Organic–Inorganic Composite Antifouling Coatings with Complementary Bioactive Effects
Abstract
1. Introduction
2. Experiments and Methods
2.1. Materials
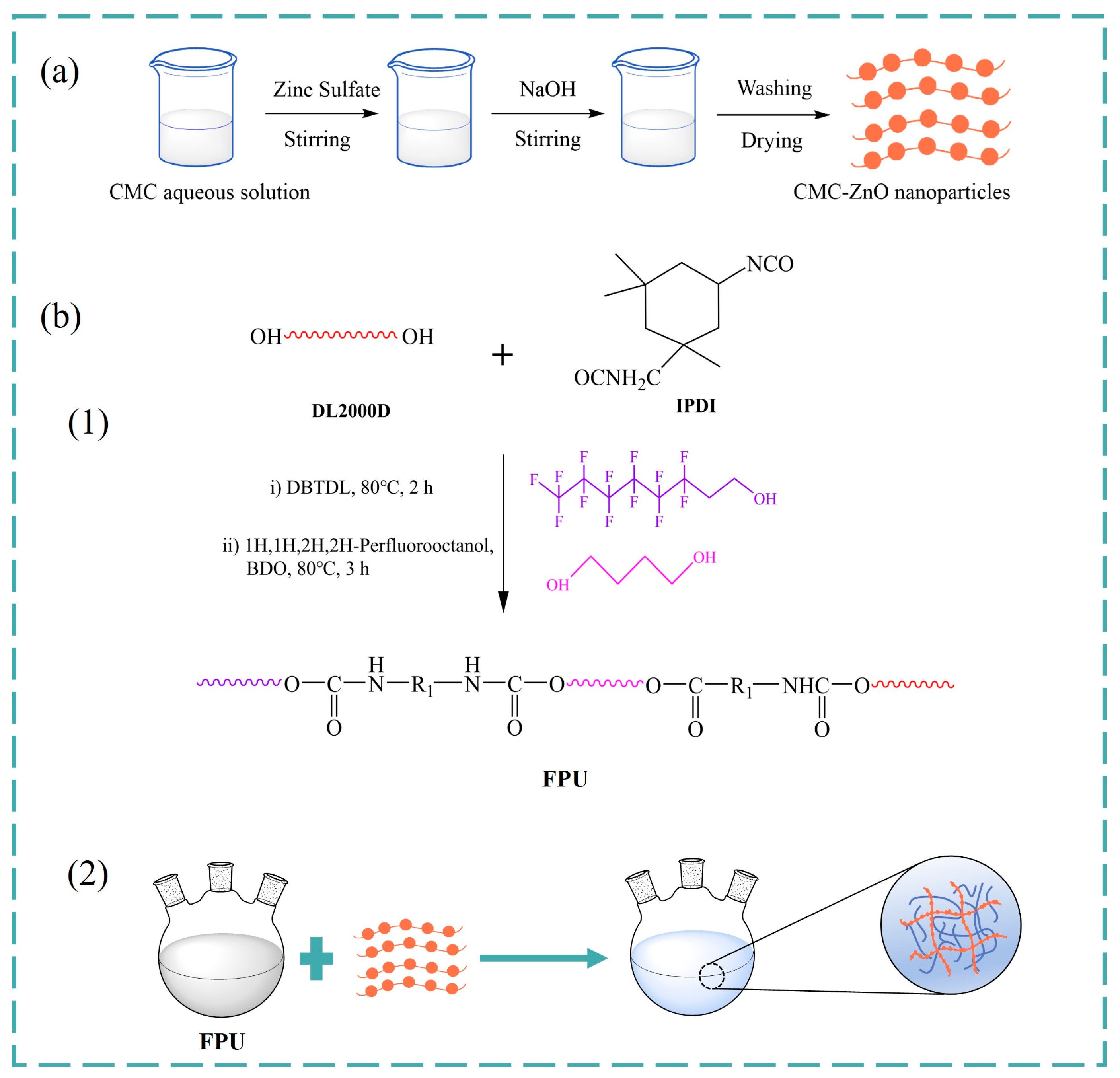
2.2. Synthesis of Carboxymethyl Chitosan-Zinc Oxide Composites (CMC-ZnO)
2.3. Preparation of FPU Antifouling Coatings
2.4. Characterization
2.4.1. Structural Characterization
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Wettability of Composite Coatings
Contact Angle Test
Water Absorption Test
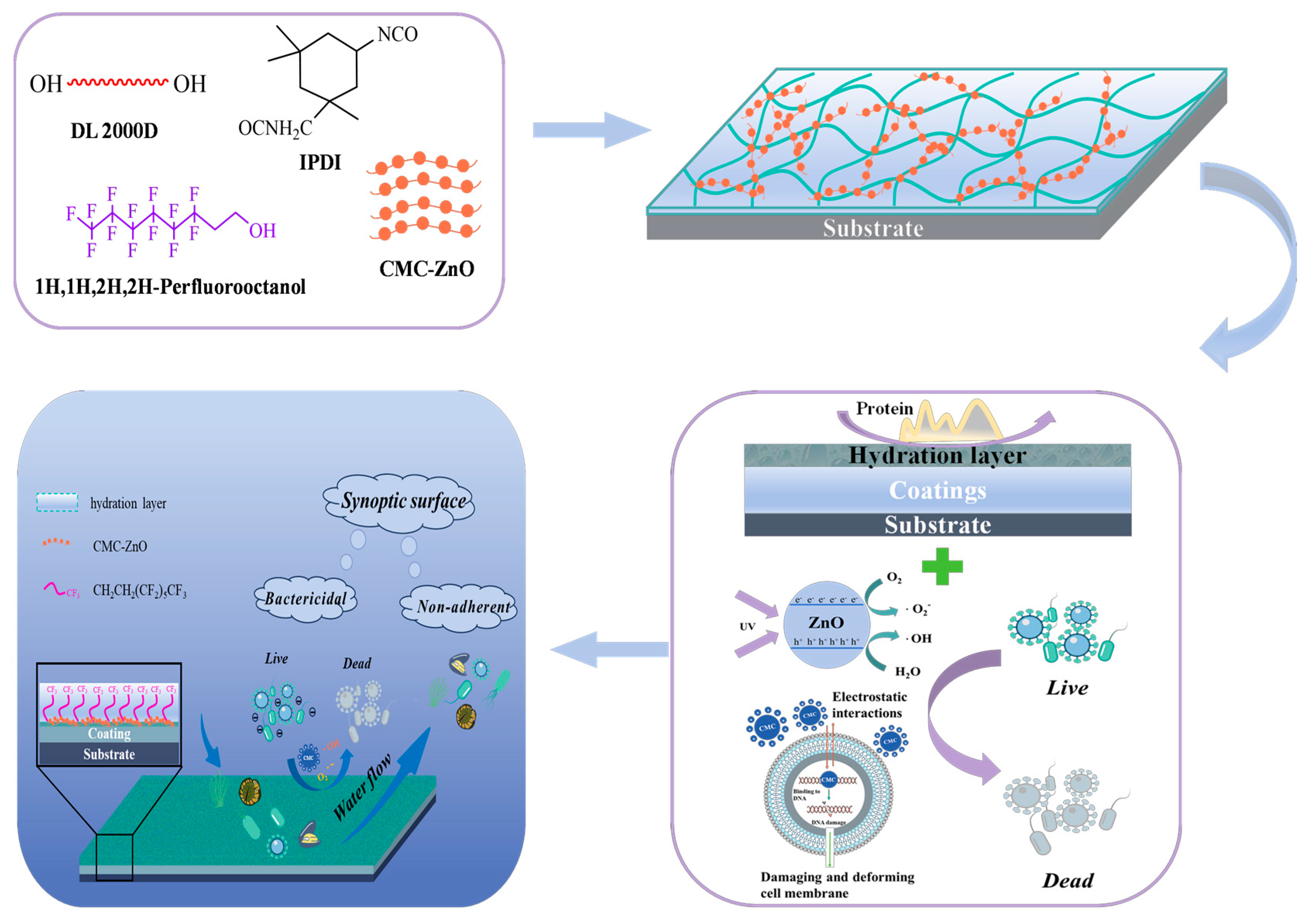
2.4.4. Anti-Fouling Experiments with Composite Coatings
Antimicrobial Experiment
Anti-Protein Adsorption Experiment
3. Results and Discussion
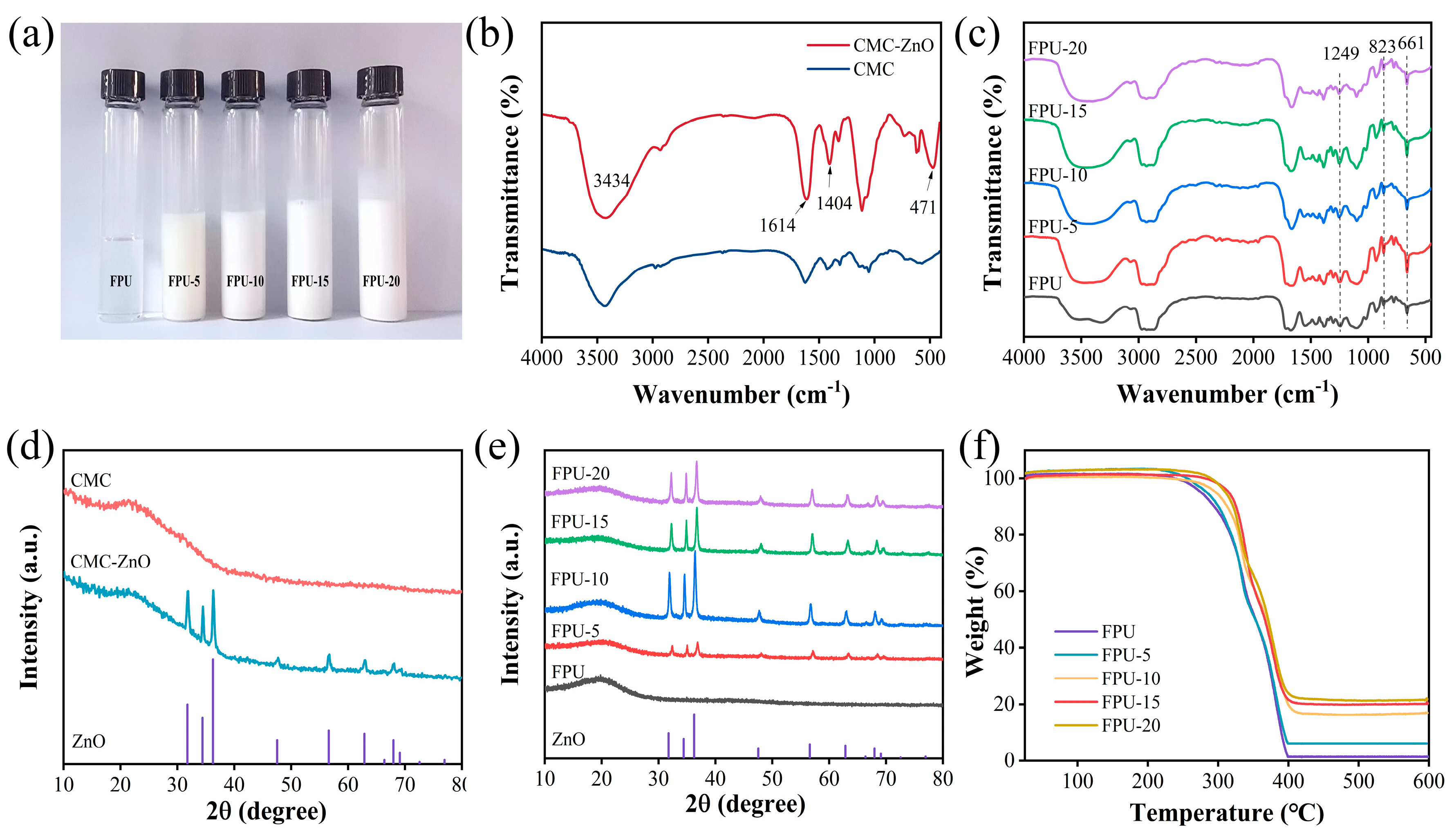
3.1. Characterizations of CMC–ZnO and Composite Coatings
3.2. Wettability of Composite Coatings
3.3. Anti-Fouling Experiments with Composite Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from Nature. Philos. Trans. Math. Phys. Eng. Sci. 2012, 370, 2381–2417. [Google Scholar] [CrossRef] [PubMed]
- Mooss, V.A.; Hamza, F.; Zinjarde, S.S.; Athawale, A.A. Polyurethane Films Modified with Polyaniline-Zinc Oxide Nanocomposites for Biofouling Mitigation. Chem. Eng. J. 2019, 359, 1400–1410. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Polymer-Based Coatings with Integrated Antifouling and Bactericidal Properties for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2021, 3, 2233–2263. [Google Scholar] [CrossRef]
- Su, Y.; Feng, T.; Feng, W.; Pei, Y.; Li, Z.; Huo, J.; Xie, C.; Qu, X.; Li, P.; Huang, W. Mussel-Inspired, Surface-Attachable Initiator for Grafting of Antimicrobial and Antifouling Hydrogels. Macromol. Rapid Commun. 2019, 40, e1900268. [Google Scholar] [CrossRef]
