TiN-Ag Multilayer Protective Coatings for Surface Modification of AISI 316 Stainless Steel Medical Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition of TiN-Ag Films by Cathodic Arc Evaporation and Magnetron Sputtering onto SS Substrate
2.2. TEM Analysis of the SS Substrate
2.3. SEM Analysis of the TiN-Ag Coatings
2.4. Wettability Measurements of the TiN-Ag Coatings
2.5. Cells’ Viability on the Surface of TiN-Ag Coatings
2.6. Osteogenic and Chondrogenic Differentiation of DPSCs in the Presence of TiN-Ag Coatings
3. Results
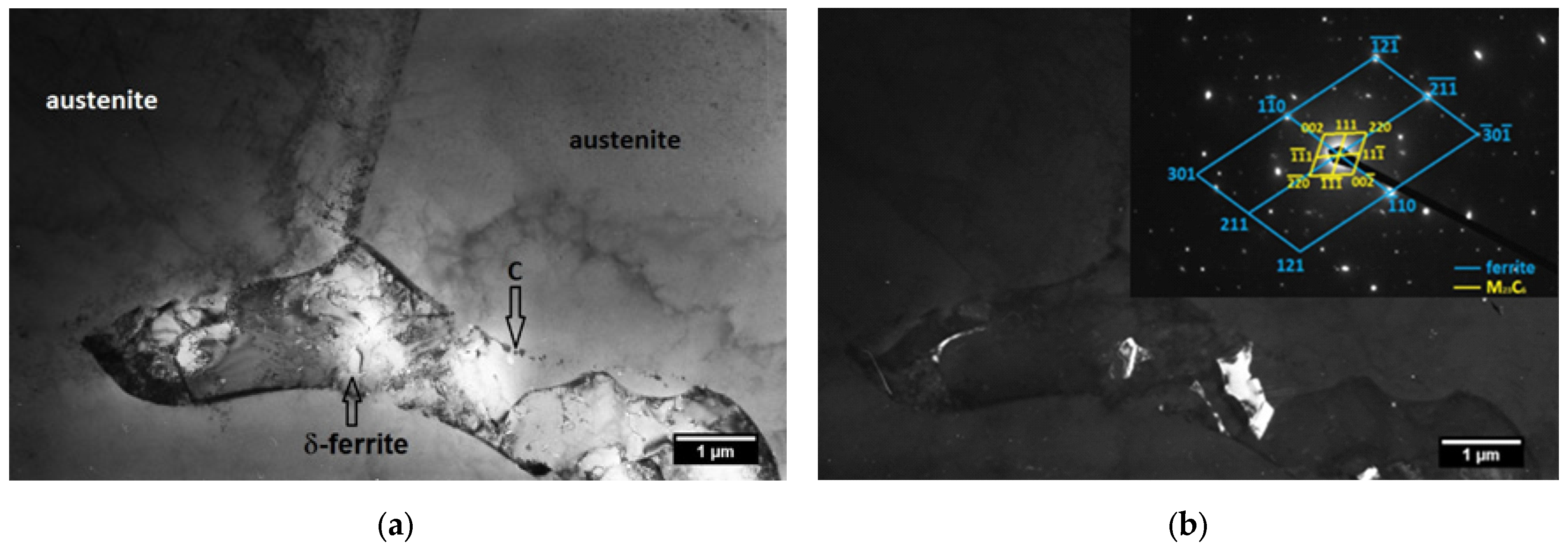
3.1. TEM Analysis
3.2. SEM Analysis
3.3. Wettability
3.4. Cells Viability
3.5. Osteogenic and Chondrogenic Differentiation
3.6. Immunocytochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | Stainless steel. |
| DC | Direct current. |
| TEM | Transmission electron microscope. |
| SEM | Scanning electron microscope. |
| EDS | Electron dispersive spectroscopy. |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
| DPSCs | Dental pulp stem cells. |
| PVD | Plasma vapour deposition. |
| PBS | Phosphate-buffered saline. |
| OCN | Osteocalcin. |
| BMP2 | Bone morphogenetic protein 2. |
| BSA | Bovine serum albumin. |
Appendix A
| Blue Motive | Yellow Motive | ||||||
|---|---|---|---|---|---|---|---|
| Diffraction Pattern | Table Values of Ferrite | Diffraction Pattern | Table Values of Carbide M23C6 | ||||
| No. | dhkl (10−10 m) | dhkl (10−10 m) | (h k l) | No. | dhkl (10−10 m) | dhkl (10−10 m) | (h k l) |
| 1. | 2.04 | 2.02 | 1. | 6.20 | 6.15 | ||
| 2. | 1.20 | 0.91 | 2. | 6.20 | 6.15 | ||
| 3. | 0.92 | 0.77 | 3. | 3.76 | 3.77 | ||
| 4. | 1.20 | 0.91 | 4. | 5.45 | 5.32 | ||
| φ1/2 | 71° | φ1-10/211 | 73.2° | φ1/2 | 70° | φ111/11-1 | 70.5° |
References
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Braic, M.; Zamfir, S.; Balaceanu, M.; Braic, V.; Pavelescu, G.; Zamfir, A.; Vladescu, A. Corrosion resistance of TiN coated 316L stainless steel in artificial physiological solution. J. Optoelectron. Adv. Mater. 2003, 5, 503–510. [Google Scholar]
- Bekmurzayeva, A.; Duncanson, W.J.; Azavedo, H.A.; Kanayeva, D. Surface Modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Mulyani, E.; Yusuf, Y. Morphological, mechanical and antibacterial properties of Ti-Cu-N thin films deposited by sputtering DC. Heliyon 2023, 10, e17170. [Google Scholar] [CrossRef]
- Staszuk, M.; Pakuła, D.; Reimann, Ł.; Kloc-Ptaszna, A.; Pawlyta, M.; Kříž, A. Structure and Properties of TiO2/nanoTiO2 Bimodal Coatings Obtained by a Hybrid PVD/ALD Method on 316L Steel Substrate. Materials 2021, 14, 4369. [Google Scholar] [CrossRef]
- Wang, Q.; Akhavan, B.; Jing, F.; Cheng, D.; Sun, H.; Xie, D.; Leng, Y.; Bilek, M.M.; Huang, N. Catalytic Formation of Nitric Oxide Mediated by Ti-Cu Coatings Provides Multifunctional Interfaces for Cardiovascular Applications. Adv. Mater. Interfaces 2018, 5, 1701487. [Google Scholar] [CrossRef]
- Braceras, I.; Brizuela, M.; Álvarez, N.; Martínez Van Geeteruyen, M.; Azkona, I. TiN-Ag as an antimicrobial and wear resistant coating. Biotribology 2021, 28, 100192. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, M.; Dong, L.; Han, X.; Li, D. Influence of Ag/Ca ratio on the osteoblast growth and antibacterial activity of TiN coatings on Ti-6Al-4V by Ag and Ca ion implantation. Surf. Coat. Technol. 2020, 403, 126415. [Google Scholar] [CrossRef]
