Abstract
A superhydrophobic surface with hierarchical micro/nano-array structures was successfully fabricated on 6061 aluminum alloy through a combination of femtosecond laser etching and anodic oxidation. Femtosecond laser etching formed a regularly arranged microscale “pit-protrusion” array on the aluminum alloy surface. After modification with a fluorosilane ethanol solution, the surface exhibited superhydrophobicity with a contact angle of 154°. Subsequently, the anodic oxidation process formed an anodic oxide film dominated by an array of aluminum oxide (Al2O3) nanopores at the submicron scale. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) analyses revealed that the nanopore structures uniformly and continuously covered the laser-ablated layer. This hierarchical structure significantly increased the surface water contact angle to 162°. Wettability analysis showed that the prepared composite coating formed an air layer accounting for 91% of the surface area. Compared with the sample only treated by femtosecond laser etching, the presence of the Al2O3 nanopore structure significantly enhanced the mechanical durability, superhydrophobic durability, and corrosion resistance of the superhydrophobic surface. The proposed multi-step fabrication strategy offers an innovative method for creating multifunctional, durable superhydrophobic coatings and has important implications for their large-scale industrial use.
1. Introduction
Superhydrophobic surfaces, widely observed in nature on biological materials such as lotus leaves, water strider legs, and shark skins, are conventionally characterized by a water contact angle (WCA) exceeding 150° and a water sliding angle (WSA) less than 10° [1,2,3]. These surfaces endow materials with excellent properties, including superior corrosion resistance, self-cleaning efficacy, and anti-fouling capabilities, rendering them highly promising for applications in diverse sectors such as aerospace engineering, marine technology, and automotive manufacturing [4,5]. Advancements in research have revealed that superhydrophobicity relies on two key factors: hierarchical micro/nano composite topographies and low surface energy states [6,7,8,9]. Consequently, the preparation strategy for superhydrophobic coatings involves constructing surfaces featuring micro-nano hierarchical architectures and concurrently low surface energy characteristics [10,11,12].
After years of industrial development and in-depth research on superhydrophobic coatings, significant theoretical breakthroughs have been achieved in the preparation of superhydrophobic coatings on metal surfaces [13,14]. Douglas et al. employed a colloidal-crystalline templating method, combining TiO2 nanoparticles with polystyrene microspheres. After spraying and thermal annealing to remove the template, a porous structure was formed. After fluorination modification, the contact angles of the coating for water and oil reached 164° and 161°, respectively [15]. Wang et al. adjusted femtosecond laser parameters to prepare superhydrophobic coatings with micro/nano composite structures on aluminum alloy surfaces. Even after five ice-melting cycles, the contact angle remained at 157°, and although the nanostructures suffered local damage, they still met the requirements for superhydrophobicity [16]. Zhou et al. controlled the electrodeposition current density, deposition time, and electrolyte composition to fabricate micro/nano needle-cone-shaped nickel-based superhydrophobic coatings on carbon steel surfaces. These coatings exhibited a high water contact angle of 168° and a sliding angle of 5°, possessing self-cleaning properties, strong water flow reflection capabilities, as well as certain mechanical durability and chemical stability [17].
Aluminum alloy, owing to its lightweight, high strength, and excellent processability, has been extensively applied in modern aerospace, automotive manufacturing, and marine engineering [18,19,20,21]. Generally, preparing superhydrophobic coatings on lightweight alloy surfaces can enhance their corrosion resistance or endow them with specific functional properties, thereby expanding their application scope [22,23,24]. Various methods are available for preparing superhydrophobic coatings on aluminum alloy surfaces, including anodic oxidation, spraying, electrodeposition, and so on [25,26,27,28,29,30,31,32,33]. For example, Emarati et al. prepared superamphiphobic coating on AA2024 aluminum alloy surfaces via anodic oxidation. The coating featured hierarchical micro/nano coral reef-like structures, achieving a water contact angle of 176°, a contact angle of 164° for glycerol, and 153° for n-decane. Moreover, the coating showed certain corrosion resistance, with a corrosion inhibition efficiency of 94% in 3.5 wt.% NaCl solution, significantly suppressing the penetration of Cl− ions into the substrate [34]. However, superhydrophobic coatings prepared by traditional single processes commonly suffer from poor corrosion resistance and insufficient mechanical durability, restricting their long-term service performance [35,36,37,38,39,40,41]. In recent years, in order to improve the superhydrophobic corrosion resistance and mechanical durability, scholars have proposed many schemes, such as two-step chemical etching, self-healing coating, and armor protection, to obtain micro-nano hierarchical structures. However, these processes have problems such as a complex preparation process, easy coating shedding, and poor controllability of the preparation process [42,43,44,45].
