Nanostructured Cellulose Acetate Membranes Embedded with Al2O3 Nanoparticles for Sustainable Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
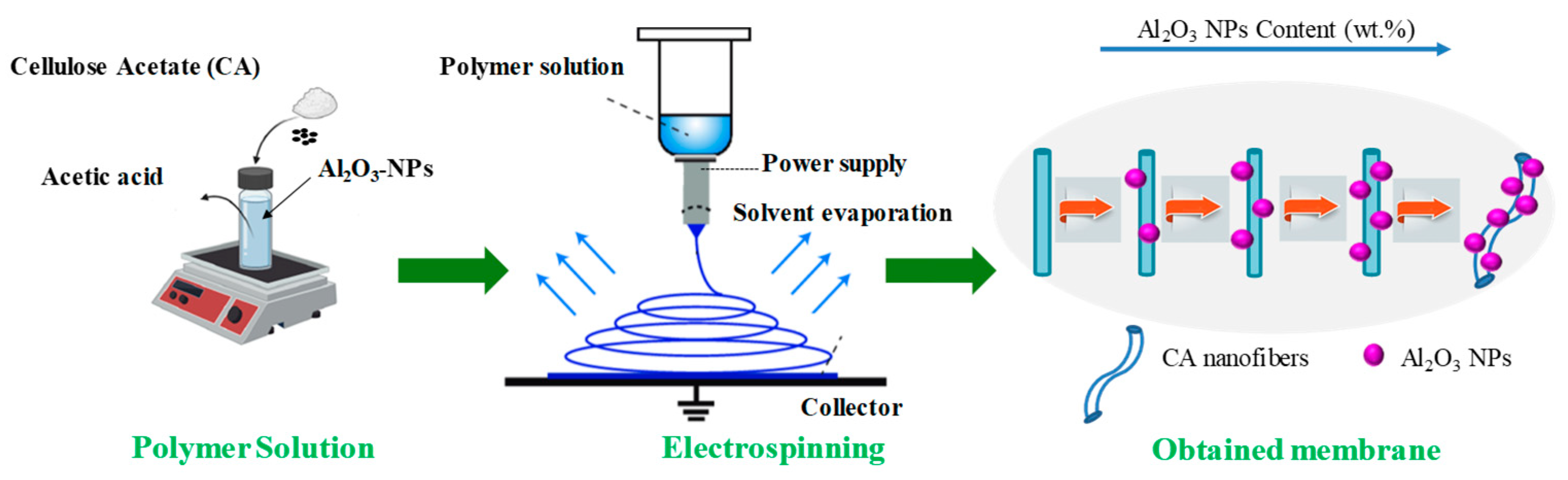
2.1. Electrospun Membrane Preparation
2.2. Membrane Characterization
2.3. Filtration Experiment
2.3.1. Pure Water Permeability
2.3.2. Porosity of CA Electrospun Nanofiber Membranes
2.3.3. Color Removal Efficiency
2.4. Phytotoxicity Test
3. Results and Discussion
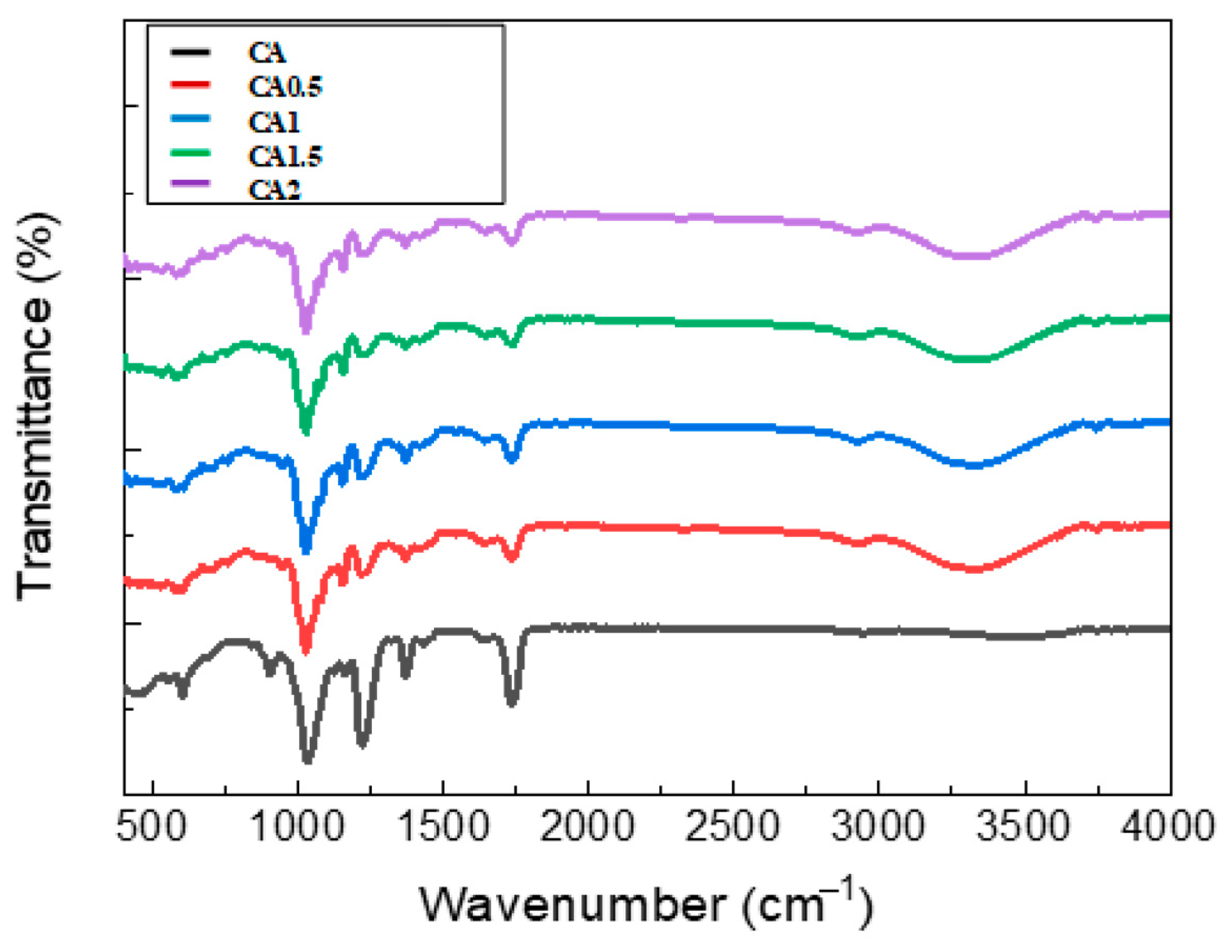
3.1. FT-IR
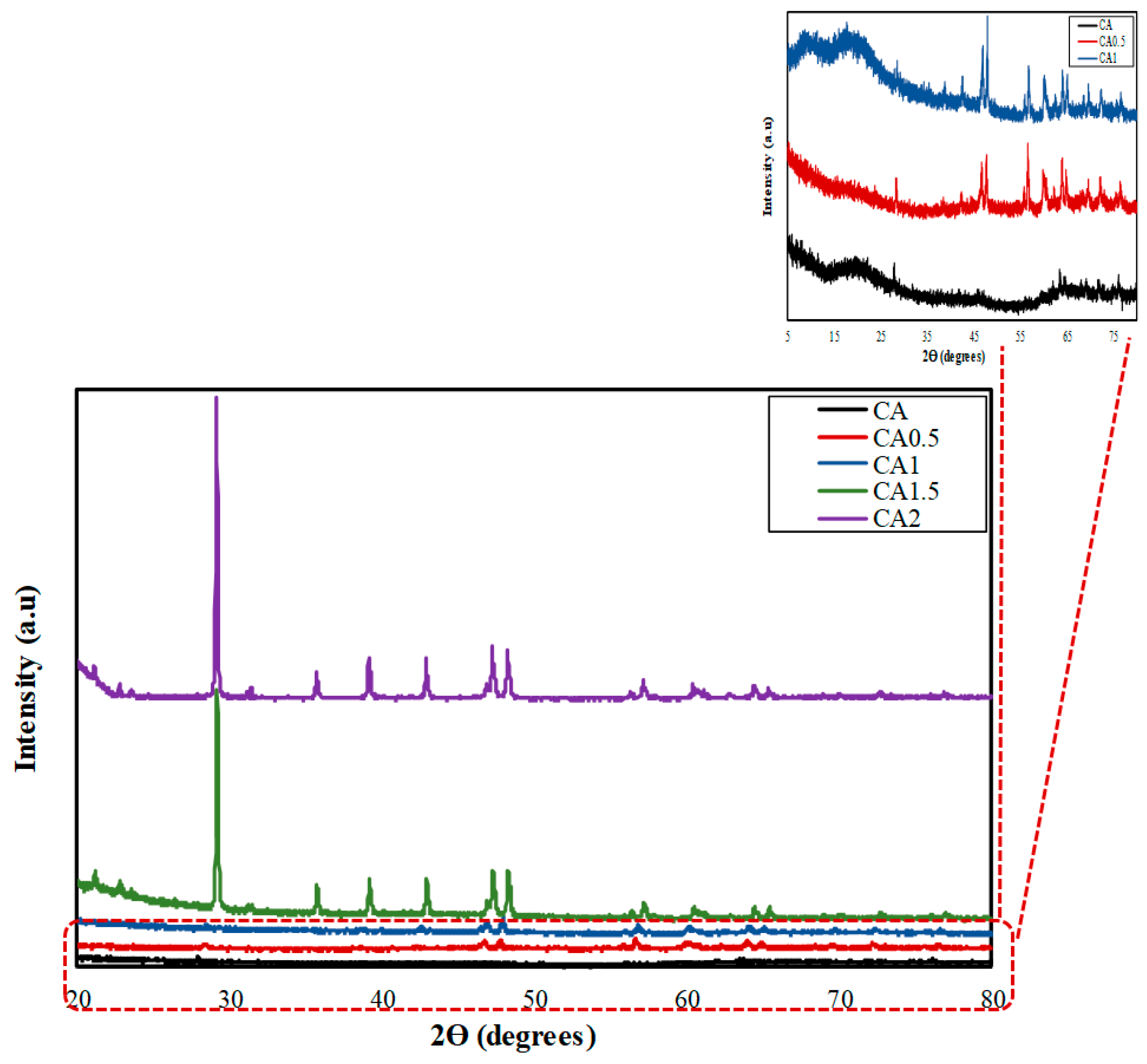
3.2. X-Ray Diffraction (XRD)
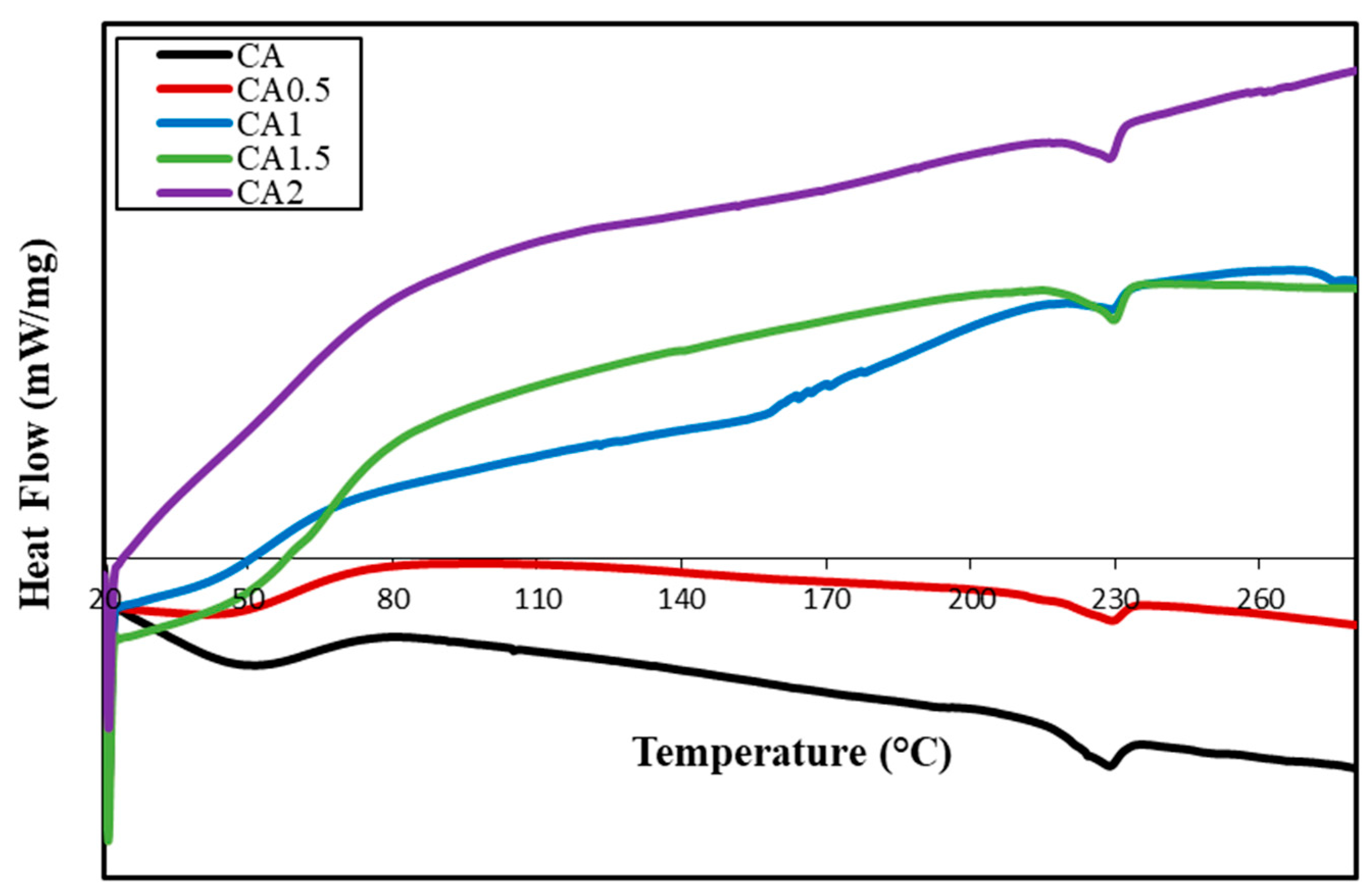
3.3. Thermal Behavior
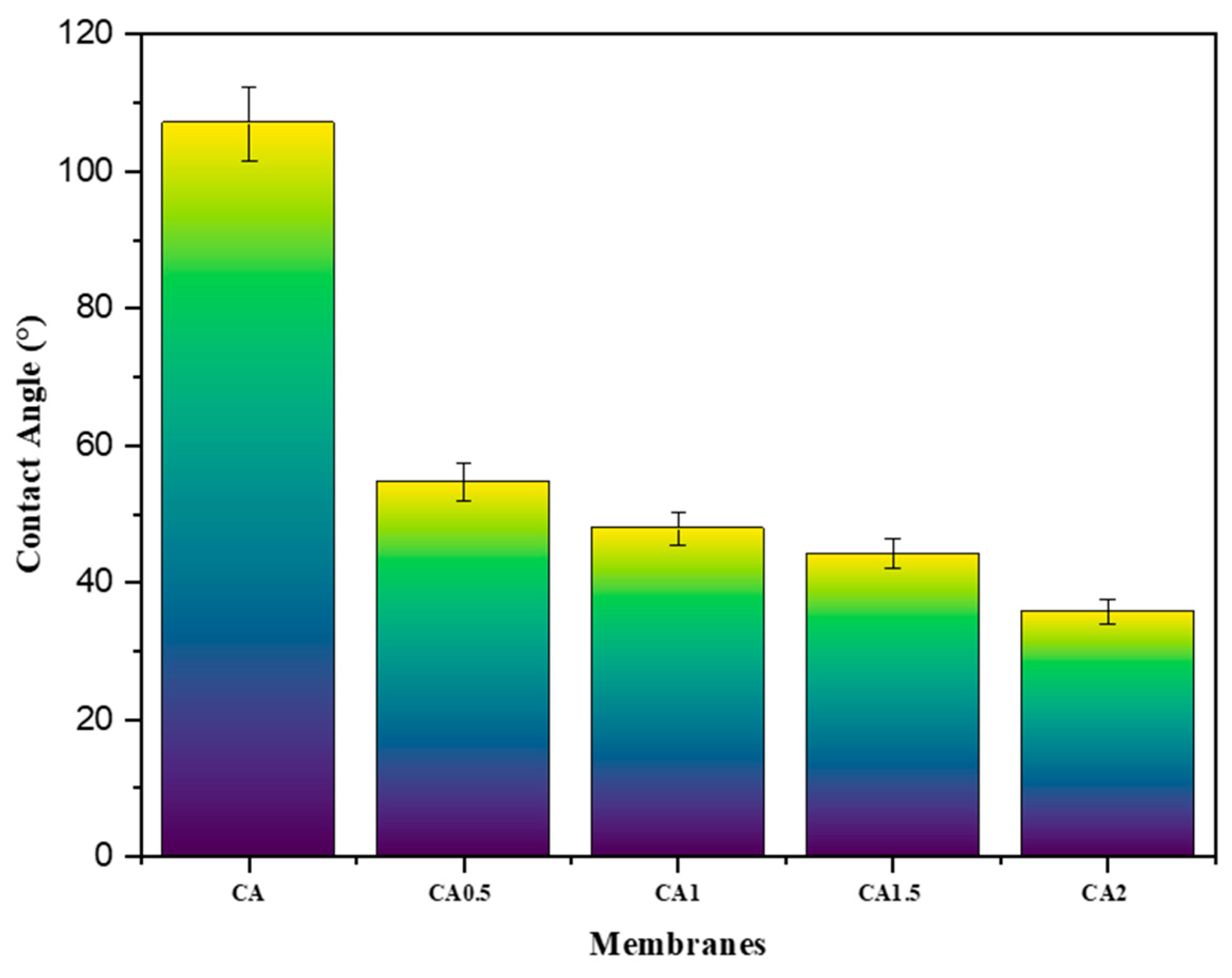
3.4. Morphology, Thickness, Contact Angle, Porosity, and Pore Size
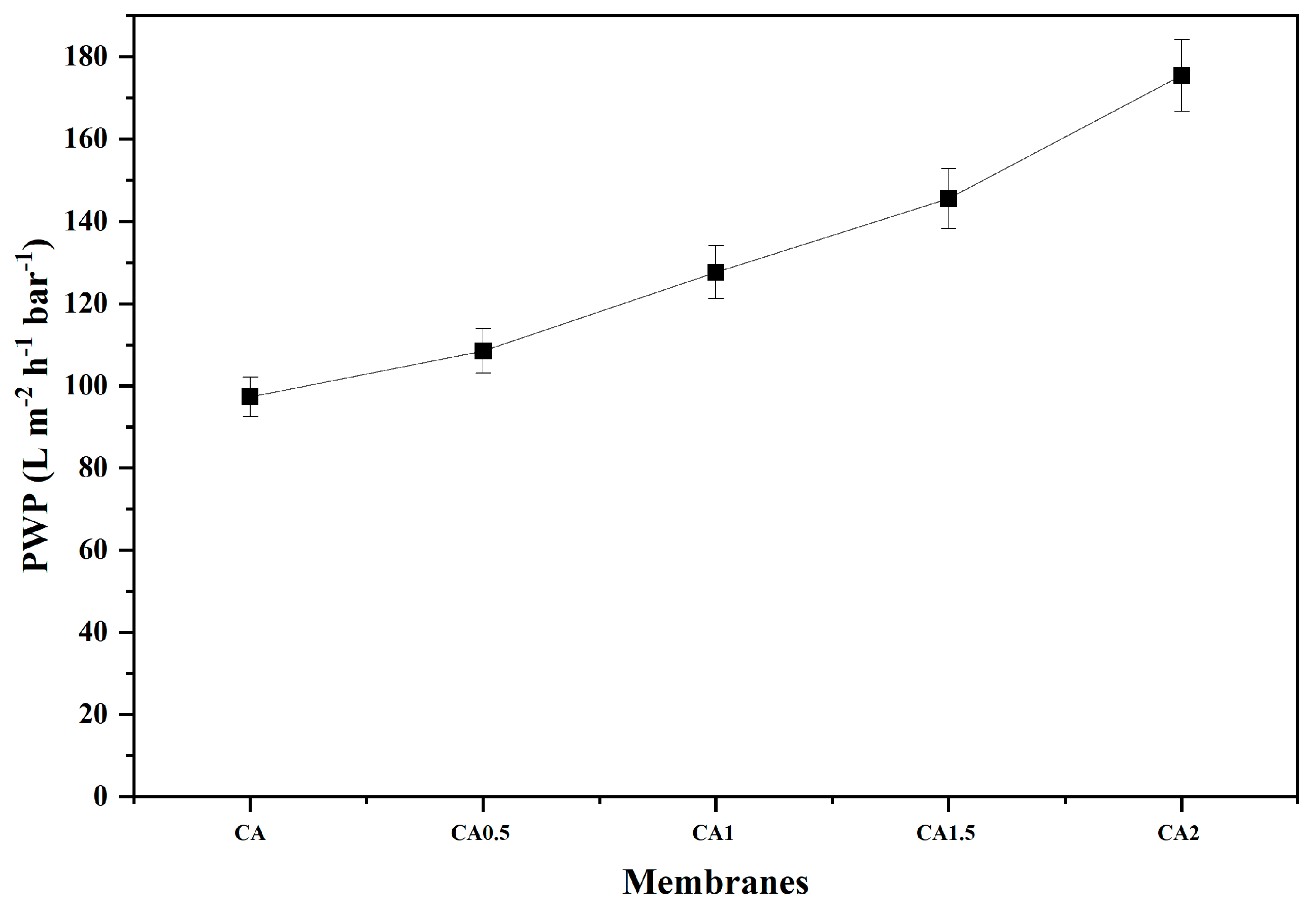
3.5. Pure Water Permeability
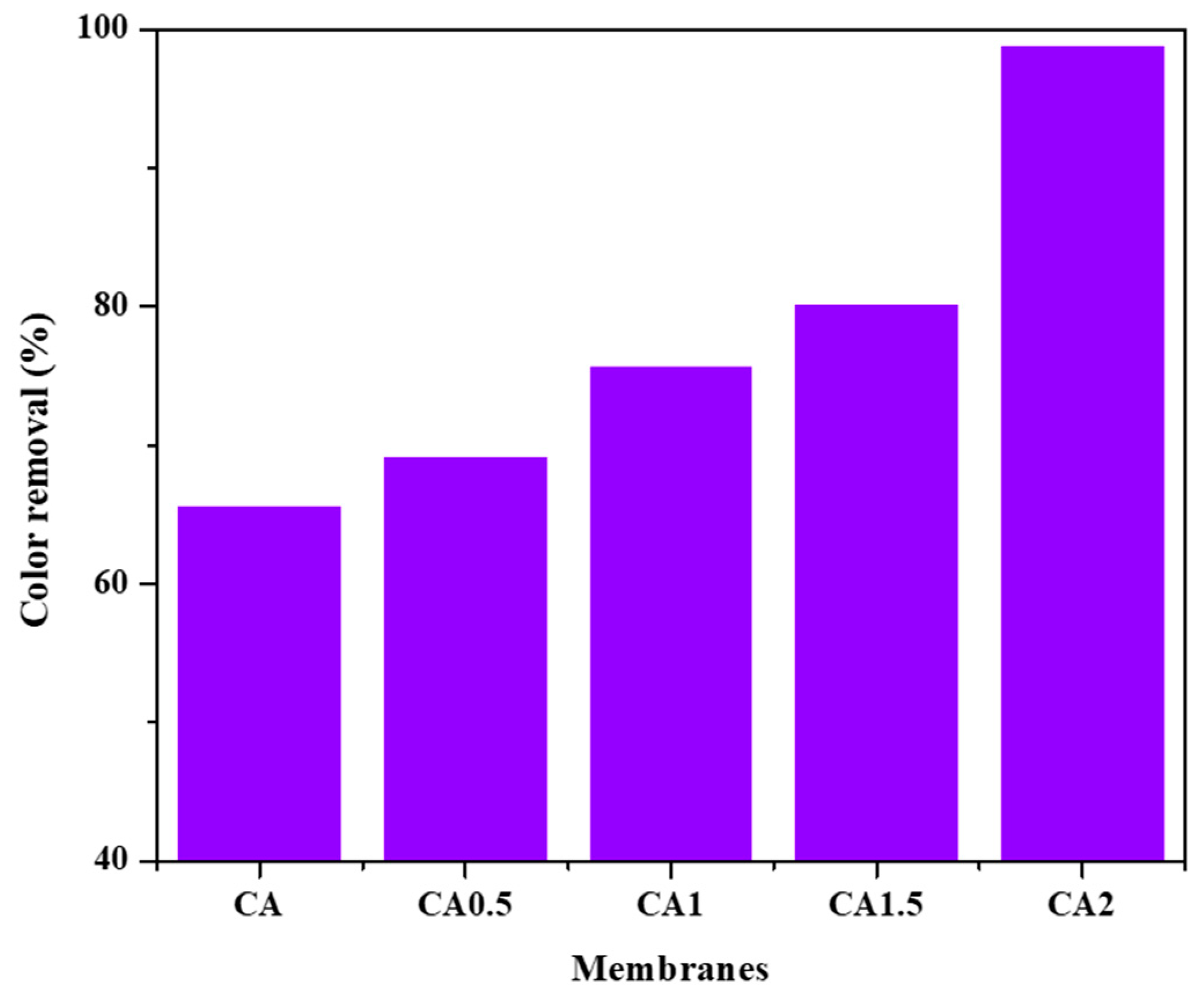
3.6. Results of Filtration Tests and Comparison with the Literature
3.7. Phytotoxicity Results (Germination Test)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amini, M.; Arami, M.; Mahmoodi, N.M.; Akbari, A. Dye Removal from Colored Textile Wastewater Using Acrylic Grafted Nanomembrane. Desalination 2011, 267, 107–113. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, Y.; Sun, W.; Shen, H.; Zheng, H.; Xu, Y.; Zhao, J.; Wu, H.; Liu, C. Advanced Treatment of Actual Textile Dye Wastewater by Fenton-flocculation Process. Can. J. Chem. Eng. 2017, 95, 1245–1252. [Google Scholar] [CrossRef]
- Samadi, S.; Khalilian, F.; Tabatabaee, A. Synthesis, Characterization and Application of Cu–TiO2/Chitosan Nanocomposite Thin Film for the Removal of Some Heavy Metals from Aquatic Media. J. Nanostruct. Chem. 2014, 4, 84. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite Membranes for Water Separation and Purification: Fabrication, Modification, and Applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Hua, Z.; Tang, L.; Wu, M.; Fu, J. Graphene Hydrogel Improves S. putrefaciens’ Biological Treatment of Dye Wastewater: Impacts of Extracellular Electron Transfer and Function of c-Type Cytochromes. Environ. Res. 2023, 236, 116739. [Google Scholar] [CrossRef]
