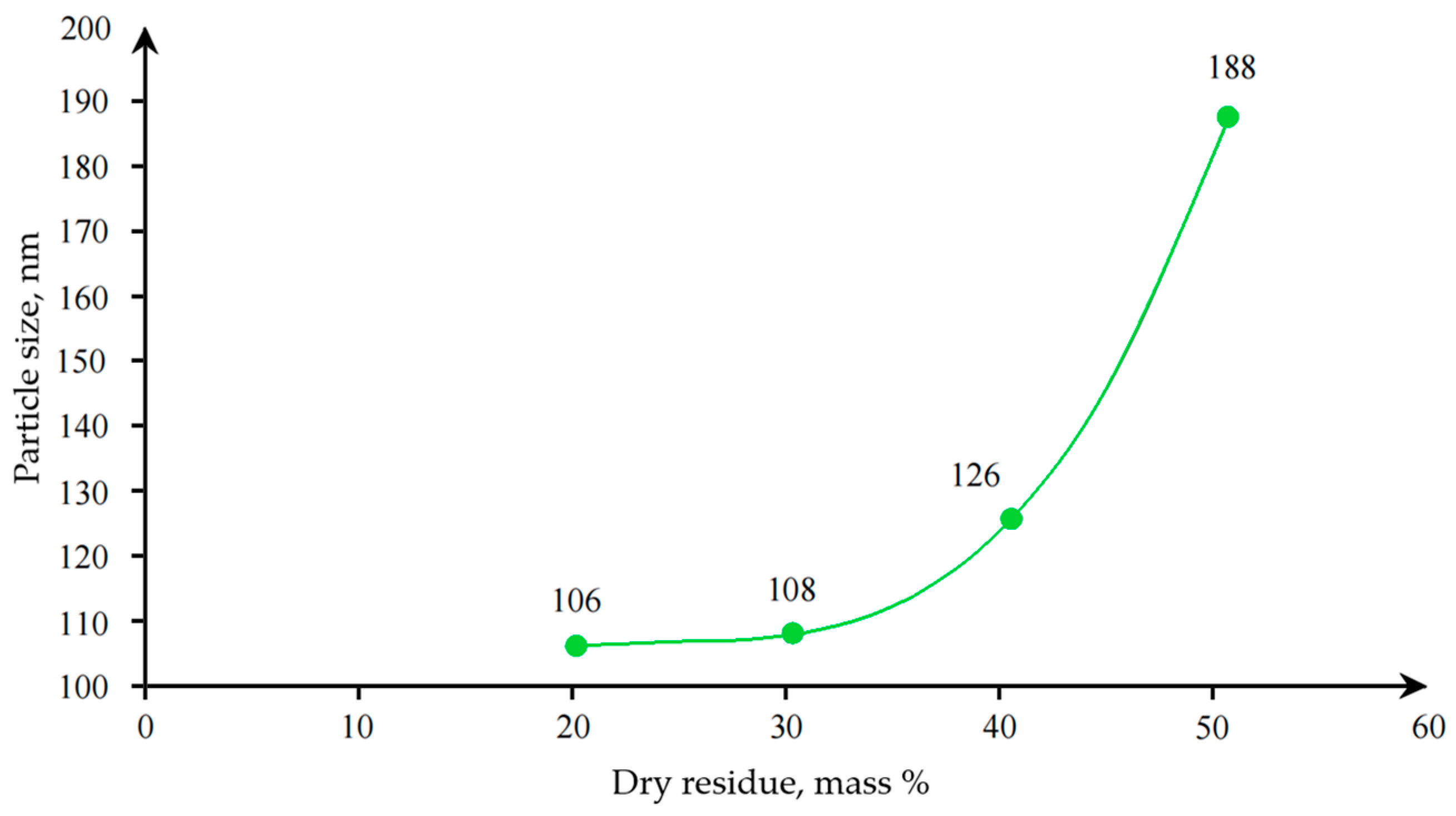
With an increase in the styrene content in the composition of the copolymer, an increase in the particle size of the dispersion was observed, as well as an increase in the hardness of the coating based on it. Upon further investigation, a relationship was obtained between the dry residue and the particle size of polyacrylic latex (
Figure 6).
It can be seen from this graph (
Figure 6) that as the dry residue increases, the particle size increases exponentially. This also leads to a sharp increase in viscosity, which limits the possibility of synthesis at higher solids content, as the stability of the entire system is compromised. In the further research process of the modified water-dispersion emulsion (
Table 8) based on synthesized emulsifier-free acrylic dispersions, the main performance characteristics such as hardness, adhesion resistance, chemical resistance, and gloss level were studied.
The production of water-dispersion paint and varnish material was carried out according to the standard procedure [
55,
56]. Compliance with high-speed operating modes, time intervals, and the sequence of component introduction is strictly mandatory in order to avoid the production of defective paint and varnish products and the destruction of hollow glass microspheres.
The properties of the coatings obtained by replacing titanium dioxide and calcium carbonate with hollow glass microspheres and modified diatomite were determined in accordance with the regulatory requirements for each indicator (
Table 9).
It has been established that when replacing both titanium dioxide and calcium carbonate with PSM, such indicators as adhesion and resistance to static water do not deteriorate.
According to the research results, there is a decrease in the dry residue and density of paint (
Figure 7), which has a positive effect on the consumption of paint and varnish material and its cost. Coatings containing microspheres are more hydrophobic than the corresponding standard paint.
