Epoxy Resin/Ionic Liquid Composite as a New Promising Coating Material with Improved Toughness and Antibiofilm Activity †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
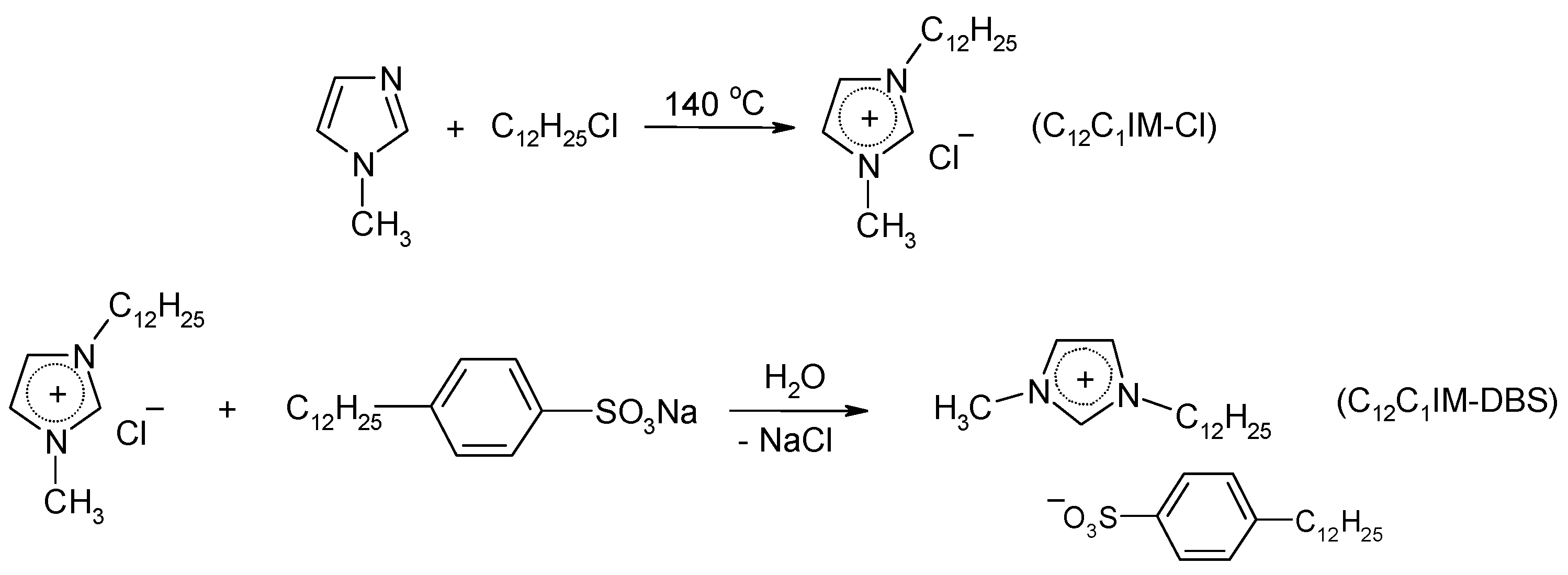
2.2. Synthesis of 1-Dodecyl-3-Methylimidazolium Dodecylbenzenesulfonate (C12C1IM-DBS)
2.3. Preparation of Modified Epoxy Coatings
2.4. Characterization of the Modified Epoxy Resin
3. Results
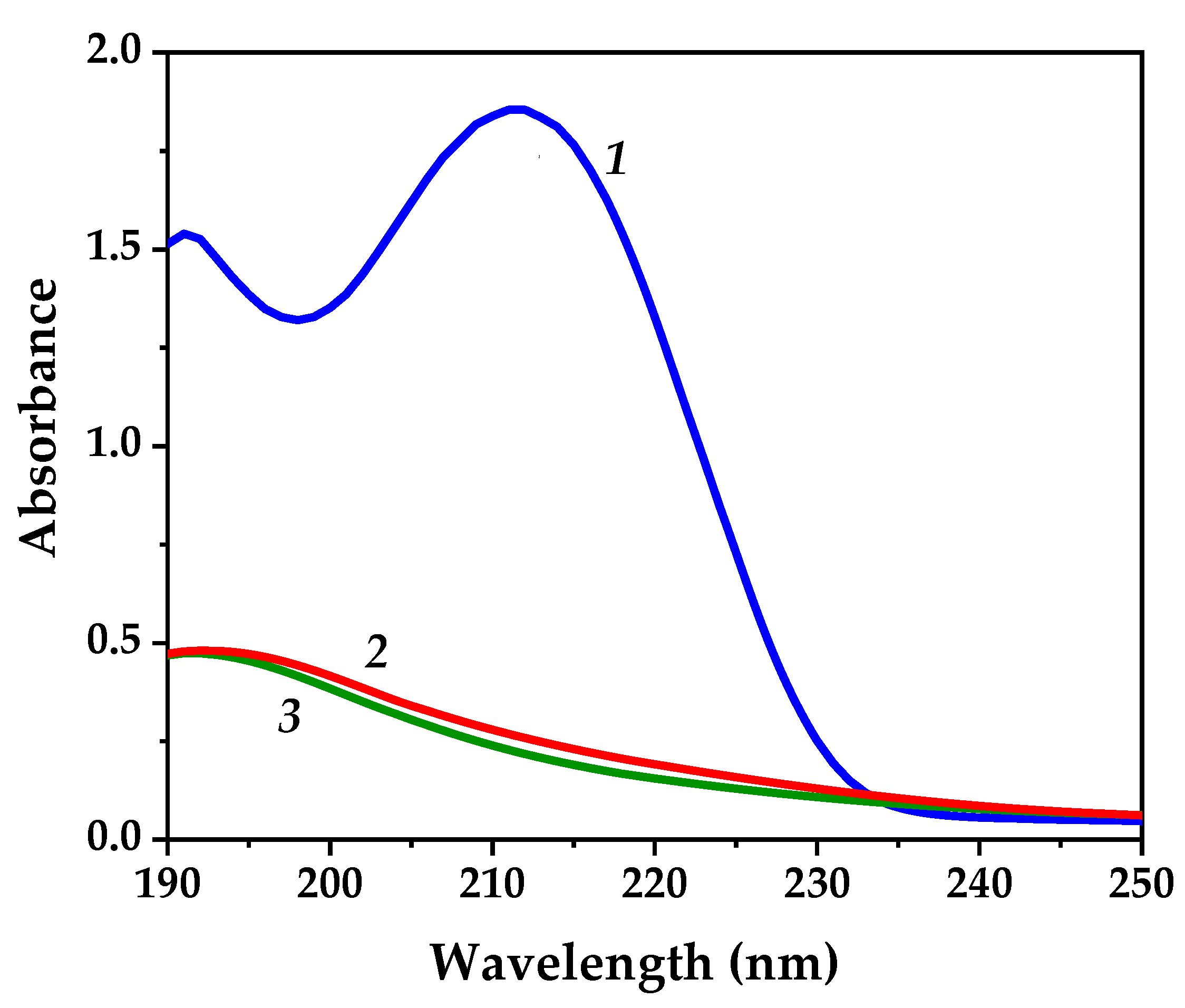
3.1. FT-IR Analysis
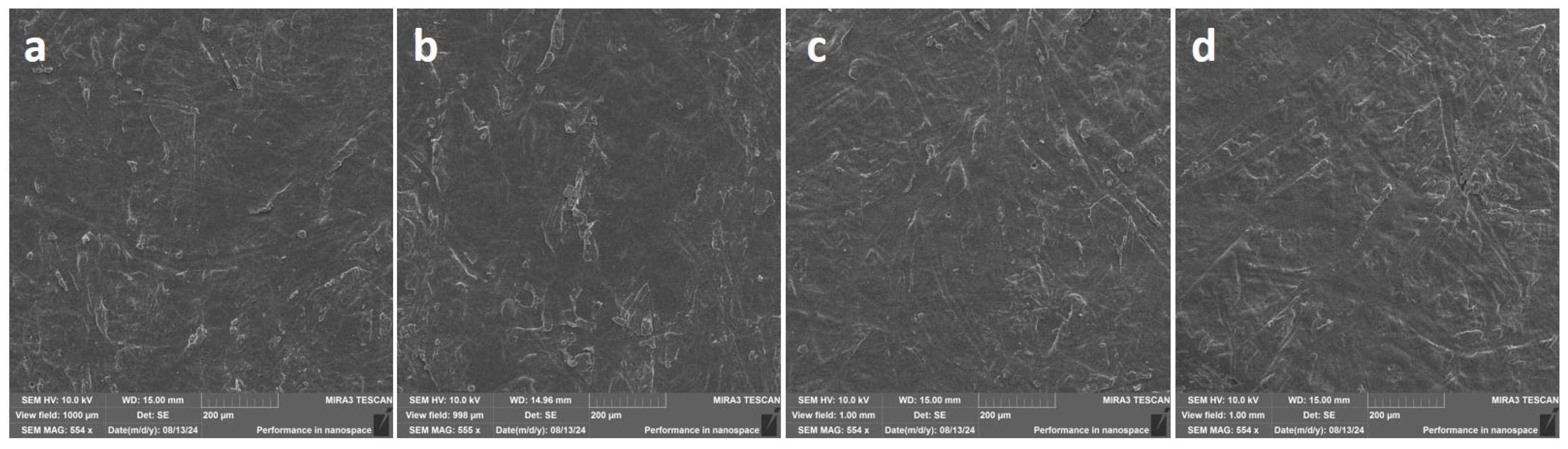
3.2. Morphological and Surface Properties of DER-331/C12C1IM-DBS Coatings
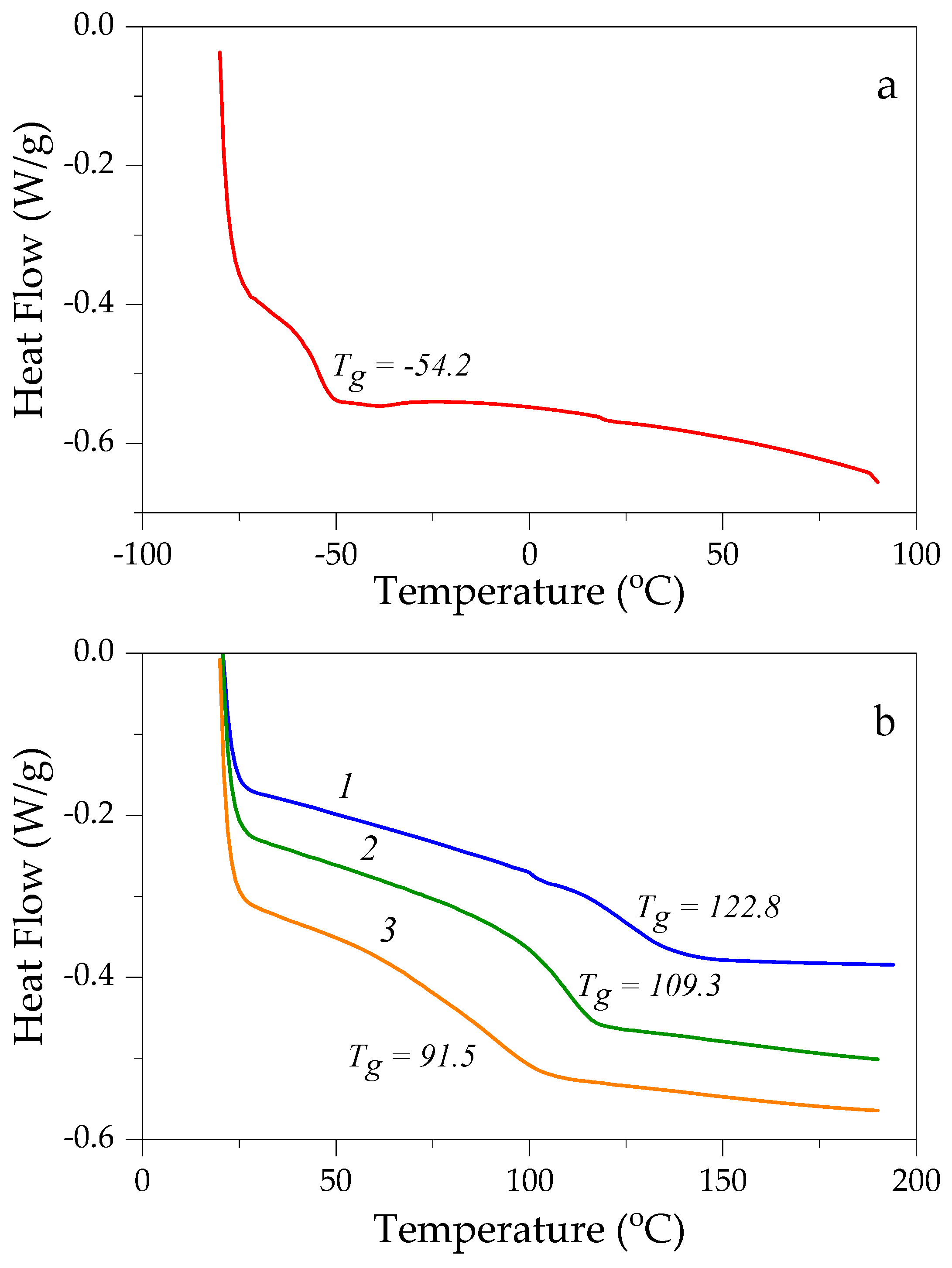
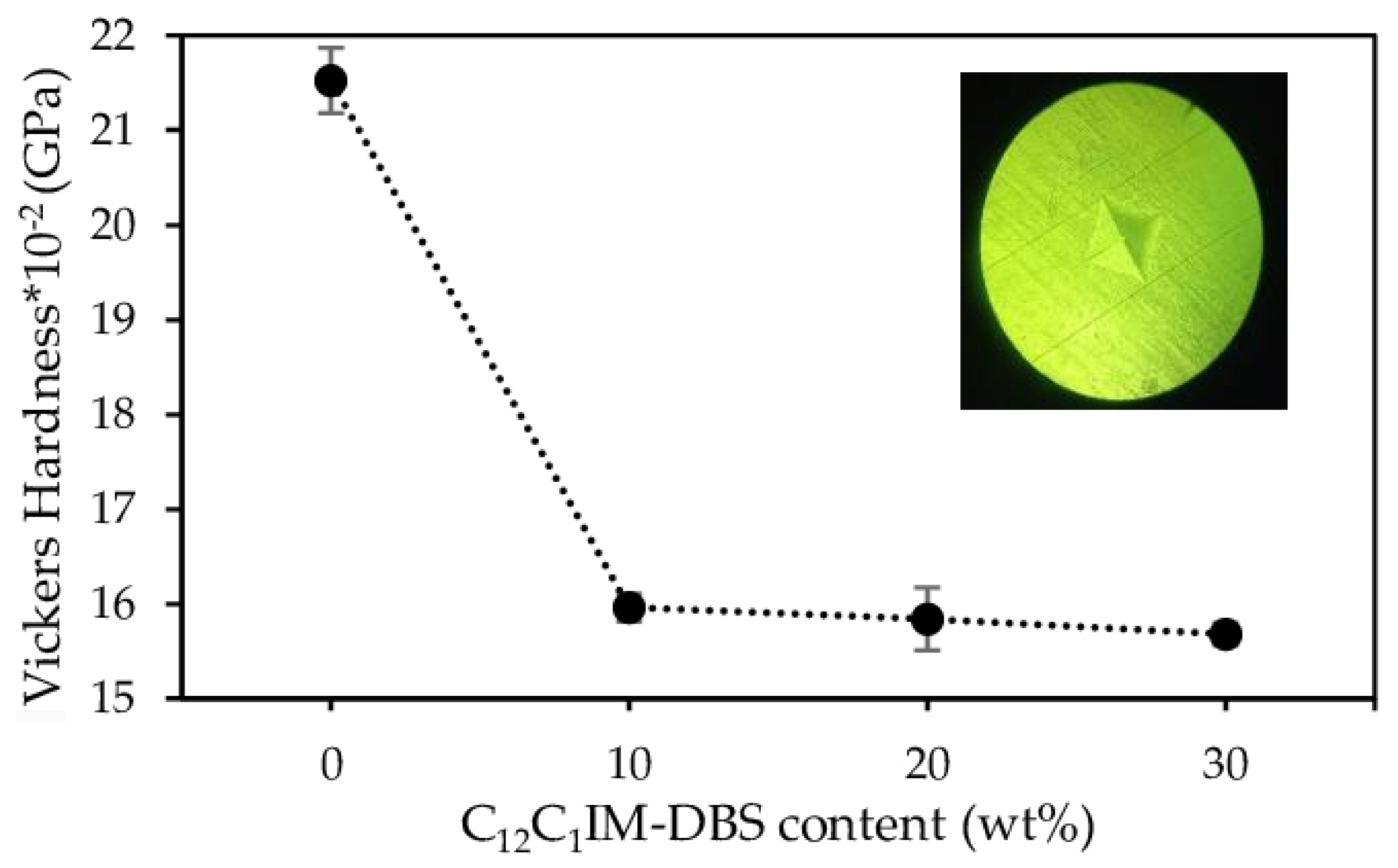
3.3. Thermal and Mechanical Properties of DER 331/C12C1IM-DBS Composites
3.4. Antibiofilm Activity of DER 331/C12C1IM-DBS Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mureşan, A.C.; Buruianã, D.L.; Istrate, G.G.; Pintilie, Ş.C. Effect of electrodeposition parameters on the morphology, topography and corrosion resistance of epoxy resin/zinc hybrid coatings. Materials 2021, 14, 1991. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Ola, S.K.; Priyanka; Dhayal, V.; Shekhawat, D.S. Recent advances in epoxy coatings for corrosion protection of steel: Experimental and modelling approach: A review. Mater. Today Proc. 2022, 62, 1658–1663. [Google Scholar] [CrossRef]
- Ramakrishnan, T.; Karthikeyan, K.R.; Tamilselvan, V.; Sivakumar, S.; Gangodkar, D.; Radha, H.R.; Singh, A.N.; Waji, Y.A. Study of various epoxy-based surface coating techniques for anticorrosion properties. Adv. Mater. Sci. Eng. 2022, 2022, 5285919. [Google Scholar] [CrossRef]