To address the above issues, this study proposes a composite process combining femtosecond laser etching and anodic oxidation. Using 6061-T6 aluminum alloy as the research object, superhydrophobic coatings with “micro-array-nanopore” hierarchical structures were fabricated on its surface. Firstly, femtosecond laser etching was utilized to construct a periodic microscale “pit-bump” skeleton. Then, anodic oxidation was applied to grow homogeneous and dense submicron-scale Al2O3 nanopores on this surface fully, forming a secondary nanostructure that further increased the surface’s ability to retain air layers. After modification with fluorosilane (FAS) to reduce surface energy, the effects of the hierarchical structure on the wettability, corrosion resistance, and mechanical durability of the coatings were systematically investigated. Compared with a single laser etching layer, the composite process not only increased the water contact angle from 154° to 162° but also significantly enhanced the corrosion resistance and mechanical durability of the coatings through the physical support and chemical barrier effects of the nanopore structures. This study provides a novel process idea for the design of superhydrophobic coatings on aluminum alloys, holding promise for promoting their practical applications in harsh environments.
2. Experimental Methods
2.1. Preparation of Experimental Samples
The substrate material used in this study was 6061-T6 aluminum alloy, purchased from Anhui Zhengying Technology Co., Ltd., with dimensions of 15 × 15 × 2 mm. The 6061 aluminum alloy sheets obtained after wire cutting were successively ground with 400#, 800#, 1000#, 1500#, and 2000# SiC sandpapers, from coarse to fine, to remove the surface oxide film and oil stains. Grinding was stopped when no obvious scratches remained on the sample surface. Subsequently, the ground 6061 aluminum alloy samples were polished to a mirror finish. After grinding and polishing, the samples were ultrasonically cleaned in absolute ethanol for 300 s to remove grinding debris and oil stains left during the previous processes. Then, the sample surfaces were rinsed with deionized water and absolute ethanol. The samples for subsequent processing were dried with a blower, while the spare samples were stored in absolute ethanol.
In this study, an ultrafast fiber laser (Femtosecond Fiber Laser, HR-Femto-GN-10-10B, Wuhan Huari Precision Laser Co., Ltd., Wuhan, China) was used to etch the surface of 6061 aluminum alloy, aiming to obtain a microtopography with a periodic array structure. The aluminum alloy surface coating prepared under the optimal femtosecond laser etching parameters was denoted as the Laser Etching (LE) layer.
The pre-treated 6061 aluminum alloy samples were placed on the processing platform, and the corresponding processing parameters were set. After the laser was focused, the laser switch was triggered. The laser emitted was focused by the galvanometer and then etched the sample surface. The laser etching scanning path adopted a vertical cross-filling mode. The detailed femtosecond laser etching processing parameters are shown in Table 1. After femtosecond laser etching, the samples were ultrasonically cleaned in absolute ethanol for 5 min and then rinsed with absolute ethanol to remove metal debris generated during the interaction between the laser and the surface. The samples were dried and used for subsequent experiments, while the spare samples were stored in absolute ethanol.

Table 1.
Detailed parameters of femtosecond laser etching processing.