- Ziebert, C.; Ulrich, S. Hard multilayer coatings containing TiN and/or ZrN: A review and recent progress in their nanoscale characterization. J. Vac. Sci. Technol. A 2006, 24, 554–583. [Google Scholar] [CrossRef]
- Zhao, M.; Gong, H.; Ma, M.; Dong, L.; Huang, M.; Wan, R.; Gu, H.; Kang, Y.; Li, D. A comparative antibacterial activity and cytocompatibility for different top layers of TiN, Ag or TiN-Ag on nanoscale TiN/Ag multilayers. Appl. Surf. Sci. 2019, 473, 334–342. [Google Scholar] [CrossRef]
- Jokanović, V.; Bundaleski, N.; Petrović, B.; Ferarra, M.; Jokanović, B.; Živković, S.; Nasov, I. Detailed phisyco-chemical characterization of the multilayered thin films based on titanium oxynitride and copper doped titanium nitride obtained by different PVD techniques. Vacuum 2022, 195, 110708. [Google Scholar] [CrossRef]
- Petrović, B.; Ilić, B.; Biočanin, B.; Potran, M.; Nikitović, A.; Nasov, I.; Jokanović, V. Physico-chemical and biological properties of titanium nitride doped with copper and silver by combined methods of cathodic arc evaporation and magnetron sputtering. Int. J. Surf. Sci. Eng. 2024, 18, 245–259. [Google Scholar] [CrossRef]
- Čolović, B.; Kisić, D.; Jokanović, B.; Rakočević, Z.; Nasov, I.; Petkoska, A.T.; Jokanović, V. Wetting Properties of Titanium Oxides, Oxynitrides and Nitrides Obtained by DC and Pulsed Magnetron Sputtering and Cathodic Arc Evaporation. Mater. Sci. Pol. 2019, 37, 173–181. [Google Scholar] [CrossRef]
- Basgul, C.; MacDonald, D.W.; Klein, G.R.; Piuzzi, N.S.; Kurtz, S.M. Retrieval Analysis of Titanium Nitride Coatings for Orthopaedic Implants. J. Arthroplast. 2024, 39 (Suppl. 1), S272–S279. [Google Scholar] [CrossRef]
- de Arruda, A.C.S.; Mansano, R.D.; Ordonez, N.; Ruas, R.; Durrant, S.F. Ag Behavior on TiN Thin Films for Decorative Coatings. Coatings 2024, 14, 322. [Google Scholar] [CrossRef]
- Vukovic, M.; Lazarevic, M.; Mitic, D.; Jaksic Karisik, M.; Ilic, B.; Andric, M.; Jevtic, B.; Roganovic, J.; Milasin, J. Acetylsalicylic-acid (ASA) regulation of osteo/odontogenic differentiation and proliferation of human dental pulp stem cells (DPSCs) in vitro. Arch. Oral Biol. 2022, 144, 105564. [Google Scholar] [CrossRef]
- Chawla, J.S.; Gall, D. Epitaxial Ag(001) grown on MgO(001) and TiN(001): Twinning, surface morphology, and electron surface scattering. J. Appl. Phys. 2012, 111, 043708. [Google Scholar] [CrossRef]
- Prieto, J.E.; Markov, I. Stranski–Krastanov mechanism of growth and the effect of misfit sign on quantum dots nucleation. Surf. Sci. 2017, 664, 172–184. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Ma, D.; Jiang, X.; Xie, D.; Leng, Y. Influence of Interlayers on Adhesion Strength of TiN Film on Mg Alloy. Coatings 2024, 14, 121. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of Wettability and Surface Functional Groups on Protein Adsorption and Cell Adhesion Using Well-Defined Mixed Self-Assembled Monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Alazzam, A. Micropatterning of Cells via Adjusting Surface Wettability Using Plasma Treatment and Graphene Oxide Deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, C.P.; Keerthi Krishnan, K.; Sudeep, U.; Ramachandran, K.K. Osteogenic and Antibacterial Properties of TiN-Ag Coated Ti-6Al-4V Bioimplants with Polished and Laser Textured Surface Topography. Surf. Coat. Technol. 2023, 474, 130058. [Google Scholar] [CrossRef]