- Anwar, S.; Li, X. A review of high-quality epoxy resins for corrosion-resistant applications. J. Coat. Technol. Res. 2024, 21, 461–480. [Google Scholar] [CrossRef]
- Bogatu, N.; Buruianã, D.L.; Mureşan, A.C.; Ghisman, V.; Lupu, A.; Mardare, L.; Herbei, E.E.; Basliu, V.; Ceoromila, A.; Florescu, S. Assessment of the effectiveness of protective coatings in preventing steel corrosion in the marine environment. Polymers 2025, 17, 378. [Google Scholar] [CrossRef]
- Santhosh, S.M.; Natarajan, K. Antibiofilm activity of epoxy/Ag-TiO2 polymer nanocomposite coatings against Staphylococcus aureus and Escherichia coli. Coatings 2015, 5, 95–114. [Google Scholar]
- Faria, S.I.; Teixeira-Santos, R.; Gomes, L.C.; Silva, E.R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J.M. Experimental assessment of the performance of two marine coatings to curb biofilm formation of microfoulers. Coatings 2020, 10, 893. [Google Scholar] [CrossRef]
- Papadopoulos, N.D.; Vourna, P.; Xafacis, S.; Stefanakis, N.; Vourna, P. Marine fouling: Factors affecting biofouling and future perspectives. Int. J. Nanomater. Nanotechnol. Nanomed. 2023, 9, 010–014. [Google Scholar]
- Rao, T.S. Biofouling in industrial water systems. In Minerals Scales and Deposits; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 123–140. [Google Scholar]
- Gormley, K.; McLellan, F.; McCabe, C.; Hinon, C.; Ferris, J.; Kline, D.I.; Scott, B.E. Automated image analysis of offshore infrastructure marine biofouling. J. Mar. Sci. Eng. 2018, 6, 2. [Google Scholar] [CrossRef]
- Mathew, N.T.; Kronholm, J.; Bertilsson, K.; Despeisse, M.; Johansson, B. Environmental and economic impacts of biofouling on marine and coastal heat exchangers. In EcoDesign and Sustainability II. Sustainable Production, Life Cycle Engineering and Management; Kishita, Y., Matsumoto, M., Inoue, M., Fukushige, S., Eds.; Springer: Singapore, 2021; pp. 385–398. [Google Scholar]