- Bahadur, N.; Das, P.; Bhargava, N. Improving Energy Efficiency and Economic Feasibility of Photocatalytic Treatment of Synthetic and Real Textile Wastewater Using Bagasse Fly Ash Modified TiO2. Chem. Eng. J. Adv. 2020, 2, 100012. [Google Scholar] [CrossRef]
- Ghaffarian Khorram, A.; Fallah, N. Comparison of Electrocoagulation and Photocatalytic Process for Treatment of Industrial Dyeing Wastewater: Energy Consumption Analysis. Environ. Prog. Sustain. Energy 2020, 39, e13288. [Google Scholar] [CrossRef]
- Panhwar, A.; Sattar Jatoi, A.; Ali Mazari, S.; Kandhro, A.; Rashid, U.; Qaisar, S. Water Resources Contamination and Health Hazards by Textile Industry Effluent and Glance at Treatment Techniques: A Review. Waste Manag. Bull. 2024, 1, 158–163. [Google Scholar] [CrossRef]
- Elaissaoui, I.; Benahmed, W.N.; Bousselmi, L.; Akrout, H. Anodic Oxidation as Promoting Technology for Treatment and Reuse of Industrial Textile Effluent Using Ruthenium-Doped Lead Dioxide (PbO2) Anode. Clean. Technol. Environ. Policy 2025, 27, 1551–1570. [Google Scholar] [CrossRef]
- Criscuoli, A.; Macedonio, F.; Brunetti, A.; Tocci, E.; Drioli, E. Impact of Membrane Engineering on the Process Engineering Progresses: Towards a Sustainable Development. Chem. Eng. Process.—Process Intensif. 2023, 189, 109385. [Google Scholar] [CrossRef]
- Elaissaoui, I.; Sayeb, S.; Ounif, I.; Ferhi, M.; Karima, H.; Ennigrou, D.J. Preparation and Characterization of Acetate Cellulose Electrospun Nanofibers Membrane: Potential Application on Wastewater Treatment. Heliyon 2024, 10, e32552. [Google Scholar] [CrossRef]
- Thomas, R.T.; Del Río de Vicente, J.I.; Zhang, K.; Karzarjeddi, M.; Liimatainen, H.; Oksman, K. Size Exclusion and Affinity-Based Removal of Nanoparticles with Electrospun Cellulose Acetate Membranes Infused with Functionalized Cellulose Nanocrystals. Mater. Des. 2022, 217, 110654. [Google Scholar] [CrossRef]
