A Methodology for Lacquer Gilding Restoration of Sandstone Sculptures: A Multidisciplinary Approach Combining Material Characterization and Environmental Adaptation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sandstone Sculpture
2.2. Restoration Materials
2.3. Preparation of the NHL2/SiO2/SF016 Composite Emulsion
2.4. Preparation of Lacquer Materials
2.5. Ground Layer Compatibility Test
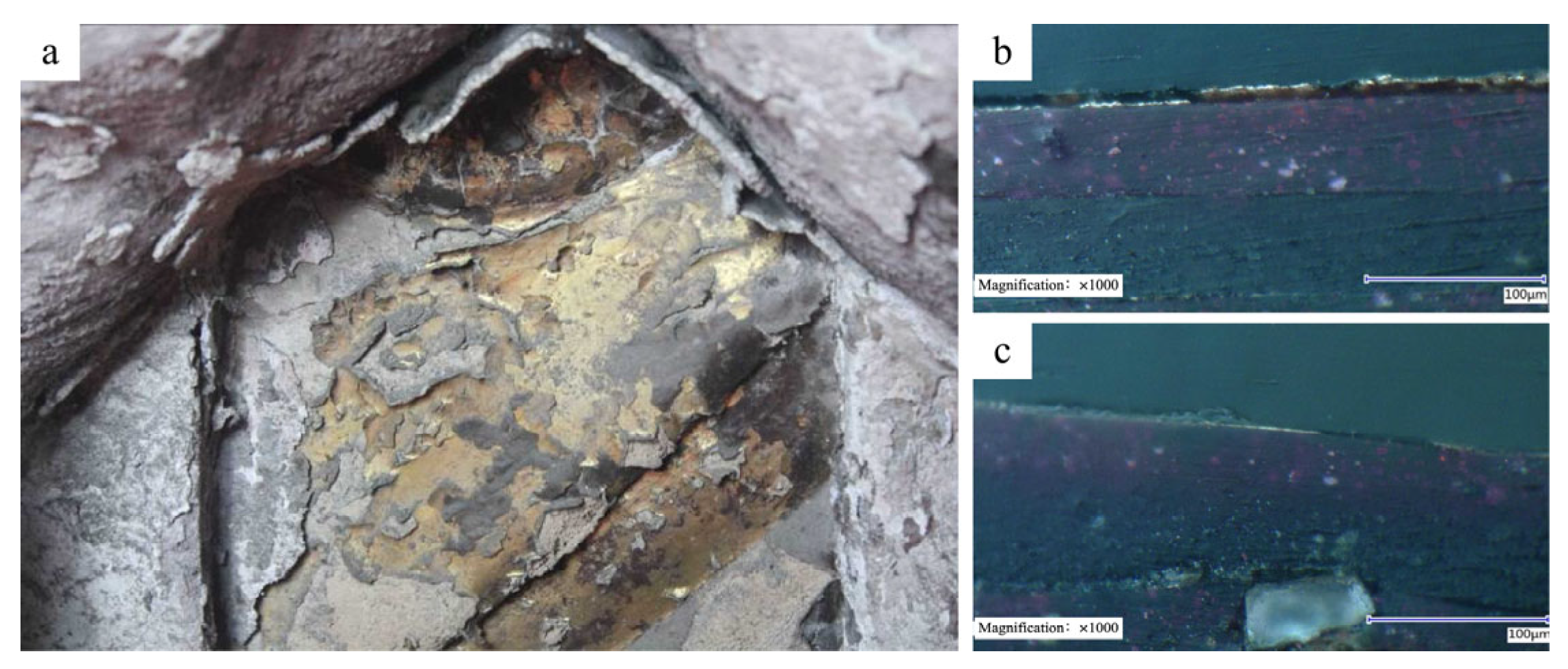
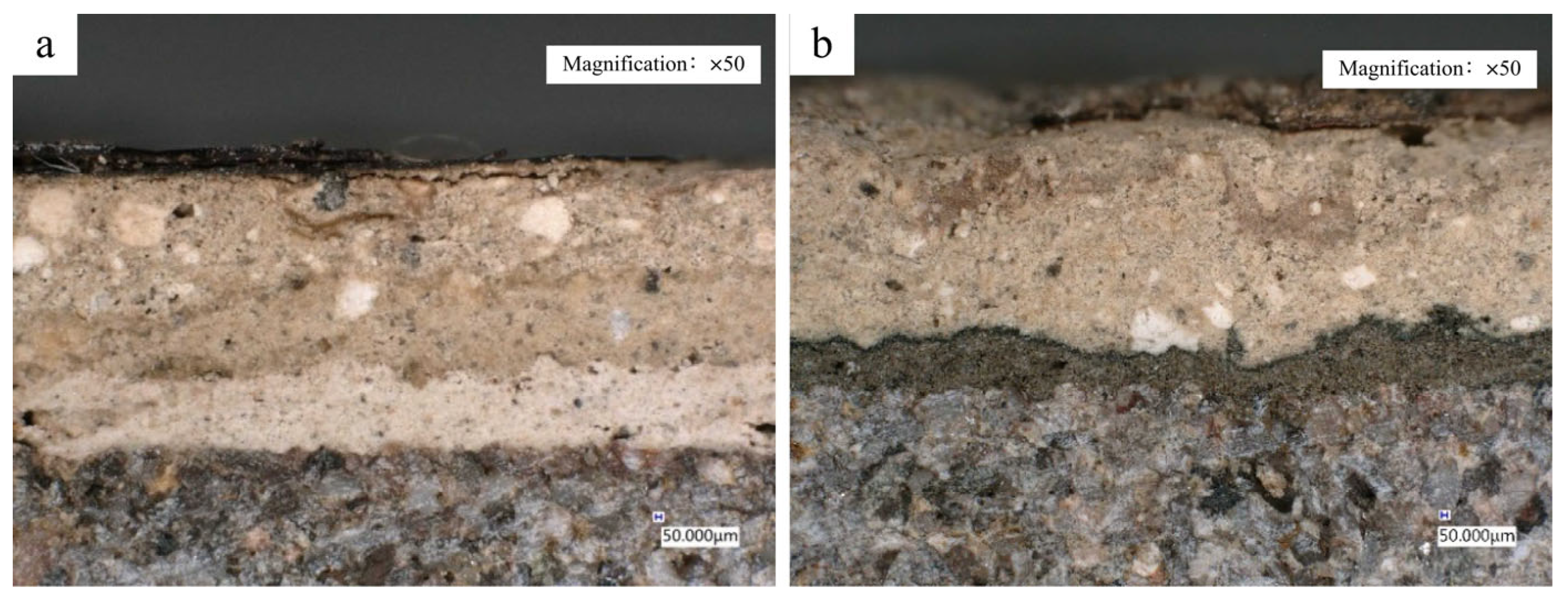
2.6. 3D Digital Microscope
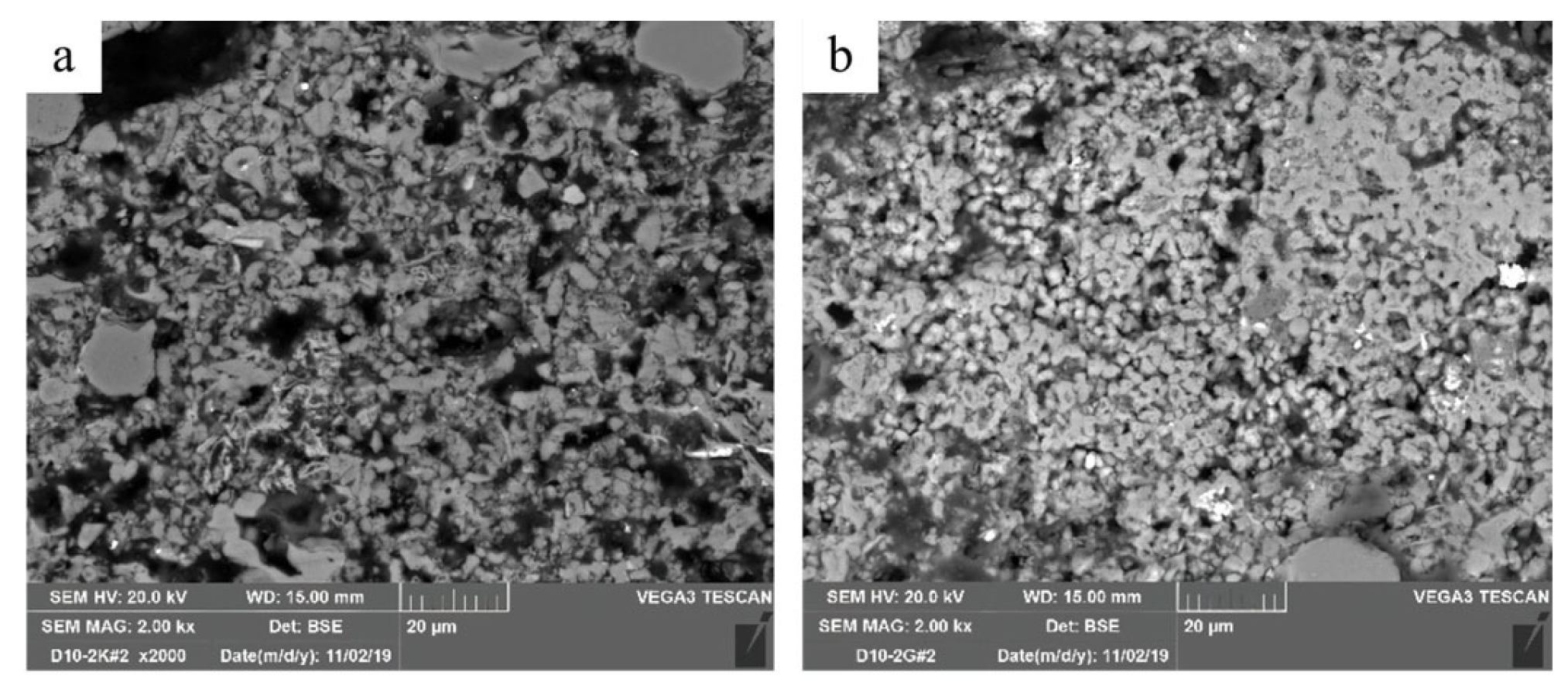
2.7. Scanning Electron Microscopy (SEM)
2.8. Pyrolysis–Gas Chromatography–Mass Spectrometry (Py-GC/MS)
2.9. X-Ray Diffraction (XRD)
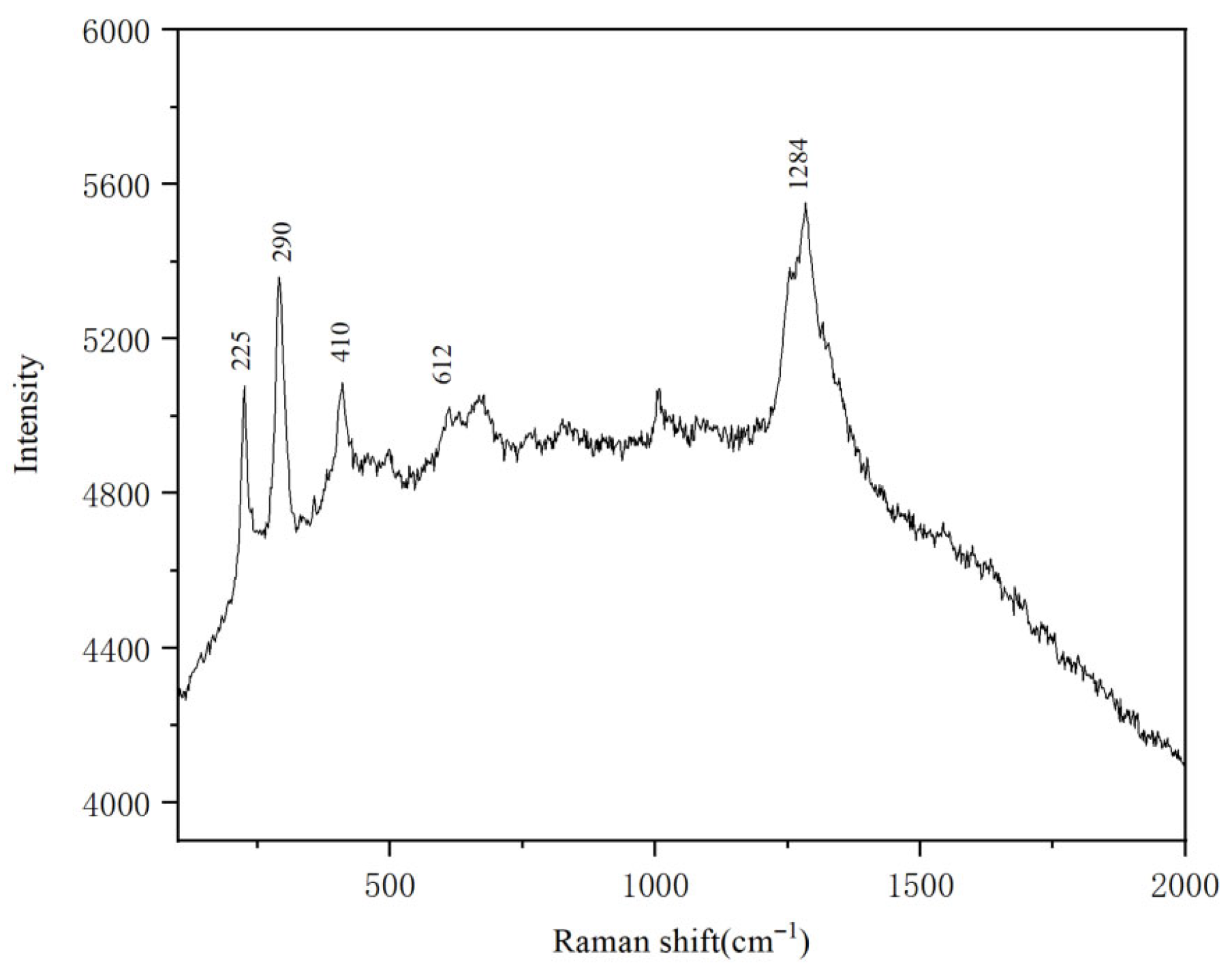
2.10. Confocal Raman Microspectroscopy (RAM)
2.11. Restoration of the Lacquered and Gilded Shakyamuni Sculpture
2.12. Methodology
3. Results
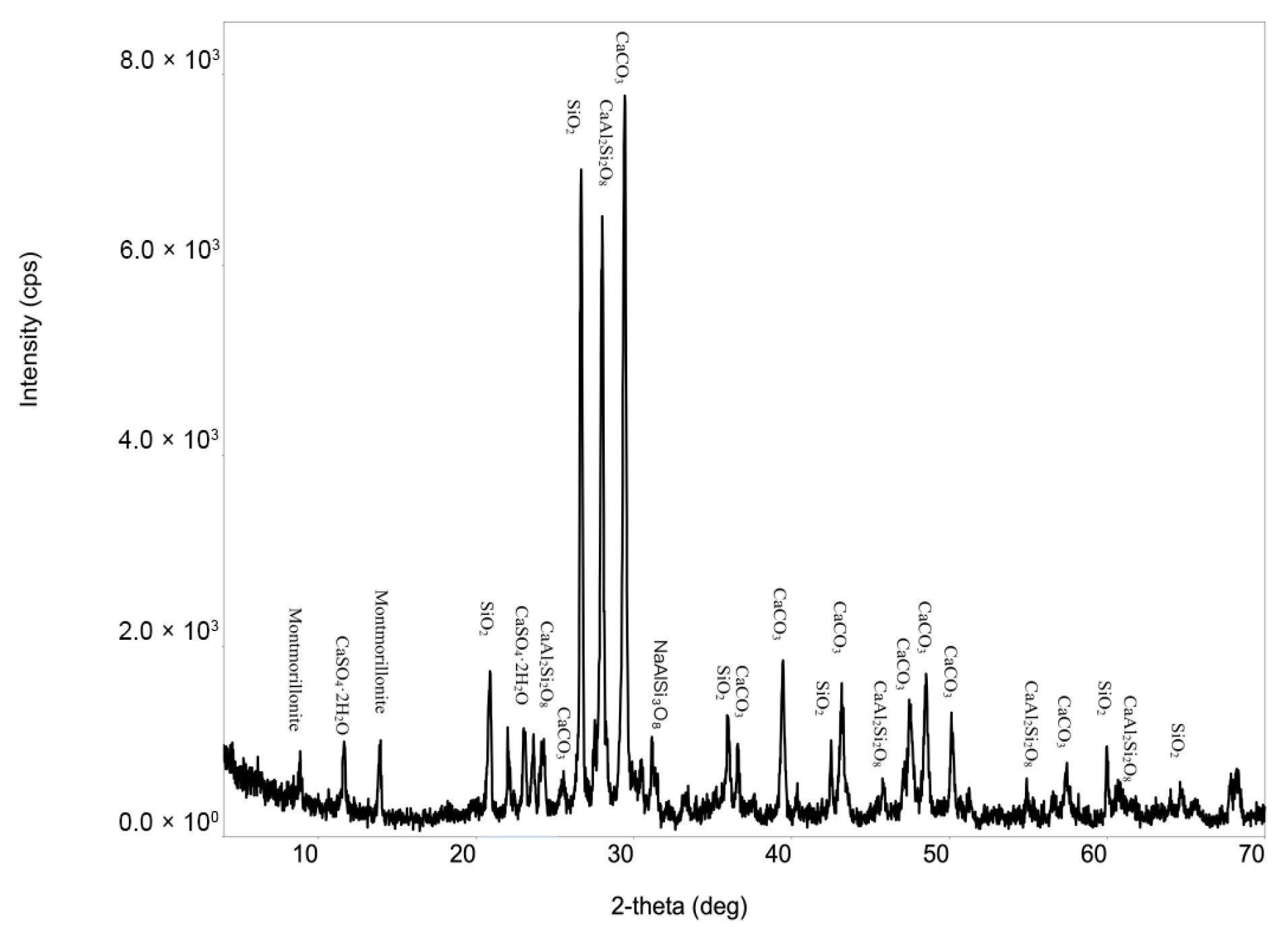
3.1. Reference Sample Characterization
3.2. Study of Restoration Techniques
3.2.1. Surface Cleaning
3.2.2. Screening of Reattachment and Consolidation Materials
3.2.3. Selection of Ground Layer Materials
3.3. On-Site Restoration
3.3.1. Optimization of the Lacquer Gilding Technique
3.3.2. Color Matching
4. Conclusions
- (1)
- On-site investigations and deterioration mapping revealed that the sculpture suffers primarily from surface contamination, gold leaf delamination, and gold leaf loss, with over 70% of the surface area affected.
- (2)
- A combination of analytical techniques including SEM-EDS, XRD, Raman spectroscopy, and Py-GC/MS was employed to systematically examine the composition and microstructural characteristics of key materials such as gold-sizing lacquer, lacquer–ash, ground layers, and pigments. The gilding structure was confirmed to exhibit a characteristic multilayer composition consistent with historical records in Xiushi Lu (Treatise on Lacquering) and to have undergone three to four phases of repair.
- (3)
- For material selection, various concentrations of NHL2/SiO2/SF016 composite emulsions were evaluated for both ground consolidation and gold leaf reattachment. The incorporation of NHL2 and nano-SiO2 effectively mitigated flowability issues and showed favorable performance. Taking interfacial compatibility as the core selection criterion, a 20% NHL2/SiO2/SF016 composite emulsion and traditional lacquer–ash were identified as the most suitable ground layer repair materials, and their compatibility with original components was thoroughly assessed.
- (4)
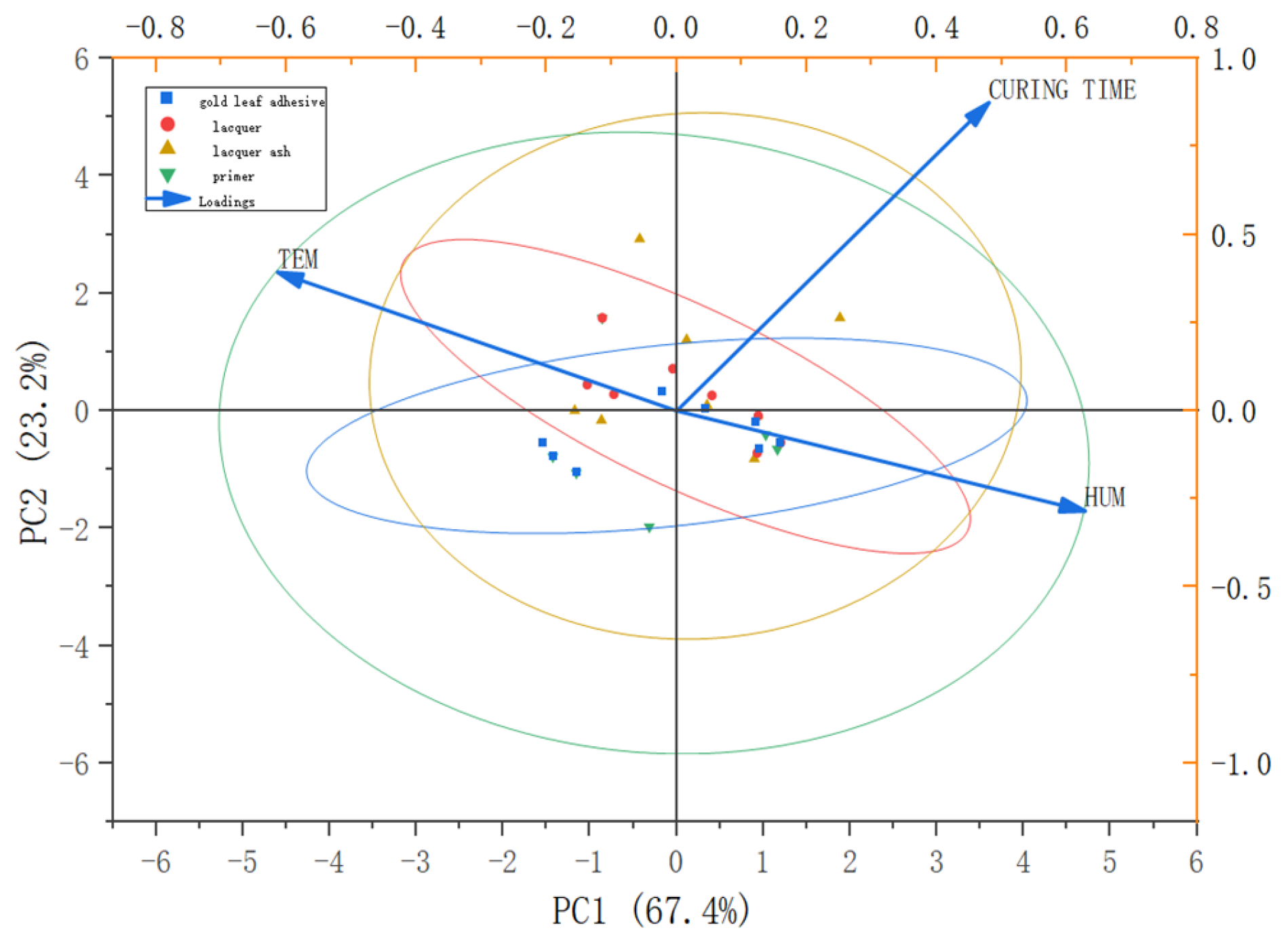
- During the restoration phase, a targeted treatment protocol was established based on identified deterioration types, including gentle cleaning, reattachment and consolidation of the gold leaf, and region-specific ground layer reconstruction. PCA was used to analyze the relationship between lacquer composition and curing behavior, supporting a strategy of modulating lacquer film quality and ground smoothness to visually harmonize the newly applied and original gold leaf.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, S.; Sun, X.; Li, R.; Hu, P.; Lu, H.; Liu, S.; Hu, G.; Hu, D.; Zhang, M. Understanding the unique corrosion phenomenon of Sanxingdui embrittled gold masks using scanning electrochemical cell microscopy (SECCM): Intergranular corrosion of Au-Ag-Pb ternary alloy. Corros. Sci. 2024, 237, 112301. [Google Scholar] [CrossRef]
