Slurry Aluminizing Mechanisms of Nickel-Based Superalloy and Applicability for the Manufacturing of Platinum-Modified Aluminide Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.2. Coating Characterization
3. Results
3.1. Aluminide Coatings
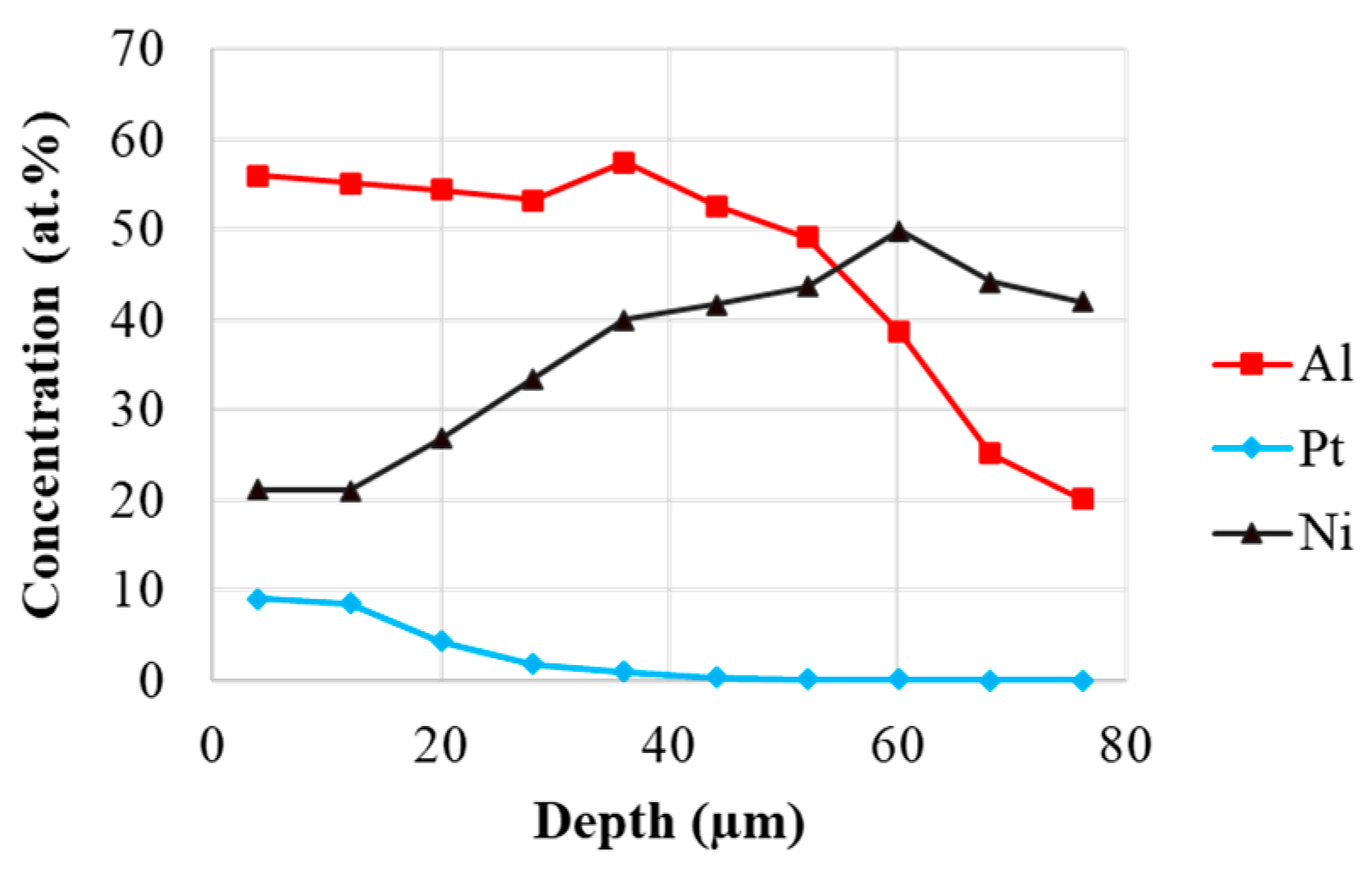
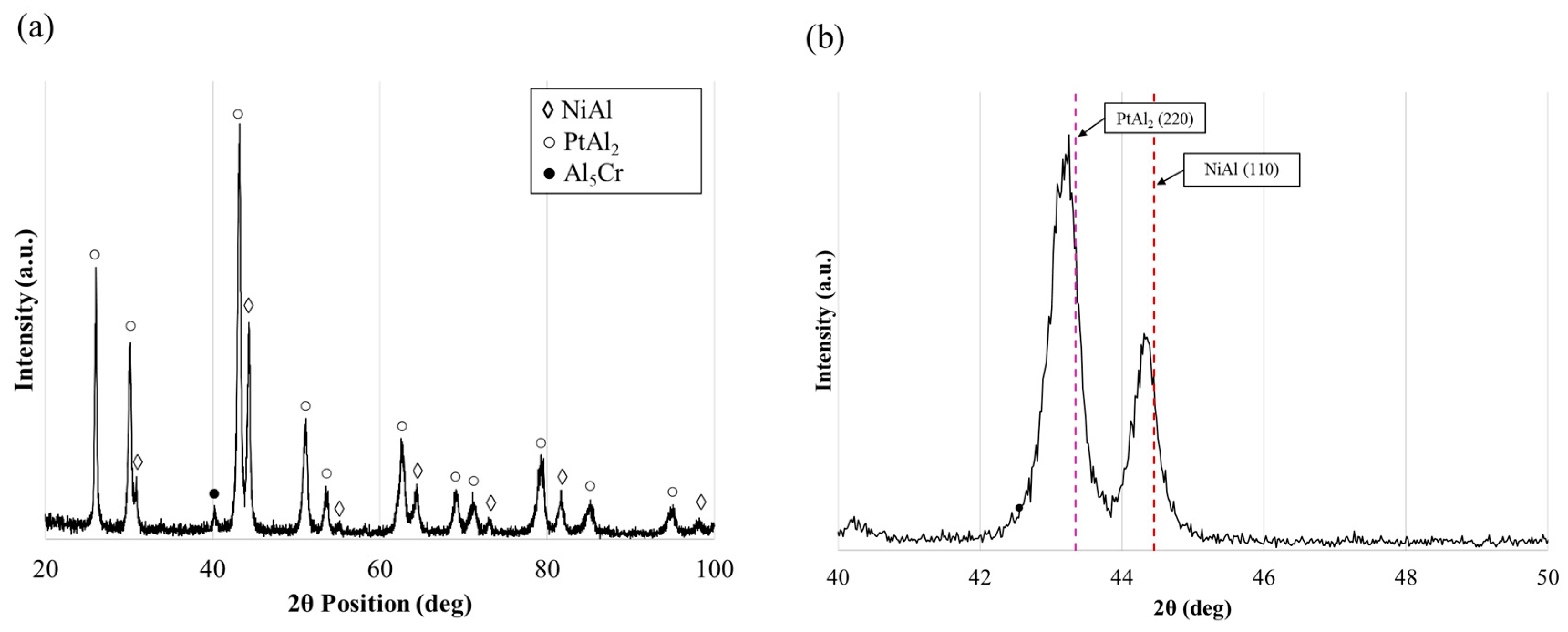
3.2. Manufacturing of Pt-Aluminides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pomeroy, M.J. Coatings for Gas Turbine Materials and Long Term Stability Issues. Mater. Des. 2005, 26, 223–231. [Google Scholar] [CrossRef]
- Darolia, R. Thermal Barrier Coatings Technology: Critical Review, Progress Update, Remaining Challenges and Prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Guo, L.; He, W.; Chen, W.; Xue, Z.; He, J.; Guo, Y.; Wu, Y.; Gao, L.; Li, D.; Zhang, Z.; et al. Progress on High-Temperature Protective Coatings for Aero-Engines. Surf. Sci. Technol. 2023, 1, 6. [Google Scholar] [CrossRef]
- Genova, V.; Pedrizzetti, G.; Paglia, L.; Marra, F.; Bartuli, C.; Pulci, G. Diffusion Aluminide Coating Modified via Electroless Nickel Plating for Ni-Based Superalloy Protection. Surf. Coat. Technol. 2022, 439, 128452. [Google Scholar] [CrossRef]
- Kopec, M. Recent Advances in the Deposition of Aluminide Coatings on Nickel-Based Superalloys: A Synthetic Review (2019–2023). Coatings 2024, 14, 630. [Google Scholar] [CrossRef]
- Kopec, M. Effect of Aluminide Coating Thickness on High-Temperature Fatigue Response of MAR-M247 Nickel-Based Superalloy. Coatings 2024, 14, 1072. [Google Scholar] [CrossRef]