- Xing, C.M.; Meng, F.N.; Quan, M.; Ding, K.; Dang, Y.; Gong, Y.K. Quantitative Fabrication, Performance Optimization and Comparison of PEG and Zwitterionic Polymer Antifouling Coatings. Acta Biomater. 2017, 59, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.H.; Guo, X.J.; Ma, J.Z.; Jia, S.T. Fabrication of Robust and Antifouling Superhydrophobic Surfaces via Surface-Initiated Atom Transfer Radical Polymerization. ACS Appl. Mater. Interfaces 2015, 7, 8251–8259. [Google Scholar] [CrossRef]
- Xie, Q.; Pan, J.; Ma, C.; Zhang, G. Dynamic Surface Antifouling: Mechanism and Systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface Design for Antibacterial Materials: From Fundamentals to Advanced Strategies. Adv. Sci. Weinh. 2021, 8, e2100368. [Google Scholar] [CrossRef]
- Faÿ, F.; Champion, M.; Guennec, A.; Moppert, X.; Simon-Colin, C.; Elie, M. Biobased Anti-Adhesive Marine Coatings from Polyhydroxyalkanoates and Polysaccharides. Coatings 2023, 13, 766. [Google Scholar] [CrossRef]
- Asha, A.B.; Peng, Y.Y.; Cheng, Q.; Ishihara, K.; Liu, Y.; Narain, R. Dopamine Assisted Self-Cleaning, Antifouling, and Antibacterial Coating via Dynamic Covalent Interactions. ACS Appl. Mater. Interfaces 2022, 14, 9557–9569. [Google Scholar] [CrossRef]
- Ji, J.; Liu, N.; Tian, Y.; Li, X.; Zhai, H.; Zhao, S.; Liu, Y.; Liu, G.; Wei, Y.; Feng, L. Transparent Polyurethane Coating with Synergistically Enhanced Antibacterial Mechanism Composed of Low Surface Free Energy and Biocide. Chem. Eng. J. 2022, 445, 136716. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, H.J.; Chen, Y.; Huang, J.J. Synthesis of Self-Matting Waterborne Polyurethane Coatings with Excellent Transmittance. Polym. Int. 2018, 67, 78–84. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, W.C.; Li, Q.; Khan, M.R.; Hu, G.H.; Liu, Y.; Wu, W.; Huang, C.X.; Li, R.K.Y. Recent Advances in Superhydrophobic Polyurethane: Preparations and Applications. Adv. Colloid. Interface Sci. 2022, 303, 102644. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jin, Y.; Shi, L. Mechanically Robust and Tough Waterborne Polyurethane Films Based on Diselenide Bonds and Dual H-Bonding Interactions with Fast Visible-Light-Triggered Room-Temperature Self-Healability. Polym. Chem. 2020, 11, 5463–5474. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Peng, X.; Ma, X.; Peng, S. Structure–Property Relationships of Novel Fluorinated Polycarbonate Polyurethane Films with High Transparency and Thermal Stability. Res. Chem. Intermed. 2019, 45, 845–862. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, C.H.; Lin, W.; Ngai, T. A Facile and Effective Approach for the Synthesis of Fluorinated Waterborne Polyurethanes with Good Hydrophobicity and Antifouling Properties. Prog. Org. Coat. 2021, 159, 106405. [Google Scholar] [CrossRef]
- Zeng, S.H.; Wang, Q.M.; Chen, P.P.; Xu, Y.; Nie, W.Y.; Zhou, Y.F. Controllable Hydrolytic Stability of Novel Fluorinated Polyurethane Films by Incorporating Fluorinated Side Chains. Prog. Org. Coat. 2022, 165, 106729. [Google Scholar] [CrossRef]
- Qiao, Z.; Xu, D.; Yao, Y.; Song, S.; Yin, M.; Luo, J. Synthesis and Antifouling Activities of Fluorinated Polyurethanes. Polym. Int. 2019, 68, 1361–1366. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional Polymer–Clay Nanotube Composites with Sustained Release of Chemical Agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Recent Development in the Synthesis of Polymer Nanocomposites Based on Nano-Alumina. Prog. Polym. Sci. 2015, 51, 74–93. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Ellinas, K.; Kaprou, G.D.; Mastellos, D.C.; Tserepi, A.; Gogolides, E. Bactericidal Action of Smooth and Plasma Micro-Nanotextured Polymeric Surfaces with Varying Wettability, Enhanced by Incorporation of a Biocidal Agent. Macromol. Mater. Eng. 2021, 306, 2000694. [Google Scholar] [CrossRef]