- Tissot, I.; Troalen, L.G.; Manso, M.; Ponting, M.; Radtke, M.; Reinholz, U.; Barreiros, M.A.; Shaw, I.; Carvalho, M.L.; Guerra, M.F. A multi-analytical approach to gold in Ancient Egypt: Studies on provenance and corrosion. Spectrochim. Acta B At. Spectrosc. 2015, 108, 75–82. [Google Scholar] [CrossRef]
- Darque-Ceretti, E.; Felder, E.; Aucouturier, M. Foil and leaf gilding on cultural artifacts: Forming and adhesion. Matéria 2011, 16, 540–559. [Google Scholar] [CrossRef]
- Oddy, A. Gilding through the Ages. Gold Bull. 1981, 14, 75–79. [Google Scholar] [CrossRef]
- Cardoso, I.P.; Pye, E. Gessoes in Portuguese Baroque gilding grounds, Part 1: A study of historical documentary sources. Stud. Conserv. 2017, 62, 185–209. [Google Scholar] [CrossRef]
- Bonaduce, I.; Colombini, M.P.; Diring, S.P. Identification of garlic in old gildings by gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1121, 226–232. [Google Scholar] [CrossRef]
- Powell, C. Original gilding and over-gilding: The examination of the layers on an important English eighteenth-century chair. Conservator 1999, 23, 25–33. [Google Scholar] [CrossRef]
- Strahan, D.; Maines, C.A. Lacquer as an Adhesive for Gilding on Copper Alloy Sculpture in Southeast Asia. In Gilded Metals: History, Technology and Conservation; Drayman-Weisser, T., Ed.; Archetype Publications: London, UK, 2000; pp. 185–201. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Wang, W.; Liu, X. Performance of Drying Oil Modified Chinese Lacquer and Its Gilding Effect. Coatings 2024, 14, 1379. [Google Scholar] [CrossRef]
- Liu, L.; Wu, H.; Liu, W.; Gong, D.; Zhu, Z. Lacquering Craft of Qing Dynasty Lacquered Wooden Coffins Excavated from Shanxi, China—A Technical Study. J. Cult. Herit. 2016, 20, 676–681. [Google Scholar] [CrossRef]
- Moffatt, E.A.; Shugar, A.N.; Sirois, P.J.; Stock, S. A Materials Investigation into the Metal Composition and Coating Structures of Four Ming Dynasty Cast Iron Statues. MRS Proc. 2001, 712, II6.5. [Google Scholar] [CrossRef]
- Lee, S.E.; Rogers, H.; Wenbin, Z.; Brinker, H. China: 5,000 Years—Innovation and Transformation in the Arts; Solomon R. Guggenheim Museum: New York, NY, USA, 1998. [Google Scholar]
- Lee, H.S.; Kim, S.H.; Kim, W.W.; Yu, Y.G.; Han, K.S. Study on the Characteristics and Production Techniques of the Clay Seated Vairocana Buddha Triad of Seonunsa Temple, Gochang(2)—Analysis of Gold Leaf Layers and Internal Structure of the Clay Buddha Statues. J. Conserv. Sci. 2021, 37, 43–54. [Google Scholar] [CrossRef]