In this study, anodic oxidation was performed on the LE layer after femtosecond laser etching. The anodic oxidation solution was a 30% (mass fraction) phosphoric acid aqueous solution. The anode was the LE layer sample, and the cathode was a lead plate, with a distance of approximately 4 cm between them. In order to obtain a uniform and regulated nanopore structure throughout the LE layer, anodic oxidation was carried out at room temperature for 30 min with a voltage of 60 V. The obtained coating was denoted as the Laser Etching and Anodic Aluminum Oxide (LE/AAO) coating.
The LE layer and LE/AAO coatings with micro/nano structures were dried and then immersed in a 1wt.% ethanol solution of 1H,1H,2H,2H-perfluorodecyltrimethoxysilane (fluorosilane, FAS) for 1 h. After immersion, the sample surfaces were rinsed with absolute ethanol and dried to remove the remaining modifier. Then, the samples were placed in an electric heating blast drying oven for heat treatment at 130 °C for 1 h. After the heat preservation process was completed, the drying oven was turned off, and the samples were taken out and stored in self-sealing bags after cooling to room temperature. The process path diagram of sample preparation is shown in Figure 1.
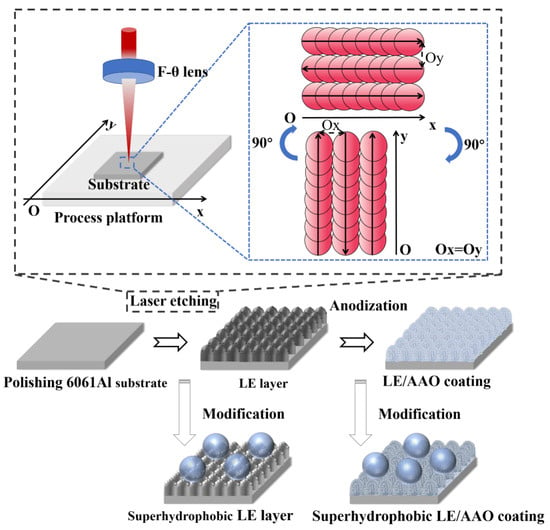
Figure 1.
Process path diagram of sample preparation.
2.2. Sample Characterization
A Phenom ProX desktop scanning electron microscope (SEM, Phenom Scientific, Eindhoven, The Netherlands) was used to analyze the microtopography of the surfaces of LE and LE/AAO samples, as well as their surfaces after durability tests. A Hitachi SU8600 cold field emission scanning electron microscope(FE-SEM, Hitachi, Tokyo, Japan) was employed to conduct a higher-magnification morphological analysis of the LE/AAO coating surface at the submicron scale. X-ray diffraction (XRD) analysis was performed on the sample surfaces using a Bruker D2 Phaser X-ray diffractometer (Bruker, Karlsruhe, Germany) with a CuKα target. The scanning angle range was 10°–90°, the scanning speed was 5°/min, and the step size was 0.02°.
An Attension Theta Flex optical contact angle measuring instrument (Biolin Scientific, Västra Frölunda, Sweden) was used to analyze the wettability of the aluminum alloy sample surfaces at room temperature. The static water contact angle (WCA) of the samples was measured by the sessile drop method. The workbench was adjusted to a horizontal position, and the sample was placed horizontally on the workbench. A 10 μL deionized water droplet was dropped onto the sample surface using a syringe, and the droplet was released manually. The liquid contour image was captured by a CCD camera, and the WCA was automatically calculated by fitting the droplet edge using the One Attension 4.0 software equipped with the instrument. The adhesion of the sample surface was qualitatively characterized based on the video recording of the dynamic process of WCA measurement. The sample platform is manually rotated, and the inclination of the sample platform is recorded when the droplet begins to slide, which is the water sliding angle (WSA).