- Barwinska, I.; Kopec, M.; Kukla, D.; Łazińska, M.; Sitek, R.; Kowalewski, Z.L. Effect of Aluminizing on the Fatigue and High-Temperature Corrosion Resistance of Inconel 740 Nickel Alloy. JOM 2023, 75, 1482–1494. [Google Scholar] [CrossRef]
- Pedraza, F.; Gómez, C.; Carpintero, M.C.; Hierro, M.P.; Pérez, F.J. On the Aluminisation of Stainless Steel by CVD in Fluidised Beds. Surf. Coat. Technol. 2005, 190, 223–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Pint, B.A.; Cooley, K.M.; Haynes, J.A. Formation of Aluminide Coatings on Fe-Based Alloys by Chemical Vapor Deposition. Surf. Coat. Technol. 2008, 202, 3839–3849. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Burnell-Gray, J.S.; Datta, P.K. Aluminide Coating Formation on Nickel-Base Superalloys by Pack Cementation Process. J. Mater. Sci. 2001, 36, 5673–5682. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, K.; Xin, X.; Zeng, X.; Guo, X.; Qiao, Y. Microstructure and Oxidation Behavior of the Aluminized Coating on K447A Nickel-Based Superalloy Prepared by AlF3-Activated Pack Cementation. Surf. Coat. Technol. 2025, 510, 132236. [Google Scholar] [CrossRef]
- Wang, C.-J.; Chen, S.-M. Microstructure and Cyclic Oxidation Behavior of Hot Dip Aluminized Coating on Ni-Base Superalloy Inconel 718. Surf. Coat. Technol. 2006, 201, 3862–3866. [Google Scholar] [CrossRef]
- Glasbrenner, H.; Wedemeyer, O. Comparison of Hot Dip Aluminised F82H-Mod. Steel after Different Subsequent Heat Treatments. J. Nucl. Mater. 1998, 257, 274–281. [Google Scholar] [CrossRef]
- Kepa, T.; Pedraza, F.; Rouillard, F. Intermetallic Formation of Al-Fe and Al-Ni Phases by Ultrafast Slurry Aluminization (Flash Aluminizing). Surf. Coat. Technol. 2020, 397, 126011. [Google Scholar] [CrossRef]