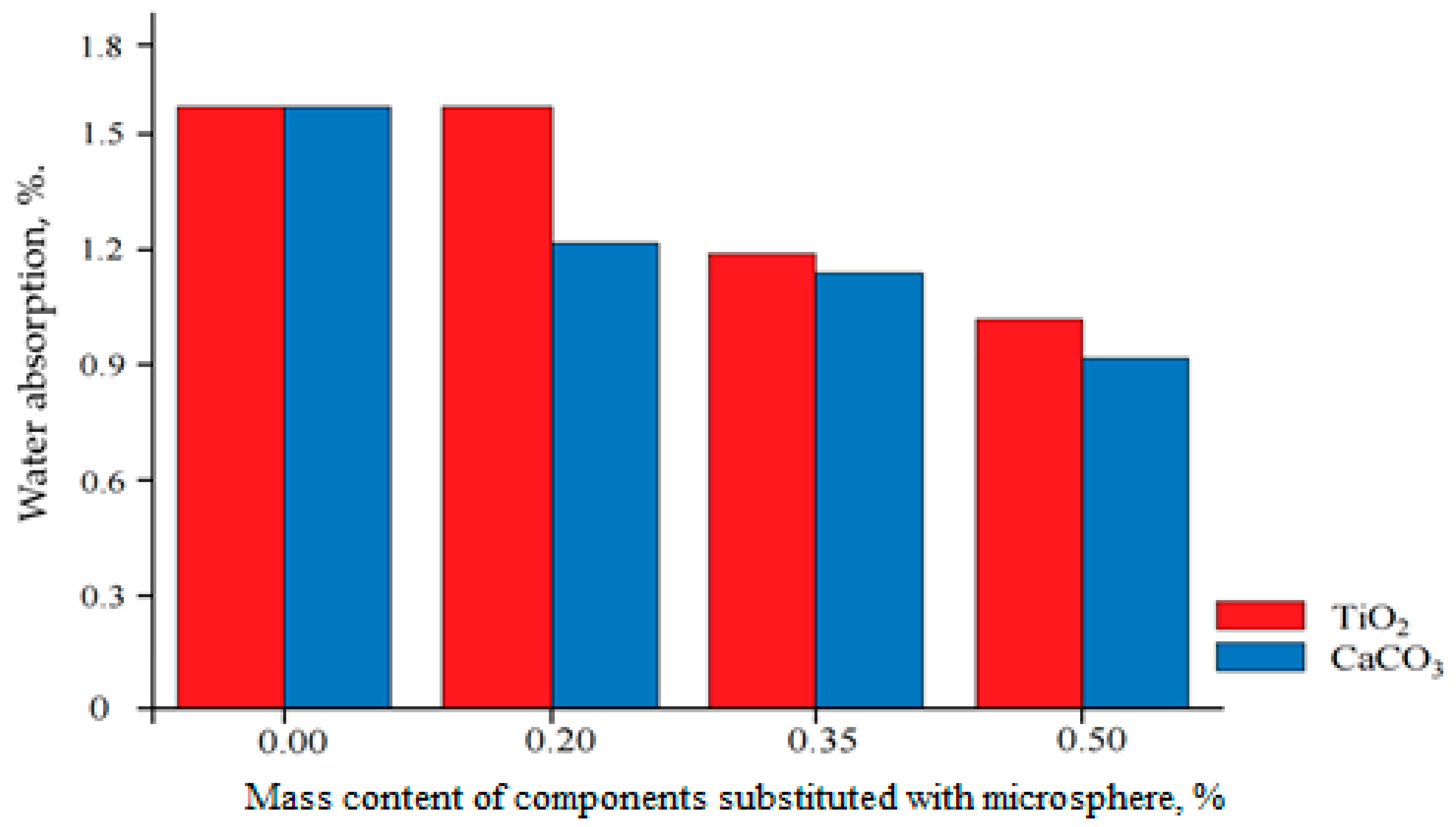
The addition of microspheres makes a positive contribution to reducing moisture absorption (
Figure 8) and thereby slows down the rate of paint degradation. However, the use of hollow glass microspheres does not allow replacing TiO
2 in large quantities.
So, when replacing 3 wt.% TiO2 on microspheres, the opacity of the dried film increases by 35%, and at 6 wt.% TiO2, it increases by 8%. When the mass content of TiO2 water-dispersion paints in the formulation is less than 3 wt.%, the opacity of the paint decreases sharply; however, when replacing CaCO3 with hollow glass microspheres, regardless of the amount, it increases.
Our study also evaluated modified water-dispersion compositions based on synthesized acrylic dispersion in terms of hardness, adhesion resistance, chemical resistance, and gloss level with a standard single-phase acrylic dispersion.
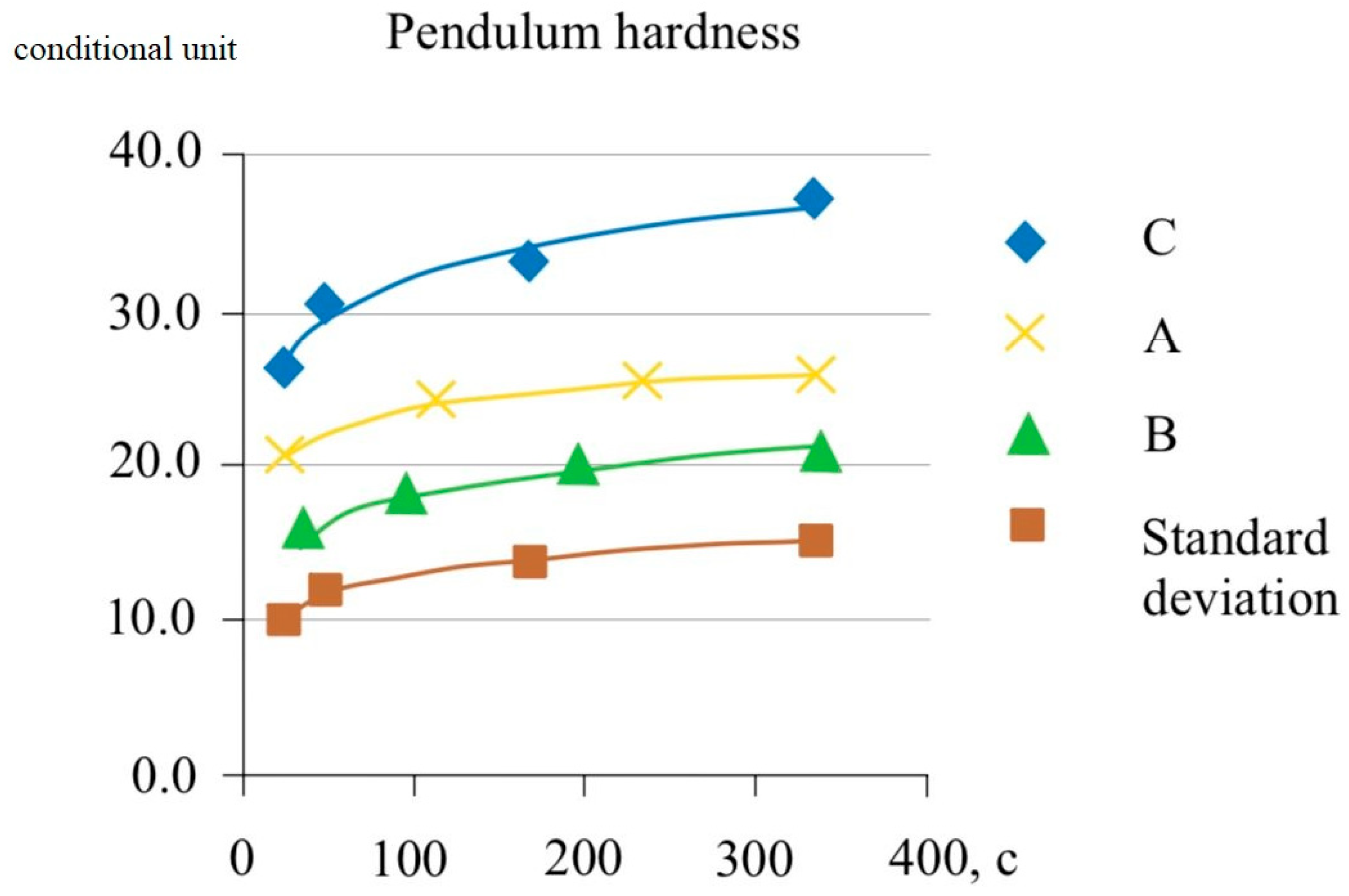