- Aquino, M.; Santoro, S.; Politano, A.; D’Andrea, G.; Siciliano, A.; Straface, S.; La Russa, M.F.; Curcio, E. Environmentally Friendly Photothermal Membranes for Halite Recovery from Reverse Osmosis Brine via Solar-Driven Membrane Crystallization. Membranes 2024, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, R.; Yin, J.; Jiao, T.; Huang, H.; Zhao, X.; Zhang, L.; Li, Q.; Zhou, J.; Peng, Q. Fabrication and Highly Efficient Dye Removal Characterization of Beta-Cyclodextrin-Based Composite Polymer Fibers by Electrospinning. Nanomaterials 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Green Membrane Technologies Towards Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780323951654.
- Galiano, F.; Santoro, S.; Castro-Muñoz, R.; Russo, F.; Figoli, A. Smart and Novel Nanofiber Membranes . In Electrospun and Nanofibrous Membranes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 603–623. [Google Scholar]
- Russo, F.; Santoro, S.; Castro-Munoz, R.; Galiano, F.; Figoli, A. Electrospun Membranes for Air Filtration. In Electrospun and Nanofibrous Membranes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 577–601. [Google Scholar]
- Torre-Celeizabal, A.; Russo, F.; Galiano, F.; Figoli, A.; Casado-Coterillo, C.; Garea, A. Green Synthesis of Cellulose Acetate Mixed Matrix Membranes: Structure–Function Characterization. ACS Sustain. Chem. Eng. 2025, 13, 1253–1270. [Google Scholar] [CrossRef]
- Cárdenas Bates, I.I.; Loranger, É.; Mathew, A.P.; Chabot, B. Cellulose Reinforced Electrospun Chitosan Nanofibers Bio-Based Composite Sorbent for Water Treatment Applications. Cellulose 2021, 28, 4865–4885. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Electrospun Cellulose-Acetate/Chitosan Fibers for Humic-Acid Removal: Improved Efficiency and Robustness with a Core-Sheath Design. Nanomaterials 2022, 12, 1284. [Google Scholar] [CrossRef]