- El Shafei, A.; Abou-Okeil, A. ZnO/Carboxymethyl Chitosan Bionano-Composite to Impart Antibacterial and UV Protection for Cotton Fabric. Carbohydr. Polym. 2011, 83, 920–925. [Google Scholar] [CrossRef]
- Lin, J.; Ding, J.; Dai, Y.; Wang, X.; Wei, J.; Chen, Y. Antibacterial Zinc Oxide Hybrid with Gelatin Coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Synthesis and Characterization of Zinc Oxide-Neem Oil-Chitosan Bionanocomposite for Food Packaging Application. Int. J. Biol. Macromol. 2015, 74, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Zarayneh, S.; Sepahi, A.A.; Jonoobi, M.; Rasouli, H. Comparative Antibacterial Effects of Cellulose Nanofiber, Chitosan Nanofiber, Chitosan/Cellulose Combination and Chitosan Alone against Bacterial Contamination of Iranian Banknotes. Int. J. Biol. Macromol. 2018, 118, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Pandey, A.C.; Verma, S.P.; Das, P.; Tewari, R.P. Efficient Water Soluble Nanostructured ZnO Grafted O-Carboxymethyl Chitosan/Curcumin-Nanocomposite for Cancer Therapy. Process Biochem. 2015, 50, 678–688. [Google Scholar] [CrossRef]
- Chen, S.-C.; Wu, Y.-C.; Mi, F.-L.; Lin, Y.-H.; Yu, L.-C.; Sung, H.-W. A Novel pH-Sensitive Hydrogel Composed of N,O-Carboxymethyl Chitosan and Alginate Cross-Linked by Genipin for Protein Drug Delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Olanipekun, E.O.; Ayodele, O.; Olatunde, O.C.; Olusegun, S.J. Comparative Studies of Chitosan and Carboxymethyl Chitosan Doped with Nickel and Copper: Characterization and Antibacterial Potential. Int. J. Biol. Macromol. 2021, 183, 1971–1977. [Google Scholar] [CrossRef]