The corrosion electrochemical performance of the prepared aluminum alloy samples was tested using an Autolab electrochemical workstation (PGSTAT302N, Metrohm, Herisau, Switzerland). A three-electrode working system (the sample under test as the working electrode, a saturated calomel electrode as the reference electrode, and a platinum sheet as the counter electrode) was connected to the electrochemical workstation. The potentiodynamic polarization curve (Tafel) test was used to characterize the corrosion resistance of the samples. Before the test, an open-circuit potential (OCP) test was first conducted, and subsequent tests were carried out after the potential stabilized. The test was performed using a 3.5 wt.% NaCl solution (simulating seawater) as the corrosive medium, with a scanning rate of 1 mV/s.
The mechanical durability of the superhydrophobic surfaces was tested using the linear friction wear test method. The prepared superhydrophobic aluminum alloy surfaces were inverted and placed on 1000# SiC sandpaper, and a 20 g weight was glued onto the surface. The sample was pushed and pulled at a constant speed, with 100 mm defined as one cycle (1 cycle). After each cycle, the wettability of the sample surface was characterized. SEM characterization was performed for representative cycle numbers.
3. Results and Discussion
3.1. Microstructural Characterization
Figure 2a–c shows the microstructure of the LE layer. The boundaries between the “bumps” and “pits” on the sample surface are very distinct. The grooves in the “pit” area have a certain depth, and the bulges formed by sputtering and backflow in the “bump” area have accumulated into regular hill-like structures. The width of the pits and bumps is about 15 μm. Figure 2d–f displays the surface morphology of the LE/AAO sample. As shown in Figure 2d, the periodic “bump-pit” morphology features after femtosecond laser etching are still clearly visible at low magnification, indicating that the anodic oxidation process adopted in this study does not cause significant damage to the laser-etched structure. From Figure 2e, it can be observed that the entire LE layer surface is completely covered with nanopores. Uniform and dense nanopores have formed on the top, side, and bottom of the grooves. When observing the area where nanopores are formed (Figure 2f), the nanopores are uniformly distributed on the LE layer surface under this anodic oxidation time, and the pores almost achieve uniform coverage of the LE layer. Observing the nanopores (inset in Figure 2f), at the submicron scale, they are still regular and ordered. Some pore walls of the pores are exposed, forming an alternating “pore wall-pore” structure. This structure is primarily governed by the anodization duration. The electrolyte thoroughly etches the pore walls, leading to lateral dissolution and the gradual merging of adjacent pores [25]. This structural feature provides favorable structural conditions for the stability of the Cassie state of the superhydrophobic coating.
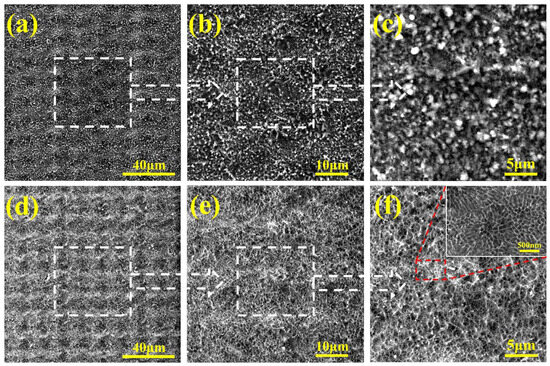
Figure 2.
SEM image of the sample surface. (a–c) LE layer, (d–f) LE/AAO coating.
3.2. Surface Composition Analysis
X-ray diffraction (XRD) was used to analyze the phases of aluminum alloy samples at different process stages, and the test results are shown in Figure 3. The polished 6061 aluminum alloy substrate and the LE layer show diffraction peaks at the same positions, and no obvious Al2O3 peaks are observed in the LE layer. Therefore, the main component of the LE layer surface is still Al. This indicates that the femtosecond laser etching process only changes the surface structure of the aluminum substrate without causing significant phase transitions. It also proves that the periodic array structure of the LE layer prepared in this study is mainly formed by the physical impact of the plasma focused by the galvanometer, rather than the high-temperature ablation of the traditional laser marking process. The XRD pattern of the LE/AAO coating in the range of 10°–90° retains the characteristic peaks of Al, and the diffraction intensities of these peaks are stronger than those of the LE layer and the aluminum substrate, which may be due to the loose “pore wall-pore” structure. In addition, diffraction characteristic peaks of Al2O3 appear in the range of 15°–35°. These experimental results indicate that after anodic oxidation treatment, aluminum oxide was successfully formed on the surface of the LE layer. The anodic oxidation process indeed caused the formation of Al2O3 on the surface of the LE layer, changing the phase composition of the coating. The main component of the uniformly distributed submicron-scale “pore wall-pore” structure is Al2O3.