- Ma, W.; Guo, Z.; Zhao, J.; Yu, Q.; Wang, F.; Han, J.; Pan, H.; Yao, J.; Zhang, Q.; Samal, S.K.; et al. Polyimide/Cellulose Acetate Core/Shell Electrospun Fibrous Membranes for Oil-Water Separation. Sep. Purif. Technol. 2017, 177, 71–85. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.; Kim, J.; Kim, J. Nanostructured Ceramic Photocatalytic Membrane Modified with a Polymer Template for Textile Wastewater Treatment. Appl. Sci. 2017, 7, 1284. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Y.; Zuhra, Z.; Butler, I.S. Synthesis of γ-Alumina (Al2O3) Nanoparticles and Their Potential for Use as an Adsorbent in the Removal of Methylene Blue Dye from Industrial Wastewater. Nanoscale Adv. 2019, 1, 213–218. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Chen, D.; Guo, J.; Zhang, L.; Shao, Y. Fabrication of SiC-Al2O3 Foam Ceramic and Its Application in Fluoride-Containing Water. Ceram. Int. 2025, 51, 2268–2277. [Google Scholar] [CrossRef]
- Yuan, M.; Kong, J.; Zhao, J.; Tang, R.; Tian, Y.; Qiao, Y. Study on the Optimized Al2O3–SO3H Catalysis for a New Formaldehyde Recovery Process. Int. J. Hydrogen Energy 2021, 46, 37824–37835. [Google Scholar] [CrossRef]
- Ding, C.; Jin, M.; Ma, J.; Chen, Z.; Shen, Z.; Yang, D.; Shi, D.; Liu, W.; Kang, M.; Wang, J.; et al. Nano-Al2O3 Can Mediate Transduction-like Transformation of Antibiotic Resistance Genes in Water. J. Hazard. Mater. 2021, 405, 124224. [Google Scholar] [CrossRef]
- Breida, M.; Younssi, S.A.; El Rhazi, M.; Bouhria, M. Removal of Heavy Metals by Tight γ-Al2O3 Ultrafiltration Membrane at Low Pressure. Desalination Water Treat. 2019, 167, 231–244. [Google Scholar] [CrossRef]
- Keirouz, A.; Galiano, F.; Russo, F.; Fontananova, E.; Castro-Dominguez, B.; Figoli, A.; Mattia, D.; Leese, H.S. Cyrene-Enabled Green Electrospinning of Nanofibrous Graphene-Based Membranes for Water Desalination via Membrane Distillation. ACS Sustain. Chem. Eng. 2024, 12, 17713–17725. [Google Scholar] [CrossRef] [PubMed]
- Tahazadeh, S.; Mohammadi, T.; Tofighy, M.A.; Khanlari, S.; Karimi, H.; Motejadded Emrooz, H.B. Development of Cellulose Acetate/Metal-Organic Framework Derived Porous Carbon Adsorptive Membrane for Dye Removal Applications. J. Memb. Sci. 2021, 638, 119692. [Google Scholar] [CrossRef]