- French, J.A. Control seawater intake biofouling. Oceanogr. Fish. Open Access J. 2022, 15, 555912. [Google Scholar] [CrossRef]
- Islam, M.R.; Parimalam, M.; Sumdani, M.G.; Taher, A.; Asyadi, F.; Yenn, T.W. Rheological and antimicrobial properties of epoxy-based hybrid nanocoatings. Polym. Test 2019, 81, 106202. [Google Scholar] [CrossRef]
- Kocijan, A.; Conradi, M.; Hočevar, M. The influence of surface wettability and topography on the bioactivity of TiO2/epoxy coatings on AISI 316L stainless steel. Materials 2019, 12, 1877. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, W.; Zhang, L.; Vinokurov, V.; Stavitskaya, A.; Lvov, Y. Development of marine antifouling epoxy coating enhanced with clay nanotubes. Materials 2019, 12, 4195. [Google Scholar] [CrossRef]
- Quan, X.; Wang, J.; Zhao, S.; Cai, W.; Wang, Z.; Wang, S.; Cui, X. Improved antibacterial, antifouling and corrosion protective performance of epoxy coatings with poly(m-aminophenol). Prog. Org. Coat. 2018, 14, 9–17. [Google Scholar] [CrossRef]
- Zavareh, S.; Darwishi, F.; Samandari, G. Preparation and characterization of epoxy/oregano oil as an epoxy-based coating material with both antimicrobial effect and increased toughness. J. Coat. Technol. Res. 2015, 12, 407–414. [Google Scholar] [CrossRef]
- Wu, C.; Yan, Y.; Wang, Y.; Sun, P.; Qi, R. Antibacterial epoxy composites with addition of natural Artemisia annua waste. e-Polymers 2020, 20, 262–271. [Google Scholar] [CrossRef]