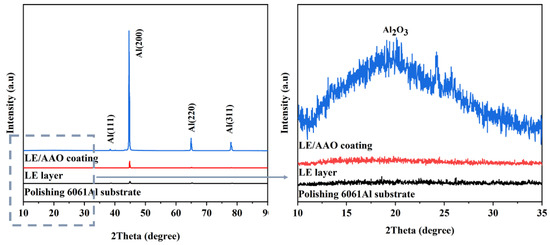
Figure 3.
Surface XRD patterns of different aluminum alloy samples.
3.3. Wettability Analysis
Figure 4a presents the results of the water contact angle (WCA) and WSA tests for the LE layer and the LE/AAO coating modified with FAS, while Figure 4b illustrates the theoretical wetting models on the surfaces of the two coatings. The WCA of the LE layer was measured to be about 154°, and the WSA was measured to be about 5°, which has reached the superhydrophobic state. Considering the morphological characteristics of this sample, the “bump-pit” periodic array structure is remarkable, with clear and continuous grooves and bulges. Such a structural feature can store a large amount of air layers, resulting in a high gas-liquid ratio at the interface. The initial static water contact angle of the LE/AAO coating was measured to be approximately 162°, an increase of about 8° compared with the LE layer. Additionally, water droplets are very easy to slide on the LE/AAO coating, and the WSA was measured to be about 2°. According to the Cassie–Baxter equation [46]
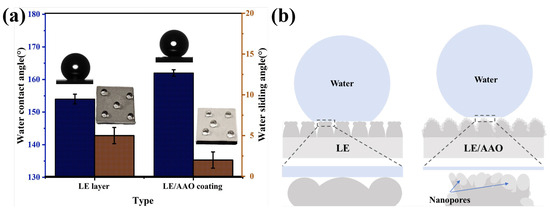
Figure 4.
Characterization of wettability. (a) Static water contact angle test of the two coatings, (b) Theoretical model of wetting on the surface of the two coatings.
In this equation, θ1 represents the intrinsic contact angle of the smooth solid surface, measured as 117° (Figure S1d), and θCB is the apparent contact angle. By substituting the measured static water contact angles of the LE and LE/AAO coatings into the equation, the ratios of solid-liquid contact area to the total surface area (f1) for the superhydrophobic LE and LE/AAO coatings were calculated to be approximately 18% and 9%, respectively. Consequently, the gas-liquid contact area ratios (f2) were 82% and 91%, respectively. This phenomenon arises because the anodic oxidation process introduced into the superhydrophobic LE/AAO coating further generates nanopores on the LE layer’s surface with distinct grooves and protrusions. After low-surface-energy modification, these nanopores could further “trap” air layers, increasing the air layer proportion from 82% (LE layer) to 91% (LE/AAO coating). This reduction in solid-liquid contact area enhances superhydrophobic performance, as evidenced by the higher measured static contact angle. Additionally, compared to our previous studies, the nanopores in this work exhibit significantly enlarged diameters, with partial pore walls collapsing and undergoing lateral dissolution, forming a composite “pore wall-pore” array structure at the submicron scale. Water droplets on this structured surface experience reduced Laplace pressure, maintaining a stable Cassie state and contributing to the higher initial static contact angle.