- Nafti-Mateur, M.; Jaouadi, M.; Van der Bruggen, B.; Naifer, K.H.; Jellouli Ennigrou, D. Development and Characterization of Porous Membranes Based on PPSU/PES Using Polyethylene Glycol as Porogen: Application for Cobalt Removal by Polyelectrolyte-enhanced Ultrafiltration. J. Chem. Technol. Biotechnol. 2024, 99, 343–354. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; da Silva, V.M.; de Oliveira Silva, J.; da Hora Machado, A.E.; Trovó, A.G. Coupling Coagulation, Flocculation and Decantation with Photo-Fenton Process for Treatment of Industrial Wastewater Containing Fipronil: Biodegradability and Toxicity Assessment. J. Environ. Manag. 2016, 174, 71–78. [Google Scholar] [CrossRef]
- Khouni, I.; Louhichi, G.; Ghrabi, A.; Moulin, P. Efficiency of a Coagulation/Flocculation–Membrane Filtration Hybrid Process for the Treatment of Vegetable Oil Refinery Wastewater for Safe Reuse and Recovery. Process Saf. Environ. Prot. 2020, 135, 323–341. [Google Scholar] [CrossRef]
- Park, J.; Yoon, J.; Depuydt, S.; Oh, J.-W.; Jo, Y.; Kim, K.; Brown, M.T.; Han, T. The Sensitivity of an Hydroponic Lettuce Root Elongation Bioassay to Metals, Phenol and Wastewaters. Ecotoxicol. Environ. Saf. 2016, 126, 147–153. [Google Scholar] [CrossRef]
- Saleh, T.A.; Tuzen, M.; Sarı, A. Polyamide Magnetic Palygorskite for the Simultaneous Removal of Hg(II) and Methyl Mercury; with Factorial Design Analysis. J. Environ. Manag. 2018, 211, 323–333. [Google Scholar] [CrossRef]
- Prakash, J.; Venkataprasanna, K.S.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-Vitro Evaluation of Electrospun Cellulose Acetate Nanofiber Containing Graphene Oxide/TiO2/Curcumin for Wound Healing Application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127166. [Google Scholar] [CrossRef]