- Bakhtiary, O.; Sarraf, S.; Soltanieh, M. Microstructure Evaluation of Si-Modified Aluminide Coatings on IN625 Deposited by Slurry Aluminizing Process. Surf. Coat. Technol. 2025, 495, 131592. [Google Scholar] [CrossRef]
- Pedraza, F.; Boulesteix, C.; Proy, M.; Lasanta, I.; de Miguel, T.; Illana, A.; Pérez, F.J. Behavior of Slurry Aluminized Austenitic Stainless Steels under Steam at 650 and 700 °C. Oxid. Met. 2017, 87, 443–454. [Google Scholar] [CrossRef]
- Bozza, F.; Bolelli, G.; Giolli, C.; Giorgetti, A.; Lusvarghi, L.; Sassatelli, P.; Scrivani, A.; Candeli, A.; Thoma, M. Diffusion Mechanisms and Microstructure Development in Pack Aluminizing of Ni-Based Alloys. Surf. Coat. Technol. 2014, 239, 147–159. [Google Scholar] [CrossRef]
- Sarraf, S.H.; Soltanieh, M.; Rastegari, S. Reactive air aluminizing of a nickel-based superalloy (IN738LC): Coating formation mechanism. Surf. Coat. Technol. 2023, 456, 129229. [Google Scholar] [CrossRef]
- Bianco, R.; Rapp, R.A. Pack Cementation Diffusion Coatings. In Metallurgical and Ceramic Protective Coatings; Springer: Dordrecht, The Netherlands, 1996; pp. 236–260. [Google Scholar]
- Wang, Y.Q.; Sayre, G. Factors Affecting the Microstructure of Platinum-Modified Aluminide Coatings during a Vapor Phase Aluminizing Process. Surf. Coat. Technol. 2009, 203, 1264–1272. [Google Scholar] [CrossRef]
- Grüters, J.; Galetz, M.C. Influence of Thermodynamic Activities of Different Masteralloys in Pack Powder Mixtures to Produce Low Activity Aluminide Coatings on TiAl Alloys. Intermetallics 2015, 60, 19–27. [Google Scholar] [CrossRef]