To further confirm the type of LE/AAO superhydrophobic coating, qualitative water adhesion analysis was performed. As shown in Figure 5, when a dropper was pressed to bring a water droplet into contact with the LE/AAO superhydrophobic coating and then lifted, the droplet detached completely without residue. If the surface were in the Wenzel state, the droplet would have infiltrated the grooves or nanopores under pressure. This experiment confirms that the LE/AAO superhydrophobic coating maintains a stable Cassie state with low adhesion, characteristic of natural “lotus leaf”-type low-adhesion superhydrophobic surfaces, justifying the use of the Cassie–Baxter equation for analysis.
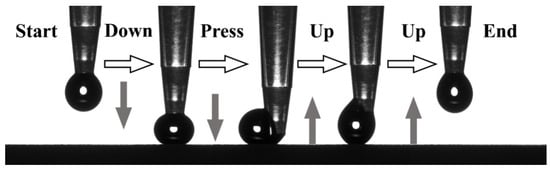
Figure 5.
Water adhesion test of superhydrophobic LE/AAO coating.
As shown in Figure 6, the superhydrophobic LE/AAO coating sample floated on water, demonstrating its 91% gas-liquid contact area ratio, where the air layer prevented water infiltration. When a water jet was applied to the surface, it deflected and rebounded, forming a distinct water column, further evidence of the air layer’s repulsive effect. Immersing the sample partially in water revealed a specular reflection effect due to total internal reflection at the air-liquid interface, confirming the air layer’s stability. These wetting behaviors collectively indicate that the LE/AAO coating surface maintains a stable air layer, validating the applicability of the Cassie model for its analysis.
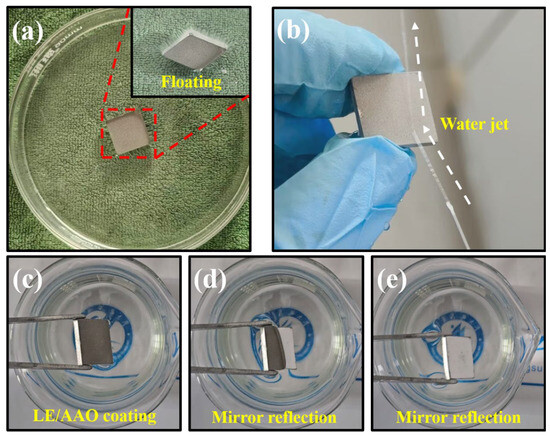
Figure 6.
Superhydrophobic phenomena of superhydrophobic LE/AAO coating. (a) Floating phenomenon in water, (b) water reflection phenomenon, (c–e) specular reflection phenomenon in water.
3.4. Corrosion Resistance Analysis
Figure 7 shows the potentiodynamic polarization curves of 6061 aluminum alloy samples after different processes. Table 2 lists the corrosion parameters calculated by Nova 2.1 software from the potentiodynamic polarization curves of aluminum alloy samples at different process stages. As shown in the table, the polished aluminum alloy substrate exhibits the most negative corrosion potential, the highest corrosion current density, and the highest corrosion rate, indicating poor corrosion resistance of the pure aluminum alloy substrate. After femtosecond laser etching, the corrosion potential of the LE layer shifts positively with a significant improvement, and the corrosion current density and corrosion rate further decrease, presumably due to the formation of a small amount of corrosion-resistant oxide on the surface from laser etching. The corrosion potential of the superhydrophobic LE layer is −6.54 × 10−1 V, which is more positive than that of the as-etched LE layer before chemical modification. The corrosion current density is 3.12 × 10−7 A/cm2, an order of magnitude lower than that of the polished substrate and the pre-modification LE layer, with a corrosion rate of 1.10 × 10−2 mm/year, confirming the better corrosion resistance of the superhydrophobic LE layer.