- Baniasadi, J.; Shabani, Z.; Mohammadi, T.; Sahebi, S. Enhanced Performance and Fouling Resistance of Cellulose Acetate Forward Osmosis Membrane with the Spatial Distribution of TiO2 and Al2O3 Nanoparticles. J. Chem. Technol. Biotechnol. 2021, 96, 147–162. [Google Scholar] [CrossRef]
- Das, M.; Konwar, M.; Sadhonider, U.; Borthakur, L.J.; Mahanta, U.J.; Saikia, L.; Deka, M. Scaling the Ionic Conductivity and Electrochemical Properties of Bio-Based Gel Polymer Electrolytes Reinforced with Al2O3 Nanofibers for Energy Storage Applications. Polym. Bull. 2024, 82, 501–522. [Google Scholar] [CrossRef]
- Chand, N.; Rai, N.; Agrawal, S.L.; Patel, S.K. Morphology, Thermal, Electrical and Electrochemical Stability of Nano Aluminium-Oxide-Filled Polyvinyl Alcohol Composite Gel Electrolyte. Bull. Mater. Sci. 2011, 34, 1297–1304. [Google Scholar] [CrossRef]
- Amar, K.M.; Manjusri, M.; Lawrence, T.D. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Liu, H.; Hsieh, Y. Ultrafine Fibrous Cellulose Membranes from Electrospinning of Cellulose Acetate. J. Polym. Sci. B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Du, J.; Hsieh, Y.-L. Cellulose/Chitosan Hybrid Nanofibers from Electrospinning of Their Ester Derivatives. Cellulose 2009, 16, 247–260. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Aitomäki, Y.; Moreno-Rodriguez, S.; Lundström, T.S.; Oksman, K. Vacuum Infusion of Cellulose Nanofibre Network Composites: Influence of Porosity on Permeability and Impregnation. Mater. Des. 2016, 95, 204–211. [Google Scholar] [CrossRef]
- Naeem, M.A.; Lv, P.; Zhou, H.; Naveed, T.; Wei, Q. A Novel In Situ Self-Assembling Fabrication Method for Bacterial Cellulose-Electrospun Nanofiber Hybrid Structures. Polymers 2018, 10, 712. [Google Scholar] [CrossRef]
- Jalalian, N.; Nabavi, S.R. Electrosprayed Chitosan Nanoparticles Decorated on Polyamide6 Electrospun Nanofibers as Membrane for Acid Fuchsin Dye Filtration from Water. Surf. Interfaces 2020, 21, 100779. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W. Comparative Assessment of Hydrophobic and Hydrophilic Membrane Fouling in Wastewater Applications. J. Memb. Sci. 2009, 339, 93–99. [Google Scholar] [CrossRef]