- Benoist, J.; Badawi, K.F.; Malié, A.; Ramade, C. Microstructure of Pt Modified Aluminide Coatings on Ni-Based Superalloys without Prior Pt Diffusion. Surf. Coat. Technol. 2005, 194, 48–57. [Google Scholar] [CrossRef]
- Voudouris, N.; Christoglou, C.; Angelopoulos, G.N. Formation of Aluminide Coatings on Nickel by a Fluidised Bed CVD Process. Surf. Coat. Technol. 2001, 141, 275–282. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of Formation of Diffusion Aluminide Coatings on Nickel-Base Superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Rannou, B.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. Comparative Isothermal Oxidation Behaviour of New Aluminide Coatings from Slurries Containing Al Particles and Conventional Out-of-Pack Aluminide Coatings. Oxid. Met. 2014, 81, 139–149. [Google Scholar] [CrossRef]
- Galetz, M.C.; Montero, X.; Mollard, M.; Günthner, M.; Pedraza, F.; Schütze, M. The Role of Combustion Synthesis in the Formation of Slurry Aluminization. Intermetallics 2014, 44, 8–17. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Mechanisms of Formation of Slurry Aluminide Coatings from Al and Cr Microparticles. Surf. Coat. Technol. 2019, 359, 323–333. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. Low-Activity Aluminide Coatings for Superalloys Using a Slurry Process Free of Halide Activators and Chromates. Surf. Coat. Technol. 2013, 222, 9–14. [Google Scholar] [CrossRef]
- Conti, M.; Paglia, L.; Genova, V.; Pedrizzetti, G.; Baiamonte, L.; Marra, F. The Effects of Deposition Parameters on the Properties of NiCr Coatings Obtained by Electroless Plating. Chem. Eng. Trans. 2023, 100, 427–432. [Google Scholar] [CrossRef]