- Zhou, Z.; Shen, L.; Li, C.; Wang, N.; Chen, X.; Yang, J.; Zhang, H. Investigation of Gilding Materials and Techniques in Wall Paintings of Kizil Grottoes. Microchem. J. 2020, 154, 104548. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, F.; Wang, X.; Hao, X.; Tong, H.; Ma, L.; Shen, X. Comprehensive Analysis of the Surface Decoration Layer of Buddha Statues from Dazu Rock Carvings in China. Anal. Lett. 2022, 55, 2058–2073. [Google Scholar] [CrossRef]
- Liu, X.; Huang, X. Gold, Skin, and Body: Chinese Buddha Statues Are Constantly Being Shaped and Stripped. Religions 2023, 14, 155. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, L.; Wang, Y.; Liu, F.; Guo, J.; Wang, R.; Nevin, A. Technical Study of the Paint Layers from Buddhist Sculptures Unearthed from the Longxing Temple Site in Qingzhou, China. Heritage 2021, 4, 2599–2622. [Google Scholar] [CrossRef]
- González-Ramírez, A. Stone Sculpture Wear: Alteration/Fragmentation Processes and Their Impact on Carving Traces of Tenon Heads of Chavín de Huántar, Peru. Adv. Archaeol. Pract. 2019, 7, 152–168. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, X.; Zhou, H.; Li, M.; Tong, H.; Liu, S. Characterization and Analysis of Sandstone Substrate, Mortar Layers, Gold Foils, and Paintings of the Avalokitesvara Statues in Dazu County (China). J. Cult. Herit. 2016, 21, 881–888. [Google Scholar] [CrossRef]
- Park, G.Y.; Lee, H.B.; Oh, Y.; Lim, J.A.; Lee, M.W. Innovativeness in Tradition: A Study on the Physical Performance of Leather Scale Armors during the Joseon Dynasty. Fash. Text. 2023, 10, 26. [Google Scholar] [CrossRef]
- Fan, X.; Liu, H.; Wang, J.; Tang, K. Investigation of double network hydrogel with controllable swelling behavior by response surface methodology. J. Appl. Polym. Sci. 2019, 137, 48805. [Google Scholar] [CrossRef]
- Freire, M.T.; Veiga, M.d.R.; Santos Silva, A.; de Brito, J. Restoration of Ancient Gypsum-Based Plasters: Design of Compatible Materials. Cem. Concr. Compos. 2021, 120, 104014. [Google Scholar] [CrossRef]
- Negri, A.; Nervo, M.; Di Marcello, S.; Castelli, D. Consolidation and Adhesion of Pictorial Layers on a Stone Substrate: The Study Case of the Virgin with the Child from Palazzo Madama, in Turin. Coatings 2021, 11, 624. [Google Scholar] [CrossRef]
- Manfredda, N.; Buscaglia, P.; Gallo, P.; Borla, M.; Aicardi, S.; Poggi, G.; Baglioni, P.; Nervo, M.; Scalarone, D.; Borghi, A.; et al. An Ancient Egyptian Multilayered Polychrome Wooden Sculpture Belonging to the Museo Egizio of Torino: Characterization of Painting Materials and Design of Cleaning Processes by Means of Highly Retentive Hydrogels. Coatings 2021, 11, 1335. [Google Scholar] [CrossRef]
- Miranda, J.; Valença, J.; Costa, H.; Júlio, E. Methodology for the restoration of heritage built in exposed concrete. The case study of ‘Piscina das Marés’, Portugal. Constr. Build. Mater. 2022, 328, 127040. [Google Scholar] [CrossRef]