- Jaid, G.M.; AbdulRazak, A.A.; Meskher, H.; Al-Saadi, S.; Alsalhy, Q.F. Metal-Organic Frameworks (MOFs), Covalent Organic Frameworks (COFs), and Hydrogen-Bonded Organic Frameworks (HOFs) in Mixed Matrix Membranes. Mater. Today Sustain. 2024, 25, 100672. [Google Scholar] [CrossRef]
- Díaz, J.C.; Kitto, D.; Kamcev, J. Accurately Measuring the Ionic Conductivity of Membranes via the Direct Contact Method. J. Memb. Sci. 2023, 669, 121304. [Google Scholar] [CrossRef]
- Shalan, A.E.; Afifi, M.; El-Desoky, M.M.; Ahmed, M.K. Electrospun Nanofibrous Membranes of Cellulose Acetate Containing Hydroxyapatite Co-Doped with Ag/Fe: Morphological Features, Antibacterial Activity and Degradation of Methylene Blue in Aqueous Solution. New J. Chem. 2021, 45, 9212–9220. [Google Scholar] [CrossRef]
- Hassan, H.S.; Elkady, M.F.; Farghali, A.A.; Salem, A.M.; El-Hamid, A.I.A. Fabrication of Novel Magnetic Zinc Oxide Cellulose Acetate Hybrid Nano-Fiber to Be Utilized for Phenol Decontamination. J. Taiwan. Inst. Chem. Eng. 2017, 78, 307–316. [Google Scholar] [CrossRef]
- ZabihiSahebi, A.; Koushkbaghi, S.; Pishnamazi, M.; Askari, A.; Khosravi, R.; Irani, M. Synthesis of Cellulose Acetate/Chitosan/SWCNT/Fe3O4/TiO2 Composite Nanofibers for the Removal of Cr(VI), As(V), Methylene Blue and Congo Red from Aqueous Solutions. Int. J. Biol. Macromol. 2019, 140, 1296–1304. [Google Scholar] [CrossRef]
- Abdrabou, D.; Ahmed, M.; Hussein, A.; El-Sherbini, T. Photocatalytic Behavior for Removal of Methylene Blue from Aqueous Solutions via Nanocomposites Based on Gd2O3/CdS and Cellulose Acetate Nanofibers. Environ. Sci. Pollut. Res. 2023, 30, 99789–99808. [Google Scholar] [CrossRef]
- Abdullahi, Y.A.; Akunna, J.C.; White, N.A.; Hallett, P.D.; Wheatley, R. Investigating the Effects of Anaerobic and Aerobic Post-Treatment on Quality and Stability of Organic Fraction of Municipal Solid Waste as Soil Amendment. Bioresour. Technol. 2008, 99, 8631–8636. [Google Scholar] [CrossRef]