- Simões, M.; Pereira, A.R.; Simões, L.C.; Cagide, F.; Borges, F. Biofilm control by ionic liquids. Drug. Discov. Today 2021, 26, 1340–1346. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Nancharaiah, Y.V. Alkylimidazolium ionic liquids as antifungal alternatives: Antibiofilm activity against Candida albicans and underlying mechanism of action. Front. Microbiol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Nancharaiah, Y.V. Alkylimidazolium ionic liquids for biofilm control: Experimental studies on controlling multispecies biofilms in natural waters. J. Mol. Liq. 2021, 336, 116859. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Rajitha, K.; Nancharaiah, Y.V. Antibiofouling potential of 1-alkyl-3-methylimidazolium ionic liquids: Studies against biofouling barnacle larvae. J. Mol. Liq. 2020, 302, 112497. [Google Scholar] [CrossRef]
- Trush, M.; Metelytsia, L.; Semenyuta, I.; Kalashnikova, L.; Papeykin, O.; Venger, I.; Tarasyuk, O.; Bodachivska, L.; Blagodatnyi, V.; Rogalsky, S. Reduced ecotoxicity and improved biodegradability of cationic biocides based on ester-functionalized pyridinium ionic liquids. Environ. Sci. Poll. Res. 2019, 26, 4878–4889. [Google Scholar] [CrossRef] [PubMed]
- Fa, K.; Liu, H.; Gong, H.; Zhang, L.; Liao, M.; Hu, X.; Ciumac, D.; Li, P.; Webster, J.; Petkov, J.; et al. In-membrane nanostructuring of cationic amphiphiles affects their antimicrobial efficacy and cytotoxicity: A comparison study between a de novo antimicrobial lipopeptide and traditional biocides. Langmuir 2022, 38, 6623–6637. [Google Scholar] [CrossRef]