- Tullio, V.D.; Proietti, N.; Gobbino, M.; Capitani, D.; Olmi, R.; Priori, S.; Riminesi, C.; Giani, E. Non-Destructive Mapping of Dampness and Salts in Degraded Wall Paintings in Hypogeous Buildings: The Case of St. Clement at Mass Fresco in St. Clement Basilica, Rome. Anal. Bioanal. Chem. 2010, 396, 1885–1896. [Google Scholar] [CrossRef]
- Nugari, M.P.; Pietrini, A.M.; Caneva, G.; Imperi, F.; Visca, P. Biodeterioration of mural paintings in a rocky habitat: The Crypt of the Original Sin (Matera, Italy). Int. Biodeterior. Biodegrad. 2009, 63, 705–711. [Google Scholar] [CrossRef]
- Fiorillo, F.; Fiorentino, S.; Montanari, M.; Roversi Monaco, C.; Del Bianco, A.; Vandini, M. Learning from the past, intervening in the present: The role of conservation science in the challenging restoration of the wall painting Marriage at Cana by Luca Longhi (Ravenna, Italy). Herit. Sci. 2020, 8, 10. [Google Scholar] [CrossRef]
- Corfield, M. The conservation of Cave 85 at the Mogao Grottoes, Dunhuang: A collaborative project of the Getty Conservation Institute and the Dunhuang Academy. J. Inst. Conserv. 2016, 39, 64–67. [Google Scholar] [CrossRef]
- Ranalli, G.; Bosch-Roig, P.; Crudele, S.; Rampazzi, L.; Corti, C.; Zanardini, E. Dry biocleaning of artwork: An innovative methodology for Cultural Heritage recovery? Microb. Cell 2021, 8, 91–105. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, T.; Wu, Z.; Liu, Y.; Zhang, Z. Reinforced Strength Evaluation of Binding Material for the Restoration of Chinese Ancient Lacquer Furniture. BioResources 2019, 14, 7182–7192. [Google Scholar] [CrossRef]
- Miranda, J.; Valença, J.; Costa, H.; Eduardo, J. Restoration Intervention of Exposed White Concrete Buildings: The Case of ‘Pavilhão do Conhecimento’, Portugal. J. Build. Eng. 2023, 69, 106175. [Google Scholar] [CrossRef]
- EN 459-1; Building Lime—Part 1: Definitions, Specifications and Conformity Criteria. European Committee for Standardization (CEN): Brussels, Belgium, 2015.
- Gulotta, D.; Saviello, D.; Gherardi, F.; Toniolo, L.; Anzani, M.; Rabbolini, A.; Goidanich, S. Setup of a Sustainable Indoor Cleaning Methodology for the Sculpted Stone Surfaces of the Duomo of Milan. Herit. Sci. 2014, 2, 6. [Google Scholar] [CrossRef]
- Albano, M.; Grassi, S.; Fiocco, G.; Invernizzi, C.; Rovetta, T.; Licchelli, M.; Marotti, R.; Merlo, C.; Comelli, D.; Malagodi, M. A Preliminary Spectroscopic Approach to Evaluate the Effectiveness of Water- and Silicone-Based Cleaning Methods on Historical Varnished Brass. Appl. Sci. 2020, 10, 3982. [Google Scholar] [CrossRef]
- Angelova, L.V.; Sofer, G.; Bartoletti, A.; Ormsby, B. A Comparative Surface Cleaning Study of Op Structure, an Op Art PMMA Sculpture by Michael Dillon. J. Am. Inst. Conserv. 2022, 61, 115–136. [Google Scholar] [CrossRef]
- Baiza, B.; Gil, M.; Galacho, C.; Candeias, A.; Girginova, P.I. Preliminary Studies of the Effects of Nanoconsolidants on Mural Paint Layers with a Lack of Cohesion. Heritage 2021, 4, 3288–3306. [Google Scholar] [CrossRef]
- Hutanu, I.; Sandu, I.; Simionescu, A.E.; Vasilache, V.; Budu, A.M.; Sandu, I.C. Study concerning the influence of acrylic consolidating agents on gold gilding and schlagmetal. Rev. Chim. 2015, 66, 1480–1484. [Google Scholar]