The results of the pendulum hardness measurements are shown in
Figure 9. Paints with a thickness of 100 microns were applied to the glass in a wet layer and stored in air at a temperature of 23 °C at a relative humidity of 50% during the entire test period. Hardness measurements were carried out on a pendulum hardness tester with fluctuations measured at an angle of 3°.
To study the resistance [
60,
61,
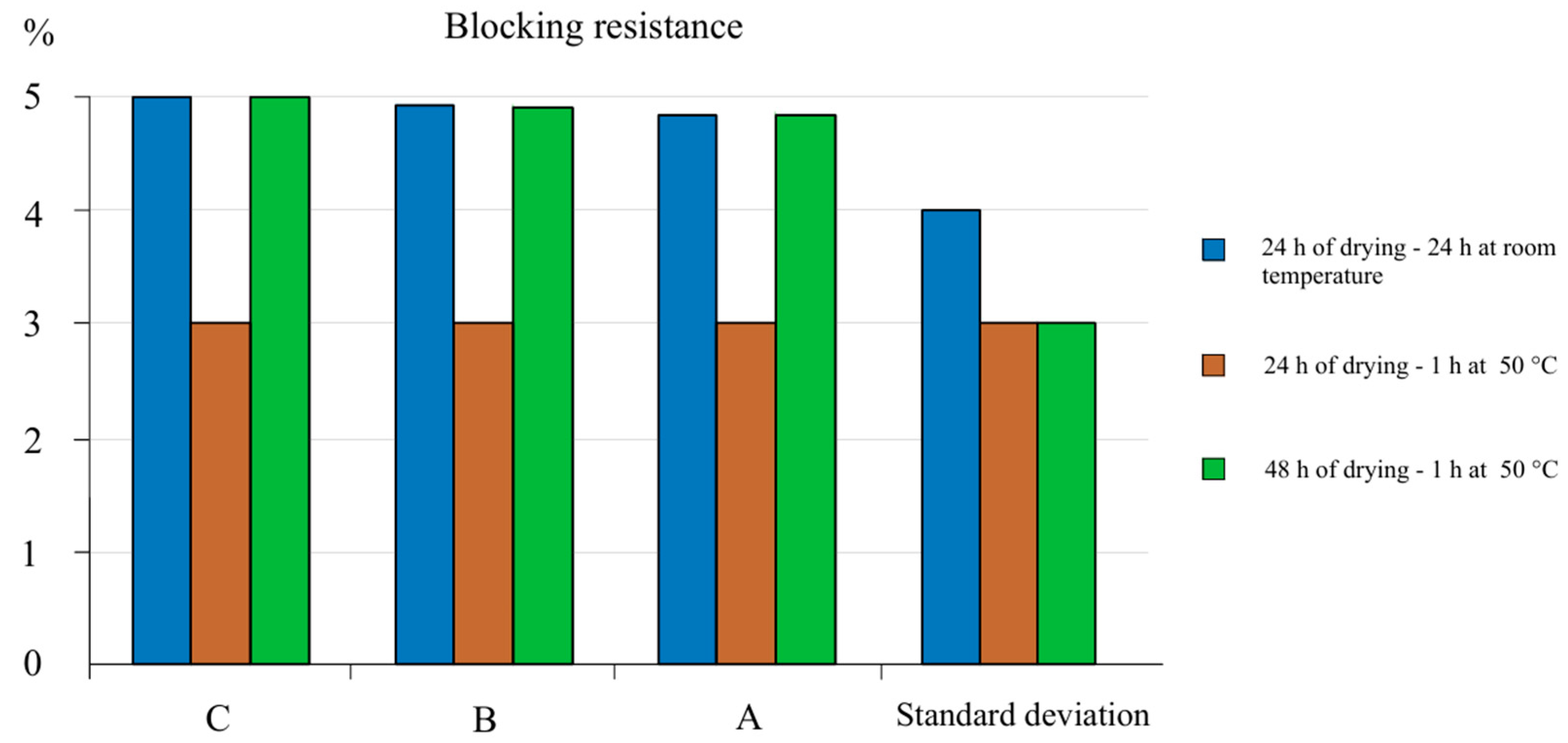
62] to sticking (
Figure 10), paint with a wet layer thickness of 100 μm was applied to a black Lenet card and dried at ambient temperature for 24 h, and then additionally for 48 h. Next, the Lenet cards with paint samples were cut into four squares measuring 2.5 × 4 cm. Two sets of squares were placed face to face with a protective layer on top and a weight of 5 kg on it. One set of samples was left in the air, while the other was placed in an oven at a temperature of 50 °C and kept there for 1 h.