- Moshynets, O.; Bardeau, J.-F.; Tarasyuk, O.; Makhno, S.; Cherniavska, T.; Dzhuzha, O.; Potters, G.; Rogalsky, S. Antibiofilm activity of polyamide 11 modified with thermally stable polymeric biocide polyhexamethylene guanidine 2-naphtalenesulfonate. Int. J. Mol. Sci. 2019, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Rogalsky, S.; Moshynets, O.; Dzhuzha, O.; Tarasyuk, O.; Hubina, A.; Darabut, A.M.; Lobko, Y.; Morozovska, I.; Protasov, O.; Bardeau, J.-F. Preparation and characterization of new antifouling coating based on alkyd paint modified with hydrophobic cationic biocide. J. Coat. Technol. Res. 2024, 21, 939–953. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Pilawka, R. Epoxy resin/ionic liquid system: The influence of imidazolium cation size and anion type on reactivity and thermomechanical properties. Ind. Eng. Chem. Res. 2012, 51, 5197–5206. [Google Scholar] [CrossRef]
- Zielinski, D.; Szpecht, A.; Pomázi, A.; Kovács, Z.; Szolnoki, B.; Pinke, B.; Toldy, A.; Smiglak, M. Multifunctional modifying systems based on ionic liquids for epoxy resin systems and composites. Appl. Sci. 2023, 13, 10661. [Google Scholar] [CrossRef]
- ISO 6272-1:2011; Paints and Varnishes—Rapid-Deformation (Impact Resistance) Tests. International Organisation for Standardisation ISO: Geneva, Switzerland, 2011.
- Baudot, A.; Moysan, J.; Payan, C.; Ylla, N.; Galy, J.; Verneret, B.; Baillard, A. Improving adhesion strength analysis by the combination of ultrasonic and mechanical tests on single lap joints. J. Adhes. 2014, 90, 555–568. [Google Scholar] [CrossRef]
- Rather, M.A.; Rather, G.M.; Pandit, S.A.; Bhat, S.A.; Bhat, M.A. Determination of cmc of imidazolium based surface active ionic liquids through probe-less UV-Vis spectrophotometry. Talanta 2015, 131, 55–58. [Google Scholar] [CrossRef]