- Das, D.K. Microstructure and High Temperature Oxidation Behavior of Pt-Modified Aluminide Bond Coats on Ni-Base Superalloys. Prog. Mater. Sci. 2013, 58, 151–182. [Google Scholar] [CrossRef]
- Vialas, N.; Monceau, D. Effect of Pt and Al Content on the Long-Term, High Temperature Oxidation Behavior and Interdiffusion of a Pt-Modified Aluminide Coating Deposited on Ni-Base Superalloys. Surf. Coat. Technol. 2006, 201, 3846–3851. [Google Scholar] [CrossRef]
- Littner, A.; Pedraza, F.; Kennedy, A.D.; Moretto, P.; Peichl, L.; Weber, T.; Schuetze, M. Performance and Thermal Stability of Pt-Modified Al-Diffusion Coatings for Superalloys under Cyclic and Isothermal Conditions. Mater. High. Temp. 2005, 22, 411–420. [Google Scholar] [CrossRef][Green Version]
- Angenete, J.; Stiller, K. A Comparative Study of Two Inward Grown Pt Modified Al Diffusion Coatings on a Single Crystal Ni Base Superalloy. Mater. Sci. Eng. A 2001, 316, 182–194. [Google Scholar] [CrossRef]
- Gleeson, B. Thermal Barrier Coatings for Aeroengine Applications. J. Propuls. Power 2006, 22, 375–383. [Google Scholar] [CrossRef]
- Rezaee, B.; Rastegari, S.; Eyvazjamadi, H. Formation mechanism of Pt-modified aluminide coating structure by out-of-the-pack aluminizing. Surf. Eng. 2020, 37, 343–350. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Ding, X.; Li, X.; Yu, J.; Li, Q.; Qu, Y. Effect of Slurry Thickness on the Quality of Aluminized Coatings. Materials 2022, 15, 6758. [Google Scholar] [CrossRef]
- Ross, E.W.; O’Hara, K.S. Superalloys; Kissinger, R.D., Deye, D.J., Anton, D.L., Cetel, A.D., Nathal, M.V., Pollock, T.M., Woodford, D.A., Eds.; The Minerals, Metals & Materials Society: Cincinnati, OH, USA; Lynn, MA, USA, 1996. [Google Scholar]