At the end of the test, the samples were removed from the oven and any additional weight was removed from all samples. All samples were then kept at ambient temperature for 30 min. The third set of samples obtained as described above was dried for 48 h and placed in an oven at 50 °C for 1 h. At the end of the test, the samples were removed from the oven and kept at ambient temperature for 30 min. All samples were then torn after 30 min of exposure to air and evaluated on a scale of 1 to 5, where 5 is the best result. A rating of 5 indicates no damage or change in gloss, 4 indicates a change in gloss only, and 3 to 1 indicates the percentage difference in paint removal.
Table 10 shows the decomposition potentials of coatings depending on the curing modes. It was found that the developed coatings, cured under normal conditions, have a potential ranging from −78.50 to −79.0 mV. When irradiating water-dispersion compositions during the curing process for 60 min, the potential shifts to a more electronegative region and ranges from −120.59 to −121.36 mV.
Corrosion tests of paint and varnish coatings were carried out under UV radiation for 120 min during the curing process. It was revealed that the decomposition potential of the studied coatings cured under natural conditions shifts toward a more electropositive region as corrosion time increases, indicating an intensification of corrosion damage. In this case, the anodic dissolution curves of the samples exposed to UV radiation during curing are shifted to the left, toward a less electropositive region, compared to those cured under natural conditions.
Table 11 shows the results of the analysis of dissolution curves during corrosion for 120 min. It has been revealed that the potential of an acrylate paint and varnish coating based on synthesized dispersions shifts to the electropositive region during UV curing and corrosion for 120 min. Thus, the potential of acrylate coatings cured under natural conditions, after corroding for 20 min, ranged from −78.50 to −79.0 mV, and after corroding for 120 min, ranged from 166.70 to 160.55 mV. It has been revealed that the potential of coatings cured by UV irradiation shifts to a less electropositive region. The potential of coatings cured by UV radiation for 60 min after 120 min of corrosion ranges from 108.42 to 107.65 mV.
It can be seen from
Table 9 that the potential of paint coatings cured under UV radiation for 60 min is less electropositive compared to the potential of paint coatings cured under UV radiation for 5 min. A potential shift to a less electropositive region indicates a decrease in the dissolution rate of the metal substrate.
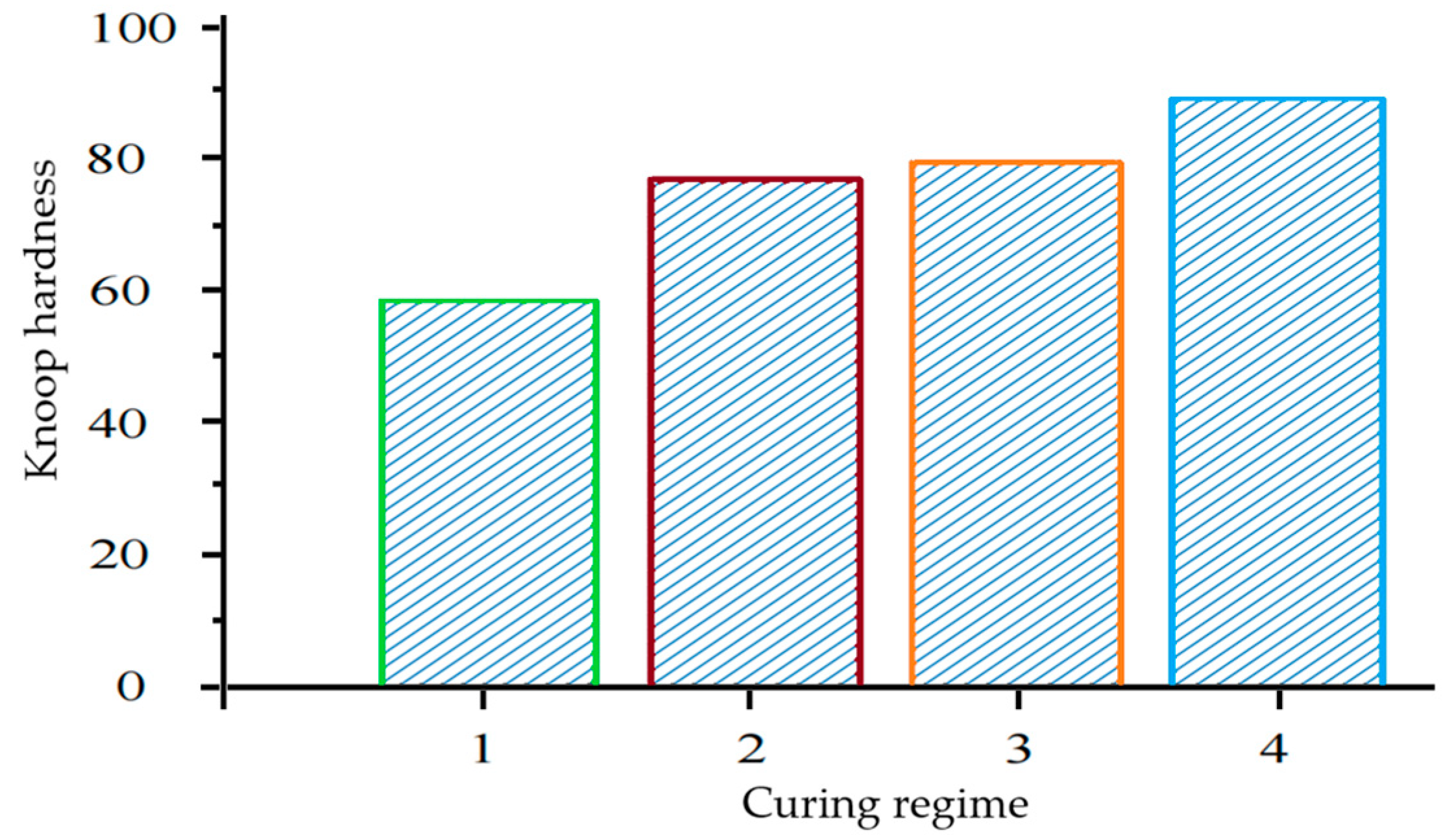
Figure 11 shows the results of a Knoop hardness testing of acrylate paint coatings based on synthesized dispersions cured under natural conditions and exposed to UV radiation for 5, 45, and 60 min.