- Ekambaram, K.; Doraisamy, M. Surface Modification of PVDF Nanofiltration Membrane Using Carboxymethylchitosan-Zinc Oxide Bionanocomposite for the Removal of Inorganic Salts and Humic Acid. Colloids Surf. A Physicochem. Eng. Asp. 2017, 525, 49–63. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/Chitosan Derivatives and Their Interactions with Microorganisms: A Comprehensive Review and Future Perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Masson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent Developments in Antibacterial and Antifungal Chitosan and Its Derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.; Fu, J.; Yu, R.; Wang, M.; Tu, J.; Wu, Q.; Zhang, X.; Niu, L.; Zhang, K. Organic–Inorganic Composite Antifouling Coatings with Complementary Bioactive Effects. Coatings 2024, 14, 741. https://doi.org/10.3390/coatings14060741
Kong H, Fu J, Yu R, Wang M, Tu J, Wu Q, Zhang X, Niu L, Zhang K. Organic–Inorganic Composite Antifouling Coatings with Complementary Bioactive Effects. Coatings. 2024; 14(6):741. https://doi.org/10.3390/coatings14060741
Chicago/Turabian StyleKong, Huixian, Jinhui Fu, Rentong Yu, Mingyu Wang, Jinchun Tu, Qiang Wu, Xuewei Zhang, Lina Niu, and Kexi Zhang. 2024. "Organic–Inorganic Composite Antifouling Coatings with Complementary Bioactive Effects" Coatings 14, no. 6: 741. https://doi.org/10.3390/coatings14060741
APA StyleKong, H., Fu, J., Yu, R., Wang, M., Tu, J., Wu, Q., Zhang, X., Niu, L., & Zhang, K. (2024). Organic–Inorganic Composite Antifouling Coatings with Complementary Bioactive Effects. Coatings, 14(6), 741. https://doi.org/10.3390/coatings14060741