- Yamada, T.; Tominari, Y.; Tanaka, S.; Mizuno, M. Infrared spectroscopy of ionic liquids consisting of imidazolium cations with different alkyl chain lengths and various halogen or molecular anions with and without a small amount of water. J. Phys. Chem. B. 2017, 121, 3121–3129. [Google Scholar] [CrossRef]
- Hodyna, D.; Bardeau, J.-F.; Metelytsia, L.; Riabov, S.; Kobrina, L.; Laptiy, S.; Kalashnikova, L.; Parkhomenko, V.; Tarasyuk, O.; Rogalsky, S. Efficient antimicrobial activity and reduced toxicity of 1-dodecyl-3-methylimidazolium tetrafluoroborate ionic liquid/β-cyclodextrin complex. Chem. Eng. J. 2016, 284, 1136–1145. [Google Scholar] [CrossRef]
- Xu, Z.P.; Braterman, P.S. High affinity of dodecylbenzene sulfonate for layered double hydroxide and resulting morphological changes. J. Mater. Chem. 2003, 13, 268–273. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier transform IR characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Nanocomposites of barium titanate nanoparticles embedded in thermosetting polymer matrices (novolac resins/unsaturated polyesters/epoxy resin): A comparative study. ChemEngineering 2019, 3, 12. [Google Scholar] [CrossRef]
- Abdi, J.; Izadi, M.; Bozorg, M. Improvement of anti-corrosion performance of an epoxy coating using hybrid UiO-66-NH2/carbon nanotubes nanocomposite. Sci. Rep. 2022, 12, 10660. [Google Scholar] [CrossRef]
- Reis, J.M.L.; Lima, R.P.; Vidal, S.D. Effect of rate and temperature on the mechanical properties of epoxy BADGE reinforced with carbon nanotubes. Compos. Struct. 2018, 202, 89–94. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; Wang, Z.-C.; Huang, W.; Huang, J.-J.; Wang, Y.-Y.; Zhao, Y.-Q. Improved mechanical properties of epoxy reinforced by low content of nanodiamond powder. Mater. Sci. Eng. A. 2011, 528, 7295–7300. [Google Scholar] [CrossRef]