- Agüero, A.; Audigié, P.; Gutiérrez, M.; Lorente, C.; Mora, J.; Rodríguez, S. Aluminide Coatings by Means of Slurry Application: A Low Cost, Versatile and Simple Technology. Coatings 2024, 14, 1243. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Pulci, G.; Pedrizzetti, G.; Pranzetti, A.; Romanelli, M.; Marra, F. Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings. Coatings 2023, 13, 1490. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Shen, X.; Liu, X.; Wang, B. Effect of Micro-Texture on Substrate Surface on Adhesion Performance of Electroless Ni–P Coating. J. Manuf. Process. 2022, 74, 296–307. [Google Scholar] [CrossRef]
- Pedraza, F.; Kennedy, A.D.; Kopecek, J.; Moretto, P. Investigation of the Microstructure of Platinum-Modified Aluminide Coatings. Surf. Coat. Technol. 2006, 200, 4032–4039. [Google Scholar] [CrossRef]
- Karunaratne, M.S.A.; Reed, R.C. Interdiffusion of the Platinum-Group Metals in Nickel at Elevated Temperatures. Acta Mater. 2003, 51, 2905–2919. [Google Scholar] [CrossRef]
- Rannou, B.; Mollard, M.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Kolarik, V.; Pedraza-Diaz, F. On the Influence of a Heat Treat for an Aluminizing Progress Based on Al Microparticles Slurry for Model Ni and Ni20Cr. Experimental and Theoretical Approaches. Defect. Diffus. Forum 2012, 323–325, 373–379. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Pulci, G.; Bartuli, C.; Marra, F. Diffusion Aluminide Coatings for Hot Corrosion and Oxidation Protection of Nickel-Based Superalloys: Effect of Fluoride-Based Activator Salts. Coatings 2021, 11, 412. [Google Scholar] [CrossRef]