- Auská, Z.; Ďoubal, J. Decision-Making Process in Polychromy Removal: Practical Application on the Stone Polychrome Sculpture of the Pensive Christ from Saint Barbara’s Cathedral in Kutná Hora. Stud. Conserv. 2025, 60, 1–23. [Google Scholar] [CrossRef]
- Rainer, L.; Bicer-Simsir, B. Field Test Methods for the Comparative Evaluation and Quality Control of Lime-based Hydraulic Injection Grouts Used for the Conservation of Architectural Surfaces. In Proceedings of the ICOM-CC 17th Triennial Conference Preprints, Building Strong Culture through Conservation, Melbourne, Australia, 17–19 September 2014; Bridgland, J., Ed.; International Council of Museums (ICOM-CC): Paris, France, 2014; pp. 295–306. [Google Scholar]
- Capasso, I.; Colella, A.; Iucolano, F. Silica-Based Consolidants: Enhancement of Chemical-Physical Properties of Vicenza Stone in Heritage Buildings. J. Build. Eng. 2023, 68, 106124. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Ortiz, P. Basic Protocol for On-Site Testing Consolidant Nanoparticles on Stone Cultural Heritage. Heritage 2019, 2, 2712–2724. [Google Scholar] [CrossRef]
- Abd El-Tawab, N.; Mahran, A.; Badr, I. Restoration and Preservation of the Wooden Ceiling of Al-Ashraf Qaytbay Madressa, Cairo, Egypt. Egypt. J. Archaeol. Restor. Stud. 2012, 2, 11–28. [Google Scholar]
- Rivers, S. Conservation of Japanese Lacquer in Western Collections: Conserving Meaning and Substance. In Proceedings of the ICOM-CC 14th Triennial Meeting Preprints, The Hague, The Netherlands, 12–16 September 2005; International Council of Museums (ICOM-CC): Paris, France, 2005; pp. 177–186. [Google Scholar]
- Nagasawa, I.; Chase, W.T.; Shida, T.; Fujita, N.; Koyano, M. Conservation of a Japanese Polychrome Buddhist Sculpture. Stud. Conserv. 2000, 45 (Suppl. S2), 23. [Google Scholar] [CrossRef]
- Dawson, T.L. Examination, Conservation and Restoration of Painted Art. Color. Technol. 2007, 123, 281–292. [Google Scholar] [CrossRef]
- Franquelo, M.L.; Duran, A.; Castaing, J.; Arquillo, D.; Perez-Rodriguez, J.L. XRF, μ-XRD and μ-Spectroscopic Techniques for Revealing the Composition and Structure of Paint Layers on Polychrome Sculptures after Multiple Restorations. Talanta 2012, 89, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Narita, C.; Yamada, K.; Tsujii, T. Solvent Influence on Crosslinking and Surface Characteristics of Urushi Films. Mater. Chem. Phys. 2017, 199, 369–378. [Google Scholar] [CrossRef]
- Babița, L.-L.; Timar, M.C. Conservation of Polychrome Wood—Principles and Case Study. Pro Ligno 2015, 11, 545–552. [Google Scholar]
- Hsu, S.; Sully, D. Fusing and Refreshing the Memory: Conserving a Chinese Lacquered Buddha Sculpture in London. J. Inst. Conserv. 2016, 61, 124–130. [Google Scholar] [CrossRef][Green Version]
- Burgio, L.; Gregory, T. Protocol for the Analysis of Cross-Sections from Gilded Surfaces. Heritage 2021, 4, 136–148. [Google Scholar] [CrossRef]