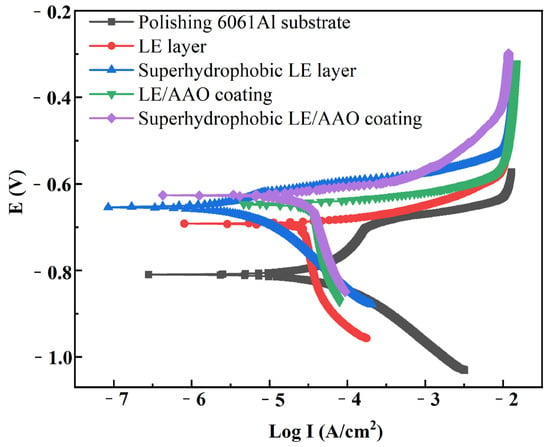
Figure 7.
Potentiodynamic polarization curves of different aluminum alloy samples in 3.5 wt% NaCl solution.

Table 2.
Corrosion potential (Ecorr) and corrosion current density (Icorr) of different aluminum alloy sample surfaces.
The LE/AAO coating shows a further positive shift in corrosion potential, reduced corrosion current density, and lower corrosion rate compared to the LE layer, indicating that the anodic oxidation process can enhance the corrosion resistance of the LE layer to some extent. Although the nanopores formed on the LE/AAO surface are structurally loose at the submicron scale, the overall structure is dense and uniform, creating an effective barrier layer that protects the substrate and LE layer. The superhydrophobic LE/AAO coating after surface chemical modification exhibits a corrosion potential of −6.27 × 10−1 V, further positively shifted compared to the pre-modification LE/AAO coating. The corrosion current density is 2.08 × 10−7 A/cm2, an order of magnitude lower than that of the polished substrate and pre-modification LE/AAO coating, with the corrosion rate reduced to 6.81 × 10−3 mm/year, demonstrating excellent corrosion resistance. The superhydrophobic LE/AAO coating surface after modification contains a large air layer that effectively isolates Cl− in the corrosive medium, significantly reducing Cl− corrosion. Additionally, compared to the superhydrophobic LE layer, the superhydrophobic LE/AAO coating has a more positive corrosion potential, lower corrosion current density, and lower corrosion rate due to its higher air layer proportion. The corrosion resistance mechanism of the superhydrophobic coating is illustrated in Figure 8.
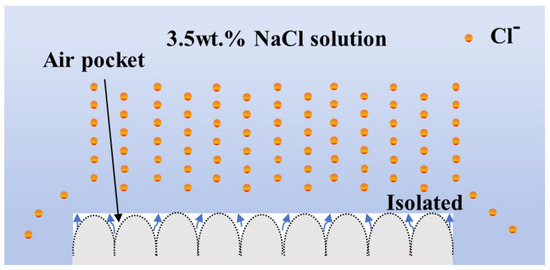
Figure 8.
Corrosion resistance mechanism diagram of superhydrophobic coating.
3.5. Mechanical Durability Analysis
Figure 9a,b displays the WCA and WSA curves of different superhydrophobic coatings subjected to varying numbers of friction cycles. These curves show that the WCA of both the superhydrophobic LE layer and the superhydrophobic LE/AAO coating decreases with an increase in friction cycles, but the WCA of the LE/AAO coating remains higher than that of the LE layer throughout the cycles. With increasing friction cycles, the WSA of both the LE layer and LE/AAO coating increases, but the LE/AAO coating consistently exhibits a lower WSA, demonstrating better hydrophobic performance throughout the entire process
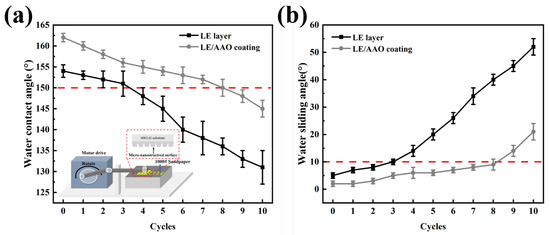
Figure 9.
Mechanical durability test of superhydrophobic coating. (a) Water contact angle curves with the number of friction cycles (b). Water sliding angle curves with the number of friction cycles.