- Bouchaud, B.; Rannou, B.; Pedraza, F. Slurry Aluminizing Mechanisms of Ni-Based Superalloys Incorporating an Electrosynthesized Ceria Diffusion Barrier. Mater. Chem. Phys. 2013, 143, 416–424. [Google Scholar] [CrossRef]
- Chien, A.; Gan, D.; Shen, P. Microstructures of Two-Stage Aluminized Coatings on Inconel 600. Mater. Sci. Eng. A 1996, 206, 215–224. [Google Scholar] [CrossRef]
- Benoist, J.; Girardeau, T.; Goudeau, P.; Badawi, K.F.; Traverse, A. Study by Complementary X-Ray Techniques of in-Depth Microstructure in Ni-Based Superalloys after Pt Diffusion Treatment. Surf. Coat. Technol. 2002, 161, 200–209. [Google Scholar] [CrossRef]
- Moretto, P.; Bressers, J.; Arrell, D.J. Evolution of a PtAl2 Coating on the Nickel-Base Alloy CMSX-6 Subjected to Thermo-Mechanical Fatigue. Mater. Sci. Eng. A 1999, 272, 310–320. [Google Scholar] [CrossRef]
- Watanabe, M.; Mumm, D.R.; Chiras, S.; Evans, A.G. Measurement of the Residual Stress in a Pt–Aluminide Bond Coat. Scr. Mater. 2002, 46, 67–70. [Google Scholar] [CrossRef]
- Das, D.K.; Joshi, S.V.; Singh, V. Evolution of Aluminide Coating Microstructure on Nickel-Base Cast Superalloy CM-247 in a Single-Step High-Activity Aluminizing Process. Metall. Mater. Trans. A 1998, 29, 2173–2188. [Google Scholar] [CrossRef]


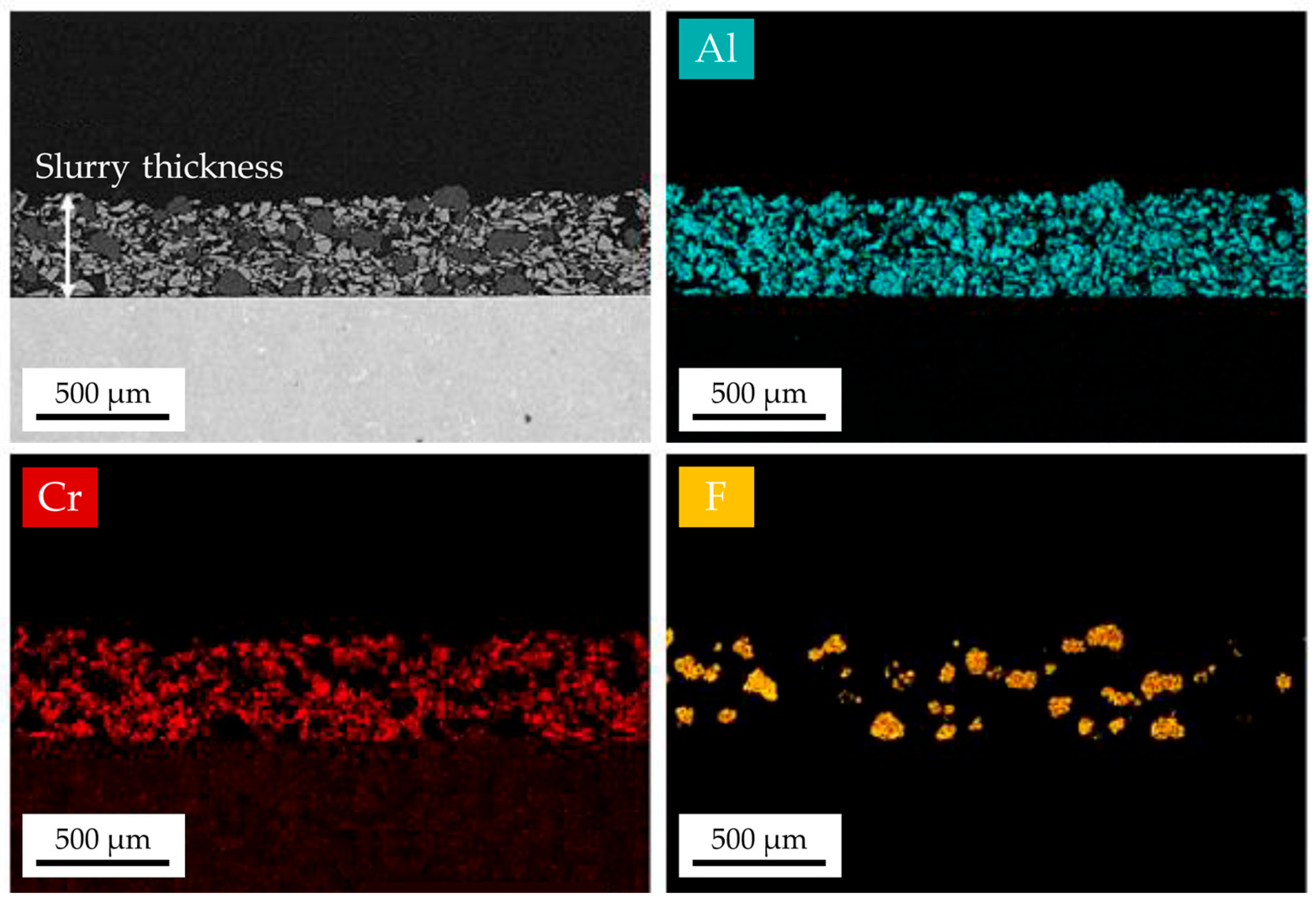
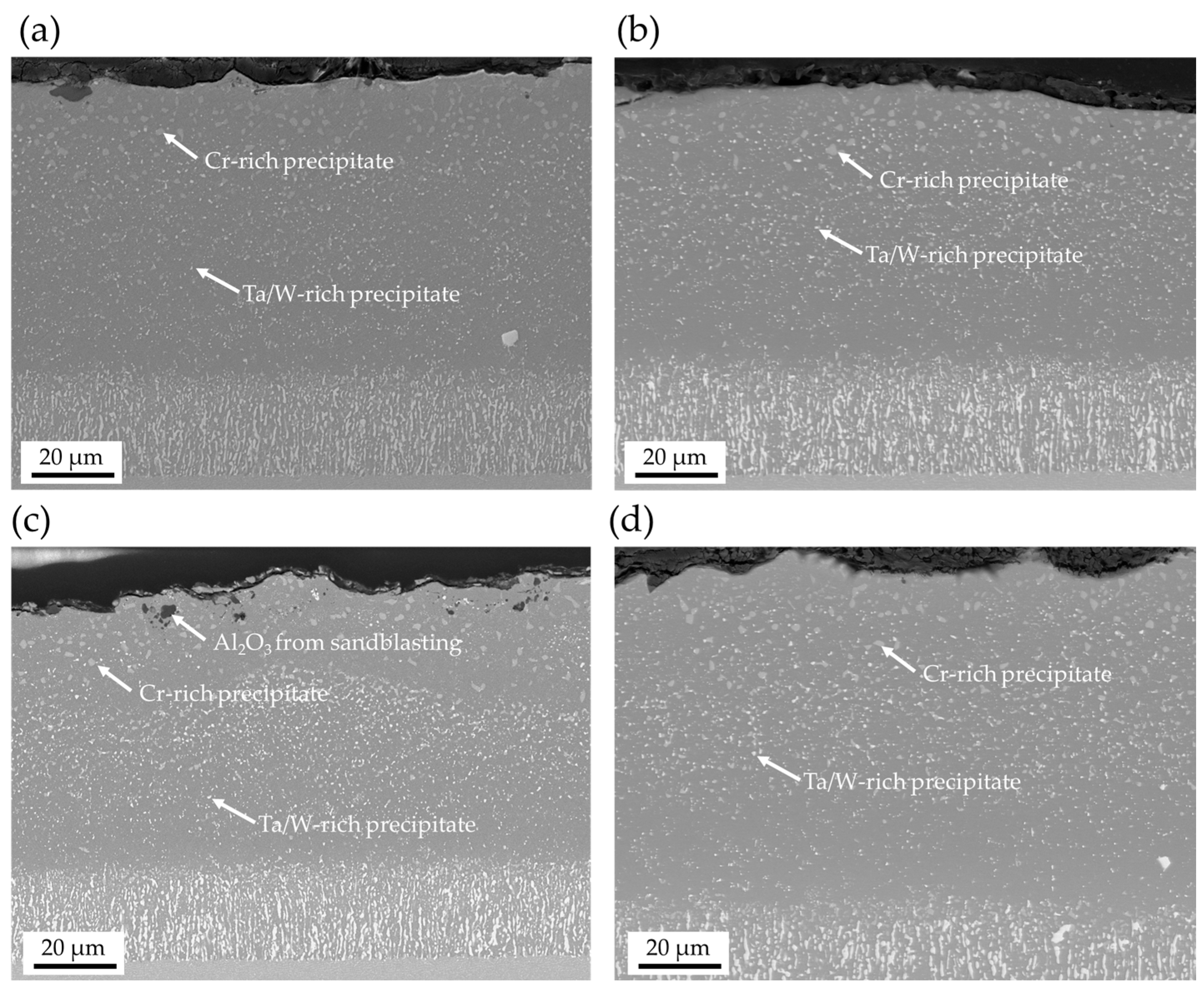


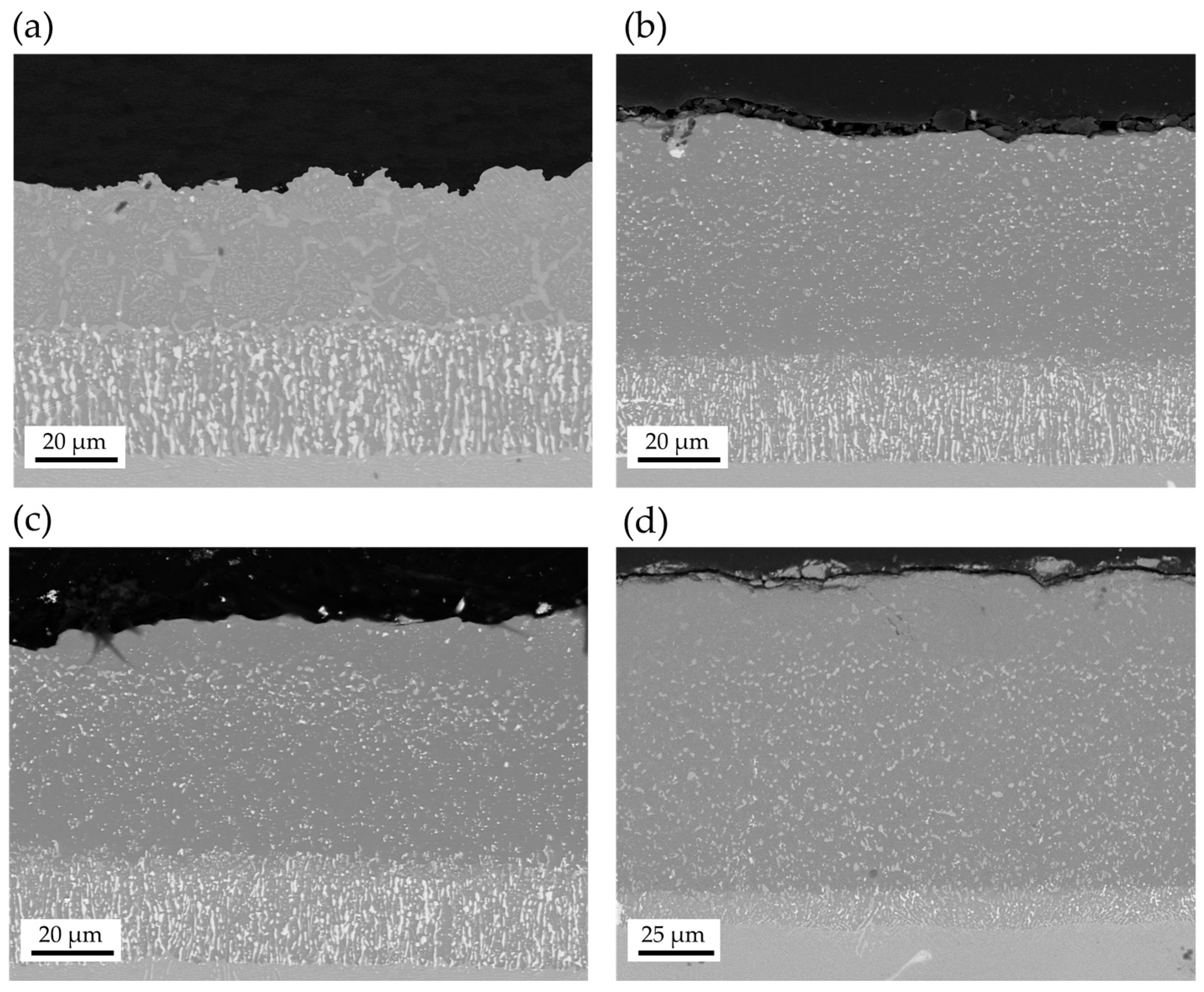
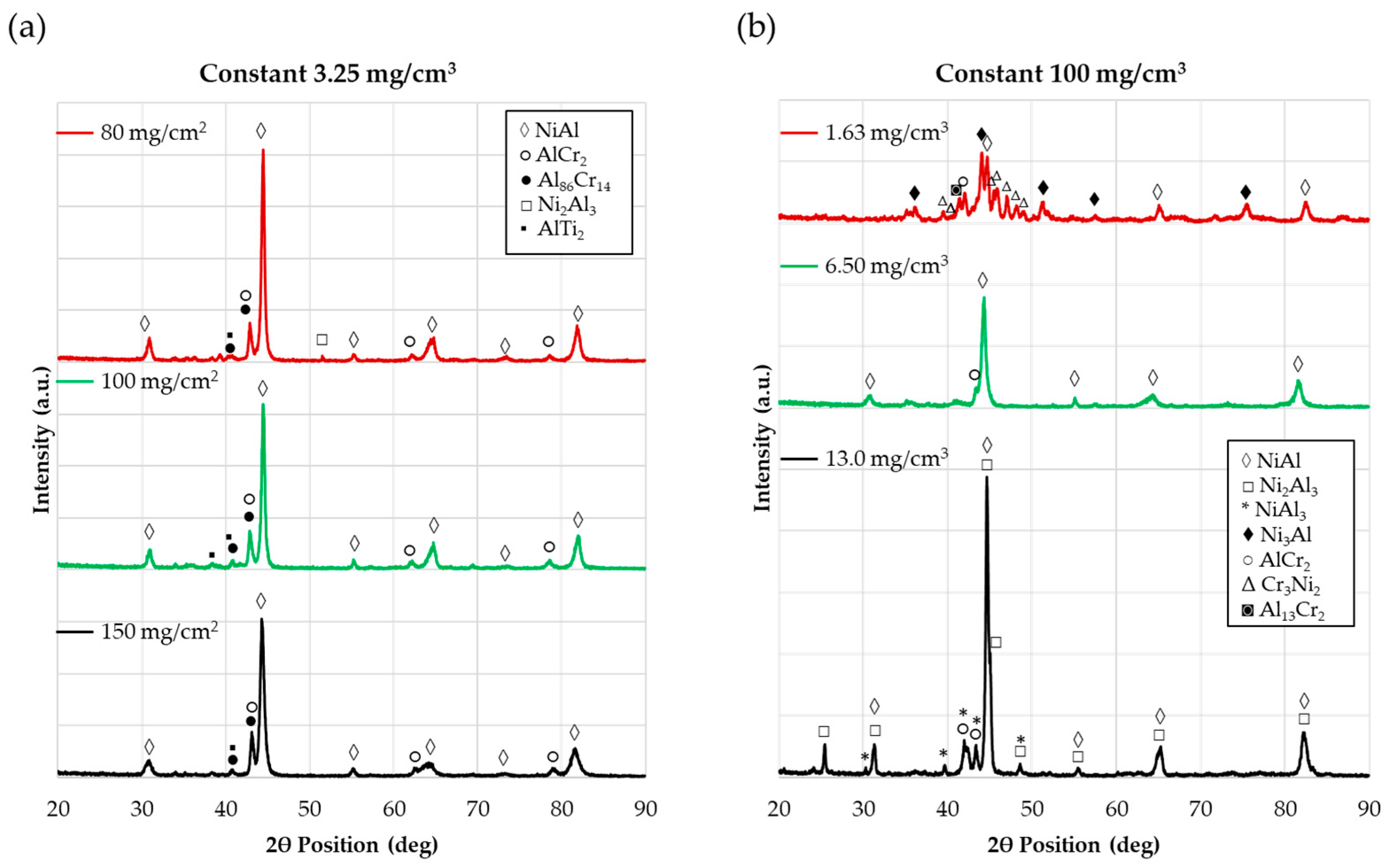

| Ni | Cr | Co | Ti | W | Al | Ta | Mo | |
|---|---|---|---|---|---|---|---|---|
| wt.% | Bal. | 10 | 7.5 | 3.5 | 6.0 | 4.2 | 4.8 | 1.5 |
| Element | Weight % | Atomic % |
|---|---|---|
| Al | 25.62 | 58.34 |