- Hao, X.; Schilling, M.R.; Wang, X.; Khanjian, H.; Heginbotham, A.; Han, J.; Auffret, S.; Wu, X.; Fang, B.; Tong, H. Use of THM-PY-GC/MS technique to characterize complex, multilayered Chinese lacquer. J. Anal. Appl. Pyrolysis 2019, 140, 339–348. [Google Scholar] [CrossRef]
- Ma, X.-M.; Lu, R.; Miyakoshi, T. Application of Pyrolysis Gas Chromatography/Mass Spectrometry in Lacquer Research: A Review. Polymers 2014, 6, 132–144. [Google Scholar] [CrossRef]
- Wong, L.; Bomin, S. The Wall Paintings in Cave 85: From Creation to Conservation; Getty Conservation Institute: Los Angeles, CA, USA, 2016. [Google Scholar]
- Fu, Y.; Chen, Z.; Zhou, S.; Wei, S. Comparative study of the materials and lacquering techniques of the lacquer objects from Warring States Period China. J. Archaeol. Sci. 2020, 114, 105060. [Google Scholar] [CrossRef]
- Zhang, K.; Sui, Y.; Wang, L.; Tie, F.; Yang, F.; Liu, Y.; Zhang, Y. Effects of sticky rice addition on the properties of lime-tile dust mortars. Herit. Sci. 2021, 9, 4. [Google Scholar] [CrossRef]
- Heginbotham, A.; Chang, J.; Khanjian, H.; Schilling, M.R. Some observations on the composition of Chinese lacquer. Stud. Conserv. 2016, 61 (Suppl. S3), 28–37. [Google Scholar] [CrossRef]
- Sparavigna, A.C. Historical Notes on the Use of Gold in Art and Culture. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Cizer, Ö.; Van Balen, K.; Elsen, J.; Van Gemert, D. Carbonation Reaction Kinetics of Lime Binders Measured Using XRD. University of Rome “La Sapienza”. 2008. Available online: https://lirias.kuleuven.be/handle/123456789/195902 (accessed on 8 July 2025).
- Chelazzi, D.; Bordes, R.; Giorgi, R.; Holmberg, K.; Baglioni, P. The use of surfactants in the cleaning of works of art. Curr. Opin. Colloid Interface Sci. 2020, 45, 108–123. [Google Scholar] [CrossRef]
- Torres-González, M.; Martín-del-Río, J.J.; Alejandre, F.J.; Macías Bernal, J.M. Guidelines for Conservation and Restoration of Historic Polychrome Plasterwork: The Church of St María la Blanca in Seville, Spain. Stud. Conserv. 2023, 68, 529–547. [Google Scholar] [CrossRef]
- Ronchin, L.; Stucchi, N.M.E.; Vavasori, A.; Traviglia, A.; De Nardi, C.; Franceschin, G. Characterisation of Innovative Mortar Formulations for the Restoration of Roman Mosaics. In Proceedings of the Cardiff University Engineering Research Conference 2023; Cardiff University Press: Cardiff, UK, 2023; pp. 58–61. [Google Scholar] [CrossRef]
- Burgos-Ruiz, C.; Martin-Sanchez, I.; Fort, R.; Alvarez de Buergo, M.; Sánchez-Moral, S.; Villegas, M.A.; Martinez-Ramirez, S. Silica-Functionalized Nanolimes for the Conservation of Stone Heritage. Small 2023, 19, 2301775. [Google Scholar] [CrossRef]
- Šantek Bajto, J.; Štirmer, N.; Baričević, A. Sustainable Hybrid Lime Mortars for Historic Building Conservation: Incorporating Wood Biomass Ash as a Low-Carbon Secondary Binder. Heritage 2023, 6, 5242–5269. [Google Scholar] [CrossRef]