Figure 11 shows that when exposed to UV radiation, the hardness of acrylate paint coatings based on synthesized dispersions increases. Thus, the Knoop hardness of coatings cured under natural conditions is 59.3, and when exposed to UV radiation for 60 min, it is 90. With an increase in the curing time by UV radiation on acrylate paint coatings based on synthesized dispersions, the hardness increases. The increase in coating hardness is associated with the intensification of polymerization processes of acrylate paint coatings based on synthesized dispersions containing hollow glass microspheres, which are part of the studied paints. It is worth noting that these data were obtained a day after exposure to UV radiation.
When examining the adhesion of paint and varnish coatings cured by UV radiation, it was found that exposure to UV radiation during curing does not affect adhesion. The adhesion of acrylate paint and varnish coatings cured under natural conditions and exposed to UV radiation for 5, 45, and 60 min is equal to 1.
Subsequently, the gloss of films applied to glass with a thickness of 100 microns in a wet layer and dried for 24 h in ambient conditions was measured, and a GL0030 TQC gloss meter was used as a measuring device. During the test, a noticeable difference was found between the two coatings, the gloss level of the film of the modified water-dispersion composition on the synthesized dispersions was 78/80 units at an angle of 600, and the gloss level of the film with the standard dispersion was 75/77 units.
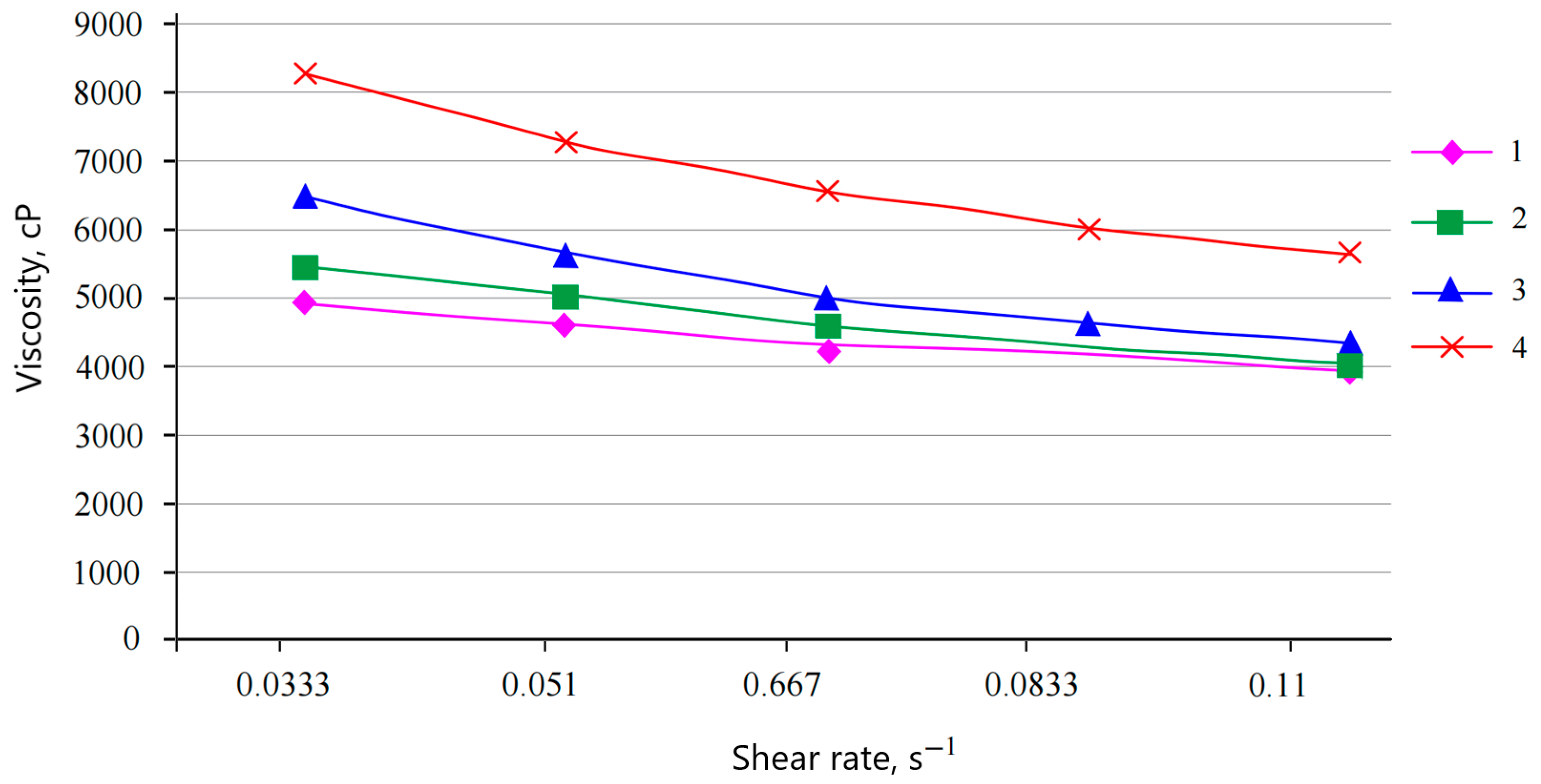
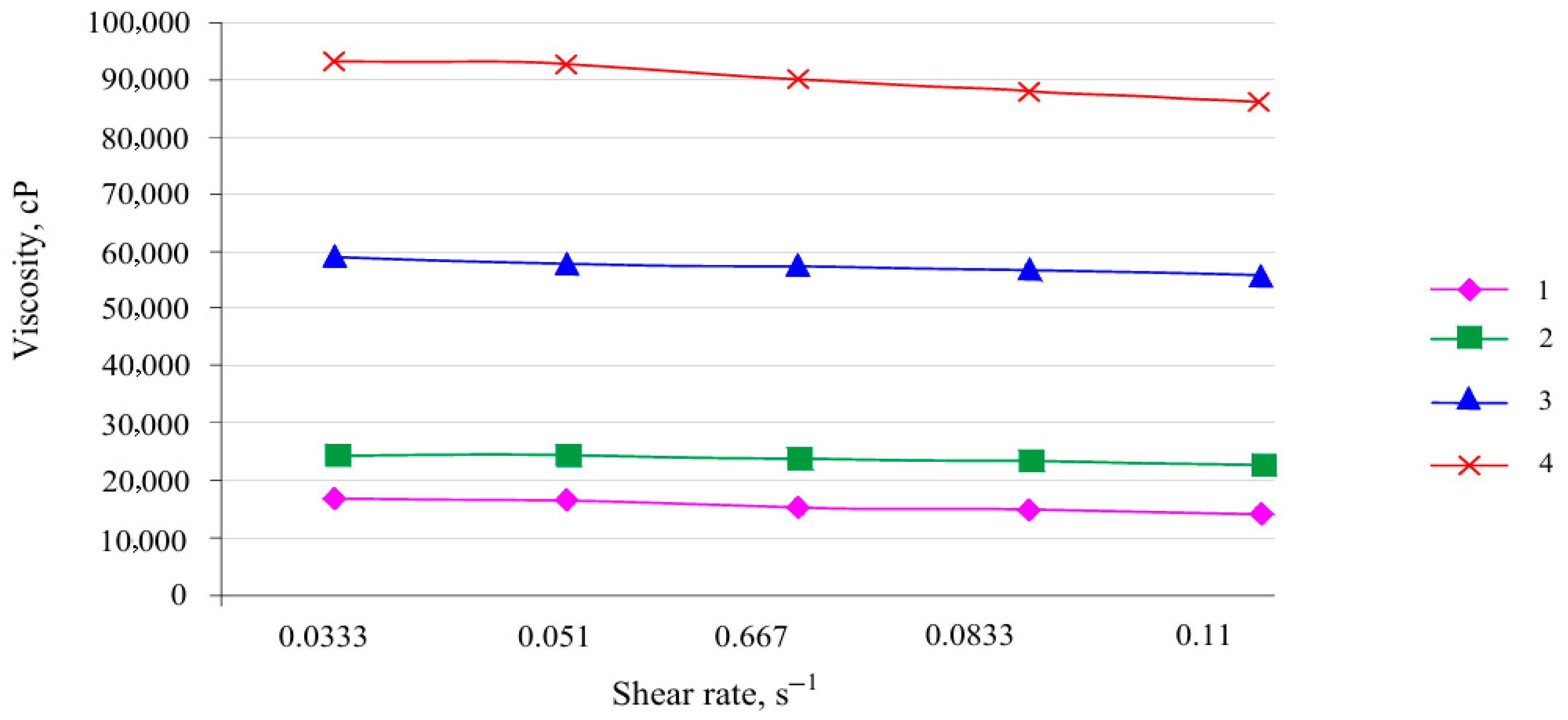
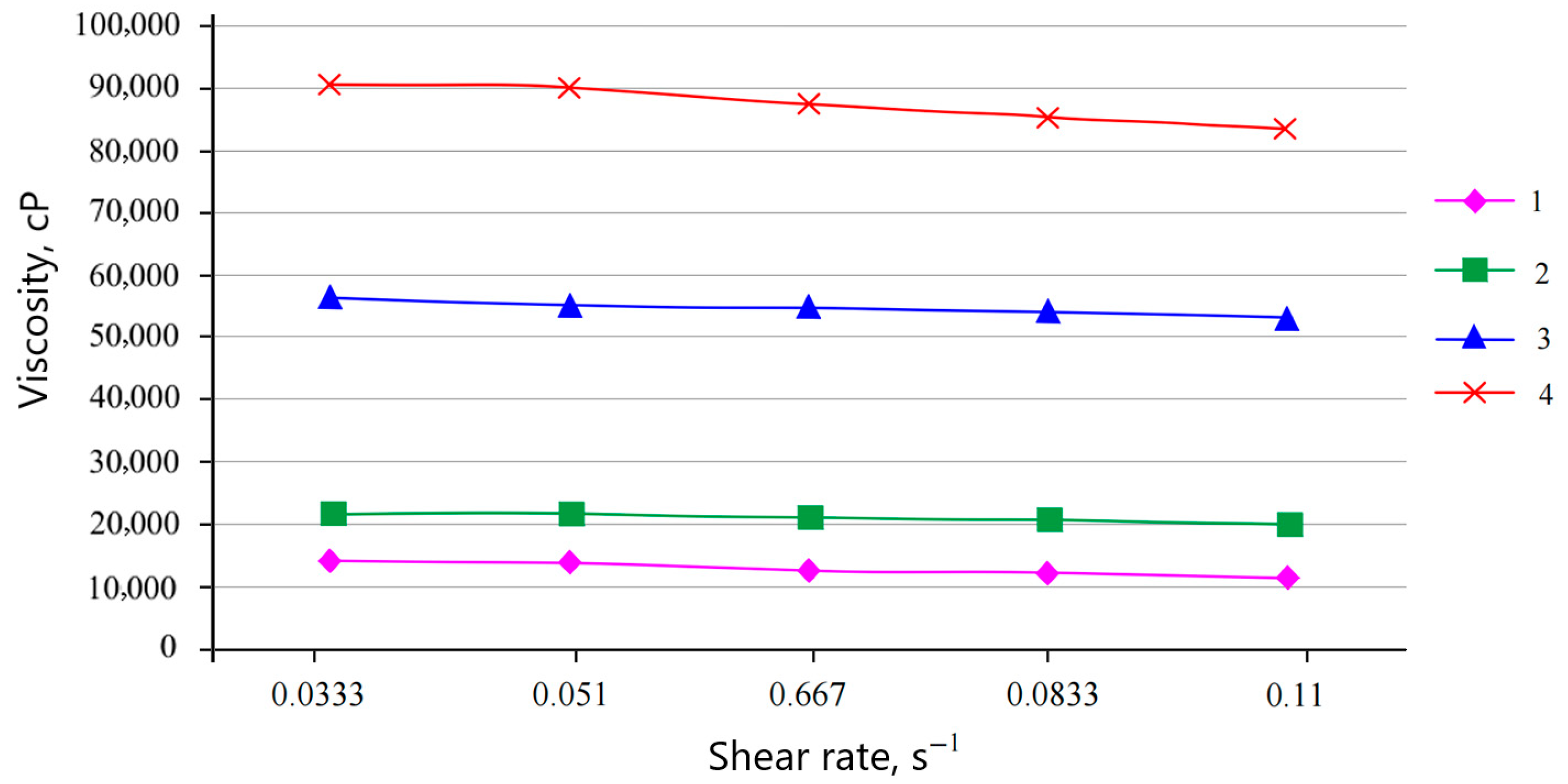
In the course of the work, the viscosity of water-dispersion compositions of compounds A, B, and C, not filled and filled with microspheres, was studied, and was measured using an AMETEK Brookfield DV–1 continuous viscometer. Based on the data obtained, viscosity dependences on shear rate were constructed (
Figure 12,
Figure 13 and
Figure 14).