| Ta + W | 0.65 | 0.22 |
| Pt | 51.40 | 16.19 |
| Ti | 2.26 | 2.90 |
| Cr | 10.08 | 11.92 |
| Co | 2.02 | 2.10 |
| Ni | 7.96 | 8.33 |
| Element | Weight % | Atomic % |
|---|---|---|
| Al | 32.78 | 53.25 |
| Ta + W | 0.07 | 0.01 |
| Pt | 7.07 | 1.59 |
| Ti | 0.68 | 0.62 |
| Cr | 1.96 | 1.65 |
| Co | 5.60 | 4.17 |
| Ni | 51.86 | 38.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrizzetti, G.; Genova, V.; Scrinzi, E.; Bottacchiari, R.; Conti, M.; Paglia, L.; Bartuli, C. Slurry Aluminizing Mechanisms of Nickel-Based Superalloy and Applicability for the Manufacturing of Platinum-Modified Aluminide Coatings. Coatings 2025, 15, 822. https://doi.org/10.3390/coatings15070822
Pedrizzetti G, Genova V, Scrinzi E, Bottacchiari R, Conti M, Paglia L, Bartuli C. Slurry Aluminizing Mechanisms of Nickel-Based Superalloy and Applicability for the Manufacturing of Platinum-Modified Aluminide Coatings. Coatings. 2025; 15(7):822. https://doi.org/10.3390/coatings15070822
Chicago/Turabian StylePedrizzetti, Giulia, Virgilio Genova, Erica Scrinzi, Rita Bottacchiari, Marco Conti, Laura Paglia, and Cecilia Bartuli. 2025. "Slurry Aluminizing Mechanisms of Nickel-Based Superalloy and Applicability for the Manufacturing of Platinum-Modified Aluminide Coatings" Coatings 15, no. 7: 822. https://doi.org/10.3390/coatings15070822
APA StylePedrizzetti, G., Genova, V., Scrinzi, E., Bottacchiari, R., Conti, M., Paglia, L., & Bartuli, C. (2025). Slurry Aluminizing Mechanisms of Nickel-Based Superalloy and Applicability for the Manufacturing of Platinum-Modified Aluminide Coatings. Coatings, 15(7), 822. https://doi.org/10.3390/coatings15070822