- Marcos, M.M. International Session Scientific Communication—Intervention in Conservation and Restoration of Wall Paintings in Theban Tombs, Egypt (2nd C, BC); Pim: Iaşi, Romania, 2020; pp. 207–222. [Google Scholar]
- Baltazar, L.G.; Cardoso, J. Preliminary Investigation of the Mechanical and Physical Properties of Natural Hydraulic Lime Grouts with Nano-Silica. CivilEng 2021, 2, 421–441. [Google Scholar] [CrossRef]
- Diaz, J.; Ševčík, R.; Mácová, P.; Menéndez, B.; Frankeová, D.; Slížková, Z. Impact of nanosilica on lime restoration mortars properties. J. Cult. Herit. 2022, 55, 210–220. [Google Scholar] [CrossRef]










| Material Name | Molecular Formula | Function |
|---|---|---|
| Tween20 (Polyoxyethylene sorbitan monolaurate) | C58H114O28 | Non-ionic surfactant for cleaning [34,35,36] |
| SF016 | Acrylic copolymer emulsion | Adhesive for gold leaf reattachment [37,38] |
| NHL2 | Hydraulic lime (Ca(OH)2, SiO2, Al2O3, etc.) | Inorganic filler and consolidant [39,40] |
| Nano-SiO2 | SiO2 | Nanofiller, strength modifier [41,42,43] |
| Raw lacquer | C15H24O2 (main component) | Traditional lacquer base [44,45] |
| Refined tung oil | C18H32O2 | Binder in gold-sizing lacquer [46] |
| Cinnabar | HgS | Gold-enhancing ground [47] |
| Turpentine | C10H16 (main component) | Solvent and thinner for lacquer [48,49] |
| Name | Formulation (% by Mass) | Effect | |||
|---|---|---|---|---|---|
| SF016 | NHL2 | Nano SiO2 | Deionized Water | ||
| 5%NHL2/SiO2/SF016 | 5 | 1 | 0.3 | 93.7 | Preliminary consolidation of ground layer |
| 15%NHL2/SiO2/SF016 | 15 | 45 | 15 | 15 | Reattachment of original gold leaf |
| 20%NHL2/SiO2/SF016 | 20 | 60 | 20 | 0 | Preparation of hydraulic lime ground layer |
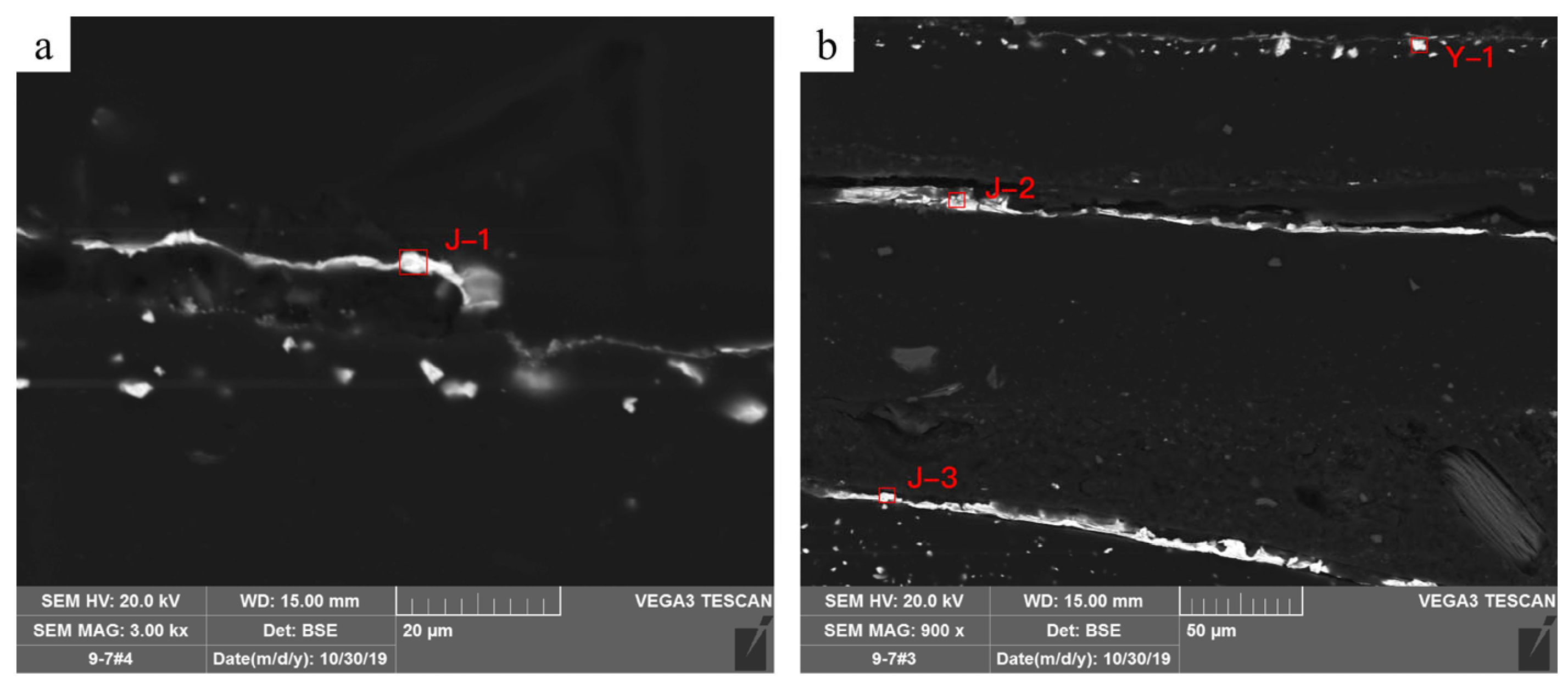
| Serial Number | Elements/wt.% | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Al | S | Ca | Au | Hg | |
| J-1 | 31.4 | 3.2 | 0.6 | 58.4 | |||
| J-2 | 18.8 | 4.6 | 0.8 | 75.8 | |||
| J-3 | 21.7 | 3.3 | 0.7 | 74.2 | |||
| Y-1 | 21.9 | 3.0 | 12.4 | 62.8 | |||
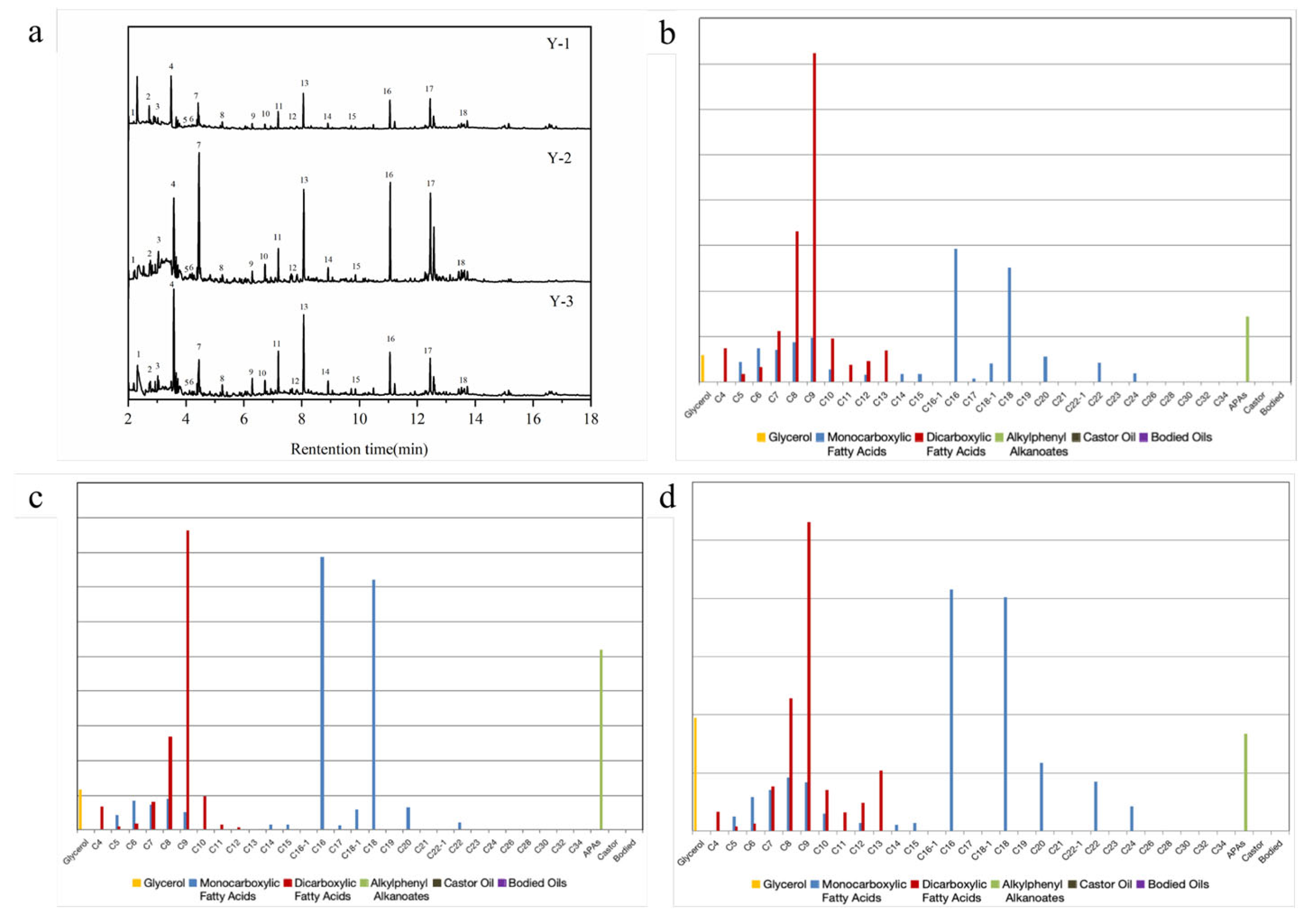
| Serial No. | Retention Time | Component | Serial No. | Retention Time | Component |
|---|---|---|---|---|---|
| 1 | 2.18 | Methyl pentanoate | 10 | 6.73 | 1-Tetradecene |
| 2 | 2.77 | Methyl 5-octenoate | 11 | 7.22 | Dimethyl azelaate |
| 3 | 3.01 | Methyl hexanoate | 12 | 7.68 | Pentadecane |
| 4 | 3.57 | Decane | 13 | 8.07 | Dimethyl azelate |
| 5 | 3.96 | 2-Methylphenol | 14 | 8.28 | Dimethyl pelargonate |
| 6 | 4.19 | 3,4-Dimethylphenol | 15 | 9.86 | Dimethyl decanoate |
| 7 | 4.45 | Methyl octanoate | 16 | 11.08 | Methyl hexadecanoate |
| 8 | 5.25 | Methyl nonanoate | 17 | 12.45 | Methyl stearate |
| 9 | 6.27 | Dimethyl sebacate | 18 | 15.23 | 1,2-Dimethoxy-3-pentadecylbenzene |
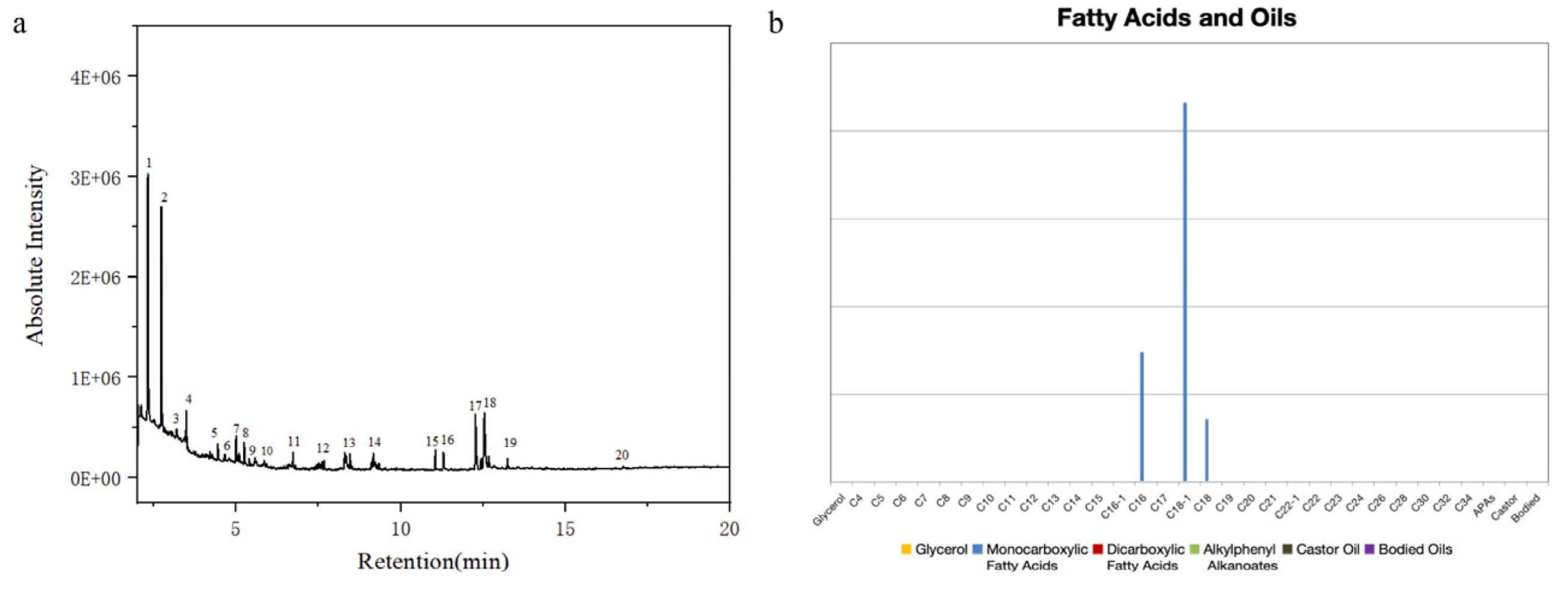
| Serial No. | Retention Time | Component | Serial No. | Retention Time | Component |
|---|---|---|---|---|---|
| 1 | 2.32 | Glycine | 11 | 7.67 | Pentadecane |
| 2 | 2.73 | Alanine | 12 | 8.31 | Octadecadienoic acid |
| 3 | 3.17 | Trimethyl phosphate | 13 | 9.19 | 1-Heptadecene |