Figure 12 shows that the initial acrylate dispersion has a viscosity of 4398 cP, and no viscosity anomaly was observed. When filling solution A with microspheres, the viscosity increases and amounts to 4685 cP at 0.20 mass parts of the filler, 5220 cP at 0.35 wt.% mass parts, and 6758 cP at 0.50 wt.%.
Figure 13 shows that the initial solution has a viscosity of 14,794 cP, and no viscosity anomaly was observed. When filling the solution in microspheres, the viscosity increases and amounts to 22,932 cP at 0.20 mass parts of the filler, 56,720 cP at 0.35 wt.%, and 89,526 cP at 0.50 wt.%.
Figure 14 shows that the initial solution has a viscosity of 14,000 cP, and no viscosity anomaly was observed. When filling the solution with microspheres, the viscosity increases and amounts to 22,531 cP at 0.20 wt.% of the filler, 55,420 cP at 0.35 wt.%, and 87,526 cP at 0.50 wt.%.
The joint influence of surfactants and film formers on the wetting ability of water–acrylic compositions was modeled using the probabilistic-deterministic planning (PDP) method [
63]. A detailed description of the process of applying the PDP method for modeling multifactorial processes is presented in [
64]. Research work using PDP consisted of the following stages:
1. The following input factors were determined: the content of the synthesized polymer dispersion in the aqueous solution (Spl, %: 0 ÷ 30) and the concentration of surfactants (The joint influence of surfactants, g/dm
3: 0 ÷ 4). The numerical values of the levels for each factor are presented in
Table 12.
2. An orthogonal plan matrix of a 4 × 6 two-factor experiment was compiled (
Table 13). Taking into account the different number of levels of the two input factors, the total number of experiments was 4 × 6 = 24. The cosine of the contact angle was taken as the response function (where i is the ordinal number of the experiment).
3. We conducted a series of experiments according to the matrix plan (
Table 13) and formed an experimental array, establishing numerical values for the response functions (output parameters) for each experiment.
4. We sampled the experimental array for each level of each factor (
Table 14).
Based on a sample of experimental data (
Table 12), we constructed partial dependencies of response functions on the content of film-forming agents and the concentration of surfactants.
5. Each specific dependence was approximated by a single variable function, and these functions were then combined into a multifactorial statistical mathematical model (generalized equation) based on the semi-empirical Formula (3) proposed in [
62]:
where Yo is the generalized equation;
Yi is the partial function;
is the product of all partial functions;
p is the number of partial functions, equal to the number of input factors;
is the arithmetic mean of all experimental values of the response function (general mean) to the power of one less than the number of partial functions.
Each individual dependency was approximated by a single variable function, and these functions were then combined into a multifactorial statistical mathematical model (generalized equation) based on the proposed semi-empirical Formula (3) [
65].
6. The accuracy of the obtained multifactorial statistical mathematical models was assessed by calculating the nonlinear multiple correlation coefficients (R) according to (2).
The significance of the calculated nonlinear multiple correlation coefficient was confirmed using Student’s t-test.
4. Discussion
When testing the compositions obtained, which are given in
Table 1, it was established that the degree of grinding (no more than 60 μm), pH (8 ÷ 9), and drying time to degree 3 (less than 1 h) for all paints comply with regulatory requirements. For the analysis of the mass fraction of non-volatile substances (dry residue), the opacity of the dried film, and the paint consumption, the viscosity according to the VZ-246 viscometer with a nozzle diameter of 4 mm was maintained within the range of 22–27 s for all compositions, which complies with the standards.
Partial replacement of TiO2 with glass microspheres reduces the cost of the paint and varnish material while improving individual coating characteristics; in particular, increasing the opacity of the coating and reducing the density of the paint. Replacing calcium carbonate with hollow glass microspheres significantly increases the opacity of the paint and varnish material, and reduces the density, which reduces paint consumption by 1 m2, which will result in savings of up to 15%. The introduction of hollow glass microspheres into the formulation of water-dispersion paints increases the hydrophobicity of the coating, which reduces moisture absorption and slows down the rate of paint degradation.
The formation of passivating layers strongly affects the anodic dissolution of metals, and it must be borne in mind that prolonged occurrence of corrosion processes on the surface of paint coatings lead to the destruction of the passivating film and, consequently, to a change in the direction of electrode processes. Passivation can be caused by the formation of both adsorption layers and phase oxide or salt films on the coating surface, which are formed due to the dissolution of the paint coating. At the same time, the formation of dense oxide films contributes to a temporary increase in the steel’s resistance to aggressive salt environments.
This study examines how ultraviolet radiation (207 nm) influences the corrosion resistance, hardness, and adhesion of acrylate paint coatings formulated with synthesized dispersions incorporating finely dispersed fillers such as modified diatomite and hollow glass microspheres. Studies of the corrosion resistance of coatings using polarographic curves in a saturated sodium chloride solution have shown that exposure to ultraviolet radiation of acrylate paint coatings leads to an increase in their corrosion resistance to a saturated sodium chloride solution due to an increase in the continuity and smoothness of the coatings. It was found that an increase in the duration of irradiation contributes to an increase in corrosion resistance.
It has been shown that the hardness of acrylate paint and varnish coatings increases with longer UV curing time. It was found that UV exposure during the curing process does not impair the adhesion of the coatings. The adhesion of acrylate paint and varnish coatings to steel grade DC01 is rated at 1 point, which meets the regulatory requirements.