- Francisco, W.; Ferreira, F.V.; Ferreira, E.V.; de Simone Cividanes, L.; dos Reis Coutinho, A.; Thim, G.P. Functionalization of multi-walled carbon nanotube and mechanical property of epoxy-based nanocomposite. J. Aerosp. Technol. Manag. 2015, 7, 289–293. [Google Scholar] [CrossRef]
- Park, H.; Choe, Y. Enhancing toughness and impact strength of epoxy resins by using hyperbranched polymers. Int. J. Polym. Sci. 2021, 9984174. [Google Scholar] [CrossRef]
- Wei, H.; Xia, J.; Zhou, W.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K.K. Adhesion and cohesion of epoxy-based industrial composite coatings. Composites 2020, 193, 108035. [Google Scholar] [CrossRef]
- Rudawska, A. Mechanical properties of selected epoxy adhesive and adhesive joints of steel sheets. Appl. Mech. 2021, 2, 108–126. [Google Scholar] [CrossRef]
- Hodyna, D.; Kovalishyn, V.; Rogalsky, S.; Blagodatnyi, V.; Petko, K.; Metelytsia, L. Antibacterial activity of imidazolium-based ionic liquids investigated by QSAR modeling and experimental studies. Chem. Biol. Drug. Des. 2016, 88, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Poverenov, E.; Klein, M. Formation of contact active antimicrobial surfaces by covalent grafting of quaternary ammonium compounds. Colloids Surf. B Biointerfaces 2018, 169, 195–205. [Google Scholar]
- Liu, C.; Wei, Z.; Huo, Z.; Fu, S.; Li, S.; Yang, Y.; Shi, J.; Wu, Q. Constructing a contact-active antimicrobial surface based on quarternized amphiphilic carbonaceous particles against biofilms. ACS Appl. Bio Mater. 2020, 3, 5048–5055. [Google Scholar] [CrossRef] [PubMed]


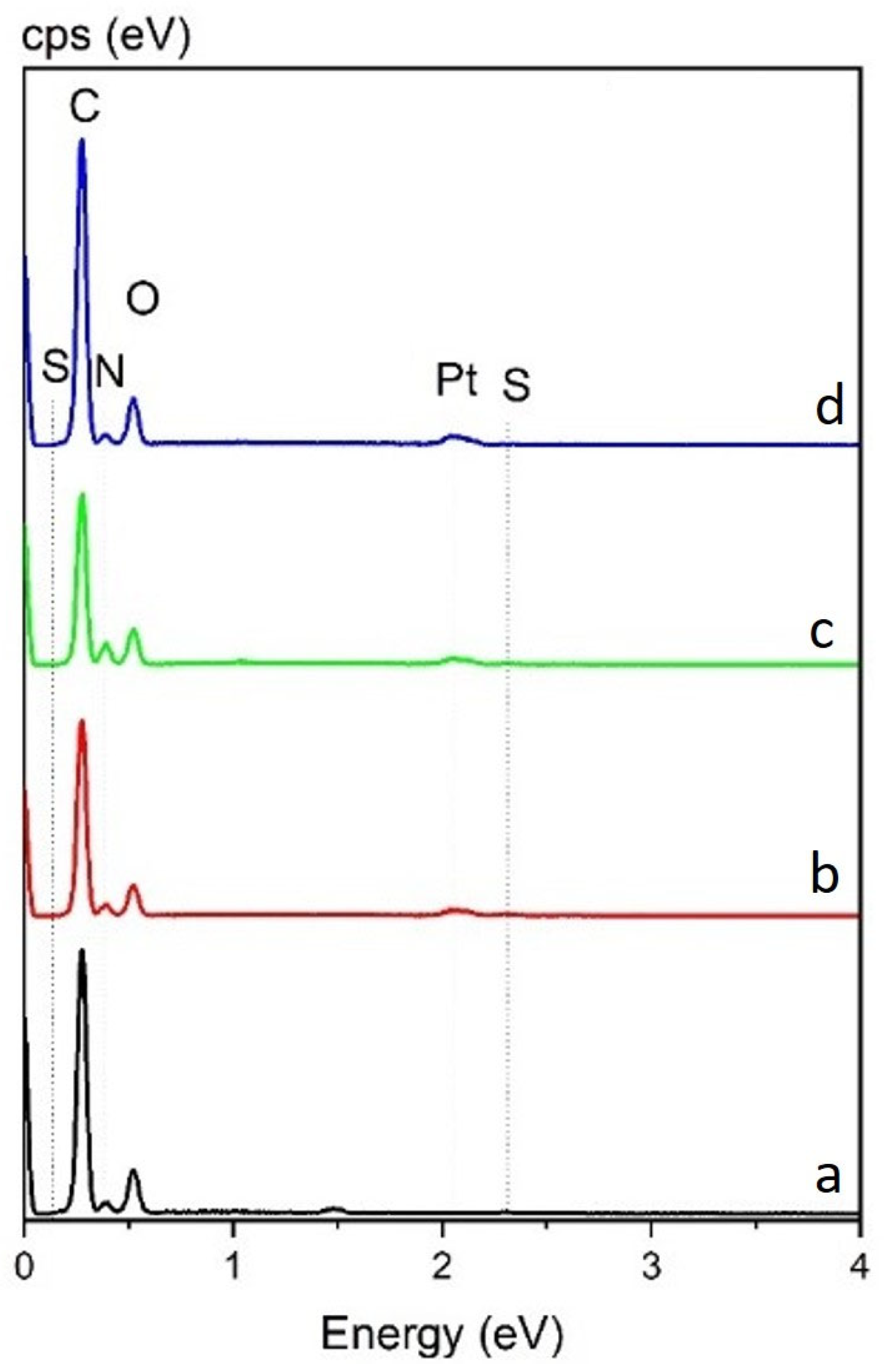
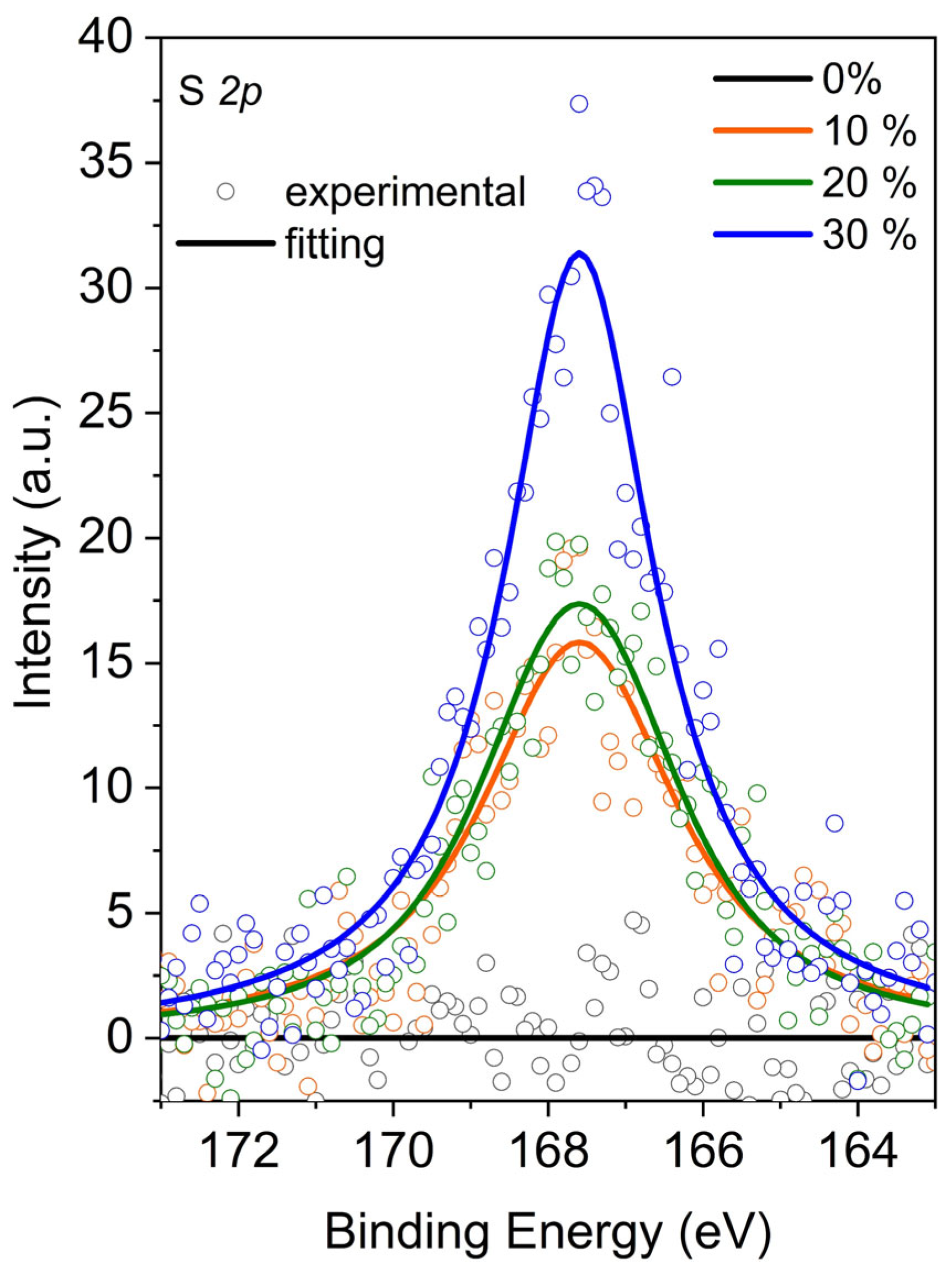