| 4 | 4.45 | Methyl octanoate | 14 | 11.04 | Methyl palmitate |
| 5 | 4.68 | Gelatin protein marker | 15 | 11.25 | n-Hexadecanoic acid |
| 6 | 5.02 | Pentylbenzene | 16 | 12.28 | Methyl stearate |
| 7 | 5.25 | Methyl methoxypropionate | 17 | 12.53 | Oleic Acid |
| 8 | 5.38 | Isoleucine amine | 18 | 12.67 | Stearic acid |
| 9 | 5.58 | 3-methylcatechol | 19 | 13.26 | Oleic Acid |
| 10 | 6.73 | 1-Tetradecene | 20 | 16.69 | 3-Heptadecylcatechol |
| Material | Concentration (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 20 | 40 | 50 | 60 | 70 | 80 | Original Solution | |
| gold-sizing lacquer | - | × | ||||||
| lacquer | - | √ | ||||||
| epoxy resin | - | √√ | ||||||
| Paraloid B72 | - | × | × | × | - | - | ||
| SF016 | × | √ | √ | √ | √ | √√ | √√ | √√ |
| polyvinyl acetate emulsion | - | × | ||||||
| Position | Before Repair | After Repair | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | ||
| 1 | 2.98 | 0.48 | −2.98 | 3.07 | 2.11 | −8.86 | 6.1 |
| 2 | 3.43 | 1.36 | −5.22 | 3.16 | 2.12 | −8.51 | 3.39 |
| 3 | 3.34 | 1.34 | −8.20 | 3.07 | 2.11 | −7.73 | 0.94 |
| 4 | 2.98 | 0.48 | −6.56 | 3.16 | 2.12 | −10.48 | 4.25 |
| 5 | 3.25 | 1.33 | −11.32 | 3.16 | 1.72 | −9.96 | 1.42 |
| 6 | 3.43 | 1.76 | −10.68 | 3.16 | 1.72 | −9.79 | 0.93 |
| 7 | 3.43 | 2.97 | −7.67 | 3.16 | 2.12 | −7.19 | 1.01 |
| 8 | 2.98 | 1.28 | −6.00 | 3.16 | 2.12 | −10.13 | 4.22 |
| 9 | 2.98 | 0.88 | −2.41 | 3.25 | 2.94 | −6.66 | 4.73 |
| 10 | 3.61 | 5.42 | −2.07 | 3.16 | 4.95 | −3.99 | 2.03 |
| 11 | 2.98 | 0.48 | −6.00 | 3.25 | 2.14 | −9.27 | 3.68 |
| 12 | 3.34 | 2.96 | −4.24 | 3.25 | 3.35 | −5.91 | 1.72 |
| 13 | 3.16 | 3.33 | −3.61 | 3.34 | 2.55 | −6.32 | 2.83 |
| 14 | 2.89 | 0.87 | −7.47 | 3.25 | 2.54 | −7.23 | 1.73 |
| 15 | 3.34 | 4.57 | −5.18 | 3.25 | 2.94 | −4.40 | 1.81 |
| 16 | 3.25 | 2.94 | −5.15 | 3.25 | 2.54 | −8.91 | 3.78 |
| 17 | 3.43 | 2.97 | −5.78 | 3.25 | 2.54 | −6.66 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, H.; Zha, J. A Methodology for Lacquer Gilding Restoration of Sandstone Sculptures: A Multidisciplinary Approach Combining Material Characterization and Environmental Adaptation. Coatings 2025, 15, 819. https://doi.org/10.3390/coatings15070819
Bu H, Zha J. A Methodology for Lacquer Gilding Restoration of Sandstone Sculptures: A Multidisciplinary Approach Combining Material Characterization and Environmental Adaptation. Coatings. 2025; 15(7):819. https://doi.org/10.3390/coatings15070819
Chicago/Turabian StyleBu, Haijun, and Jianrui Zha. 2025. "A Methodology for Lacquer Gilding Restoration of Sandstone Sculptures: A Multidisciplinary Approach Combining Material Characterization and Environmental Adaptation" Coatings 15, no. 7: 819. https://doi.org/10.3390/coatings15070819
APA StyleBu, H., & Zha, J. (2025). A Methodology for Lacquer Gilding Restoration of Sandstone Sculptures: A Multidisciplinary Approach Combining Material Characterization and Environmental Adaptation. Coatings, 15(7), 819. https://doi.org/10.3390/coatings15070819