The results obtained are of practical importance in the development of technological modes for the production of paint and varnish coatings with enhanced performance properties by irradiation with UV radiation, which will increase the service life of painted products and reduce maintenance costs.
Studies have been conducted to determine the rheological characteristics of synthesized acrylate dispersions not filled and filled with hollow glass microspheres. It has been found that the amount of filler content affects the viscosity, and with an increase in the filler content in polymer matrices, the effect of viscosity anomaly is manifested in two cases.
The results of the studies show the possibility of using synthesized polymer dispersions in paint and varnish compositions based on an aqueous dispersion of acrylic polymer and finely dispersed fillers (lime, diatomite, and microspheres) as surfactants, which are modifying additives with a dispersing effect. The minimum average diameter of fillers with a film-forming content of 10 and 20% is achieved when 0.5 g/dm3 is added. In more concentrated suspensions with a concentration of 30%, the amount of surfactant added must be reduced by almost half (0.25 g/dm3). Generalized models have been developed and a sample of experimental data has been taken for each level of each factor to determine the degree of dispersion of fillers from the quantitative contents of film-forming agents in water–acrylic compositions.
5. Conclusions
An aqueous polyacrylic dispersion was synthesized using the surfactant-free emulsion polymerization method, and the kinetics of this process were studied. Samples of aqueous dispersions of polyacrylic copolymers were obtained, along with coatings based on them, and water-dispersion compositions were investigated. Theoretical glass transition temperatures of the copolymers were calculated, and the relationships between the properties of the dispersions and the coatings based on them and the copolymer composition were established. It was found that the use of hollow glass microspheres in combination with modified diatomite in the production of water-dispersion paints is of practical interest. Based on the research results, the dependencies of property changes in water-dispersion coatings based on synthesized dispersions, as well as modified diatomite and hollow glass microspheres, have been established.
1. As a result of research, the most effective time and temperature for synthesis by the emulsifier-free emulsion polymerization method were established, with a conversion rate of over 99%. The optimal time for emulsion polymerization was 2 h at a temperature of 85 °C, at which the highest conversion rate was achieved.
2. It was established that when replacing both titanium dioxide and calcium carbonate with PSM, such indicators as adhesion and resistance to static water exposure do not deteriorate. Studies have shown a decrease in dry residue and paint density, which has a positive effect on the consumption of paint and varnish material and its cost. Coatings containing microspheres are more hydrophobic than the corresponding standard water-dispersion paint.
Thus, when replacing 3% (by weight) of TiO2 with microspheres, the opacity of the dried film increases by 35%, and with 6% TiO2, by 8%. When the mass content of TiO2 in the formulation of water-dispersion paints is less than 3%, the covering power of the paint decreases sharply, but when CaCO3 is replaced with hollow glass microspheres, regardless of the amount, it increases.
3. The results of pendulum hardness measurements showed that at a temperature of 23 °C and relative humidity of 50% throughout the entire test period, the modified water-dispersion composition based on synthesized dispersions and hollow glass microspheres had increased performance indicators by almost 2.5 times.
4. The water-dispersion paint’s resistance to sticking showed the best results, which were rated from 4.75 to 5.0%, indicating no destruction or change in gloss.
5. It was found that the potential of an acrylate paint coating based on synthesized dispersions, when cured by UV radiation and corroded for 120 min, shifts to the electropositive region. The potential of acrylate paint coatings cured under natural conditions after 20 min of corrosion ranged from −78.50 to −79.0 mV, and after 120 min of corrosion ranged from 166.70 to 160.55 mV. The potential of paint coatings cured by UV radiation for 60 min after 120 min of corrosion ranges from 108.42 to 107.65 mV.
6. The Knoop hardness of coatings cured under natural conditions is 59.3, and when exposed to UV radiation for 60 min, it is 90.0 conventional units. With an increase in the curing time under UV radiation, an increase in hardness is observed in acrylic paint coatings, which is associated with the intensification of the polymerization processes of the coatings due to the synthesized dispersions and hollow glass microspheres included in the composition of the studied paints.
7. When studying the adhesion of paint coatings cured by UV radiation, it was found that when exposed to UV radiation for 5, 45, and 60 min, it is equal to 1. The results obtained are of practical importance in the development of technological modes for obtaining paint coatings with improved operational properties through UV irradiation, which will increase the service life of painted products and reduce maintenance costs.
8. Studies were conducted to determine the rheological characteristics of synthesized acrylate dispersions, both unfilled and filled with hollow glass microspheres. It was found that the amount of filler affects viscosity, and that an increase in filler content in polymer matrices in two cases results in a viscosity anomaly effect.
9. The developed formulations for obtaining water-dispersion paint and varnish compositions based on synthesized polymer dispersions, activated diatomite, and hollow glass microspheres meet all the performance requirements for paint and varnish materials, and in terms of economic indicators, the cost of 1 kg of paint is 30% lower than the standard.
10. The stabilizing effect of titanium dioxide dispersions in water–acrylic compositions is an additive value determined by the contribution of the film-forming and surface-active substances. Polymer dispersions synthesized by an emulsifier-free method are effective stabilizing and dispersing surfactants, which allow us, when used in measured doses, to obtain sedimentation-stable compositions without delamination and sedimentation. It has been established that the sedimentation rate significantly decreased with the addition of additives and reached a minimum value. Separation is reduced to zero due to an increase in dispersion processes. In the formulations of water–acrylic dispersions, it is recommended to introduce 0.25 g/dm3 of surfactants, which reduce sedimentation by 3.5 ÷ 3.9 times compared to unmodified suspensions.
11. The content of synthesized polymer dispersions (A, B, and C) in the modified water-dispersion composition increases the protective properties of coatings and improves performance. It has been established that as the content of finely dispersed fillers and synthesized dispersion in coatings increases, the gloss increases by almost 50% (from 18.1 to 26.9%).
The versatility of the developed dispersions allows water-dispersion compositions based on them to be used in various areas of construction work. The developed acrylic dispersions are intended for use in both architectural and industrial coatings for wood, metal, and mineral surfaces.