| Membrane Code | CA Content (wt.%) | Al2O3 NPs Content (wt.%) |
|---|---|---|
| CA | 16 | 0 |
| CA0.5 | 0.5 | |
| CA1 | 1 | |
| CA1.5 | 1.5 | |
| CA2 | 2 |
| Membrane | Modification Agents | Dye | Color Removal (%) | Qmax (mg g−1) | Ref. |
|---|---|---|---|---|---|
| CA CA CA/chitosan | Al2O3 (2%) | IC | 99 | 45.59 | Present work |
| Ag/Fe | Methylene Blue (MB) | 90 | - | [50] | |
| Single Walled Carbon Nanotubes/ferrite/ titanium dioxide (SWCNT/Fe3O4/TiO2) | Methylene blue and Congo Red | 99 | 94.3/ 70.2 | [52] | |
| CA/Chitosan CA/Cadmium sulfate (CdS) | TiO2 | Methyl Orange | 98 | 76.22 | [51] |
| Gadolinium oxide (Gd2O3) and combined with graphene oxide (GO) nanoparticles | Methylene Blue (MB) | 91.07 | 8.33 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elaissaoui, I.; Sayeb, S.; Mekki, M.; Russo, F.; Figoli, A.; Horchani-Naifer, K.; Ennigrou, D.J. Nanostructured Cellulose Acetate Membranes Embedded with Al2O3 Nanoparticles for Sustainable Wastewater Treatment. Coatings 2025, 15, 823. https://doi.org/10.3390/coatings15070823
Elaissaoui I, Sayeb S, Mekki M, Russo F, Figoli A, Horchani-Naifer K, Ennigrou DJ. Nanostructured Cellulose Acetate Membranes Embedded with Al2O3 Nanoparticles for Sustainable Wastewater Treatment. Coatings. 2025; 15(7):823. https://doi.org/10.3390/coatings15070823
Chicago/Turabian StyleElaissaoui, Ines, Soumaya Sayeb, Mouna Mekki, Francesca Russo, Alberto Figoli, Karima Horchani-Naifer, and Dorra Jellouli Ennigrou. 2025. "Nanostructured Cellulose Acetate Membranes Embedded with Al2O3 Nanoparticles for Sustainable Wastewater Treatment" Coatings 15, no. 7: 823. https://doi.org/10.3390/coatings15070823
APA StyleElaissaoui, I., Sayeb, S., Mekki, M., Russo, F., Figoli, A., Horchani-Naifer, K., & Ennigrou, D. J. (2025). Nanostructured Cellulose Acetate Membranes Embedded with Al2O3 Nanoparticles for Sustainable Wastewater Treatment. Coatings, 15(7), 823. https://doi.org/10.3390/coatings15070823