- He, P.; Zhang, Q.; Motiwala, F.I.; Shanti, R.M.; Chang, B.M.; Le, A.D. Potential application of dental stem cells in regenerative reconstruction of oral and maxillofacial tissues: A narrative review. Front. Oral Maxillofac. Med. 2022, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiong, W.; Li, J.; Feng, H.; Jing, S.; Liu, Y.; Zhou, H.; Li, D.; Fu, D.; Xu, C.; et al. Application of dental pulp stem cells for bone regeneration. Front. Med. 2024, 11, 1339573. [Google Scholar] [CrossRef]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J. Bone Miner. Metab. 2015, 33, 371–382. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Cortez de Santanna, J.P.; Frisene, I.; Gazarini, J.P.; Gomes Pinheiro, C.C.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Franco Bueno, D. Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration. Tissue Eng. Part. B Rev. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef]
- Wan, R.; Chu, S.; Wang, X.; Lei, L.; Tang, H.; Hu, G.; Dong, L.; Li, D.; Gu, H. Study on the osteogenesis of rat mesenchymal stem cells and the long-term antibacterial activity of Staphylococcus epidermidis on the surface of silver-rich TiN/Ag modified titanium alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3008–3021. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Q.; Lin, X.; Hu, N.; Liao, J.Y.; Lin, L.B.; Zhao, C.; Hu, Z.M.; Liang, X.; Xu, W.; et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016, 366, 101–111. [Google Scholar] [CrossRef]
- Damle, A.; Sundaresan, R.; Rajwade, J.M.; Srivastava, P.; Naik, A. A concise review on implications of silver nanoparticles in bone tissue engineering. Biomater. Adv. 2022, 141, 213099. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, B.; Mitić, D.; Miličić Lazić, M.; Lazarević, M.; Trajkovska Petkoska, A.; Nasov, I.; Živković, S.; Jokanović, V. TiN-Ag Multilayer Protective Coatings for Surface Modification of AISI 316 Stainless Steel Medical Implants. Coatings 2025, 15, 820. https://doi.org/10.3390/coatings15070820
Petrović B, Mitić D, Miličić Lazić M, Lazarević M, Trajkovska Petkoska A, Nasov I, Živković S, Jokanović V. TiN-Ag Multilayer Protective Coatings for Surface Modification of AISI 316 Stainless Steel Medical Implants. Coatings. 2025; 15(7):820. https://doi.org/10.3390/coatings15070820
Chicago/Turabian StylePetrović, Božana, Dijana Mitić, Minja Miličić Lazić, Miloš Lazarević, Anka Trajkovska Petkoska, Ilija Nasov, Slavoljub Živković, and Vukoman Jokanović. 2025. "TiN-Ag Multilayer Protective Coatings for Surface Modification of AISI 316 Stainless Steel Medical Implants" Coatings 15, no. 7: 820. https://doi.org/10.3390/coatings15070820
APA StylePetrović, B., Mitić, D., Miličić Lazić, M., Lazarević, M., Trajkovska Petkoska, A., Nasov, I., Živković, S., & Jokanović, V. (2025). TiN-Ag Multilayer Protective Coatings for Surface Modification of AISI 316 Stainless Steel Medical Implants. Coatings, 15(7), 820. https://doi.org/10.3390/coatings15070820