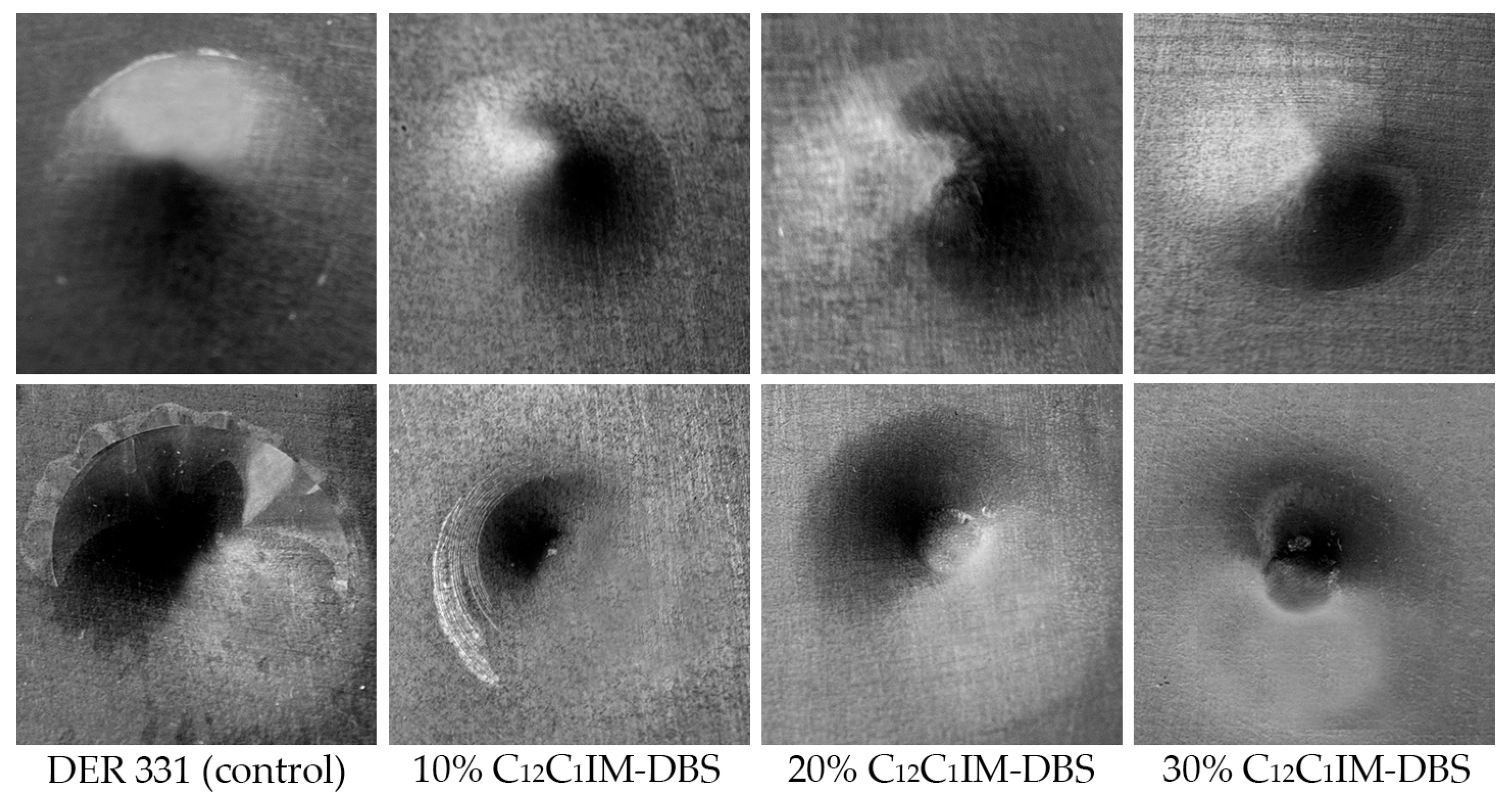


| DER 331 (Control) | DER 331/ C12C1IM–DBS (10%) | DER 331/ C12C1IM–DBS (20%) | DER 331/ C12C1IM–DBS (30%) | |
|---|---|---|---|---|
| Element | Weight % | |||
| C | 73.7 | 74.3 | 73.5 | 74.30 |
| O | 20.7 | 19.8 | 20.0 | 19.20 |
| N | 5.5 | 5.4 | 5.8 | 5.20 |
| S | 0.1 | 0.6 | 0.8 | 1.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalsky, S.; Moshynets, O.; Dzhuzha, O.; Lobko, Y.; Hubina, A.; Darabut, A.M.; Romanenko, Y.; Tarasyuk, O.; Potters, G. Epoxy Resin/Ionic Liquid Composite as a New Promising Coating Material with Improved Toughness and Antibiofilm Activity. Coatings 2025, 15, 821. https://doi.org/10.3390/coatings15070821
Rogalsky S, Moshynets O, Dzhuzha O, Lobko Y, Hubina A, Darabut AM, Romanenko Y, Tarasyuk O, Potters G. Epoxy Resin/Ionic Liquid Composite as a New Promising Coating Material with Improved Toughness and Antibiofilm Activity. Coatings. 2025; 15(7):821. https://doi.org/10.3390/coatings15070821
Chicago/Turabian StyleRogalsky, Sergiy, Olena Moshynets, Oleg Dzhuzha, Yevheniia Lobko, Anastasiia Hubina, Alina Madalina Darabut, Yaroslav Romanenko, Oksana Tarasyuk, and Geert Potters. 2025. "Epoxy Resin/Ionic Liquid Composite as a New Promising Coating Material with Improved Toughness and Antibiofilm Activity" Coatings 15, no. 7: 821. https://doi.org/10.3390/coatings15070821
APA StyleRogalsky, S., Moshynets, O., Dzhuzha, O., Lobko, Y., Hubina, A., Darabut, A. M., Romanenko, Y., Tarasyuk, O., & Potters, G. (2025). Epoxy Resin/Ionic Liquid Composite as a New Promising Coating Material with Improved Toughness and Antibiofilm Activity. Coatings, 15(7), 821. https://doi.org/10.3390/coatings15070821