After four friction cycles, the WCA of the LE/AAO coating decreases to 155°, and the WSA increase to 6°, still maintaining excellent superhydrophobicity (superior to the initial state of the LE layer), whereas the LE layer loses its superhydrophobicity with a WCA of approximately 148° after four cycles (the WSA increase to about 14°). At seven friction cycles, the LE/AAO coating retains superhydrophobicity with a WCA of 152° and a WSA of 8°, while the LE layer’s WCA drops below 150° and its WSA increases to above 10°. By nine cycles, the LE/AAO coating loses superhydrophobicity, showing a contact angle of 148°, representing a 125% improvement in mechanical durability (5 additional cycles, 500 mm increased distance) compared to the LE layer’s four cycles. This demonstrates that the introduction of the AAO layer not only enhances the superhydrophobic performance but also significantly improves the mechanical durability of the LE-based superhydrophobic surface.
The microstructural characterization of the superhydrophobic LE and LE/AAO coatings before and after mechanical durability tests is shown in Figure 10a1–b2, comparing morphologies after seven friction cycles. Before testing, both coatings exhibit intact structures, with clear nanopores at the boundaries of protrusions and pits in the LE/AAO coating (initial values of WCA and WSA are 162° and 2°). After seven cycles, the LE layer shows severe wear with scratches penetrating between adjacent protrusions, accompanied by a significant WCA decrease to 136° (the WSA increases to about 34°). In contrast, the LE/AAO coating only shows minor wear on the protrusion tops, likely due to the AAO coating’s increased hardness and wear resistance, which also aids in debris entrapment. The uniform nanopores in the LE/AAO coating allow the hierarchical “pore wall-pore” structure to sustain water droplet support and air layer retention even after top surface abrasion, maintaining superhydrophobicity (WCA and WSA are about 152° and 8°, respectively). Conversely, the LE layer’s pure Al-based structure lacks robust support after protrusion wear, leading to rapid loss of superhydrophobicity. The wetting state and durability enhancement mechanisms of the two coatings before and after friction are illustrated in Figure 10c.
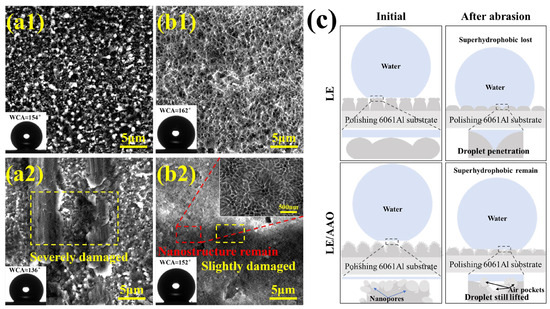
Figure 10.
Wear resistance mechanism analysis. (a1,a2) Morphology of superhydrophobic LE layer before and after friction, (b1,b2) Morphology of superhydrophobic LE/AAO coating before and after friction, (c) Ideal model of wear of two coatings (The interpolated image is the water contact angle characterization result.).
4. Conclusions
This study fabricated superhydrophobic aluminum alloy surfaces with enhanced performance by combining femtosecond laser etching and anodic oxidation to construct uniform periodic micro/nano composite arrays at the microscale and submicron scales. After anodic oxidation, a barrier-dominant oxide film with “pore wall-pore” nanopore structures grew uniformly on the laser-etched LE layer, imparting additional superhydrophobicity with a contact angle of 162°. The anodic oxidation process also improved the corrosion resistance of the samples, attributed to the enhanced surface uniformity and superhydrophobicity. In linear friction wear tests, the superhydrophobic LE/AAO coating exhibited excellent abrasion resistance. The uniform nanopore arrays effectively protected the surface structural features, enabling the samples to maintain superior superhydrophobic performance after sustained friction, with superhydrophobic durability improved by 125% compared to the LE layer.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/coatings15070816/s1: Figure S1. Surface morphology and water contact angles of samples before and after FAS modification. (a) Polished 6061Al substrate before FAS modification; (b) LE layer before FAS modification; (c) LE/AAO coating before FAS modification; (d) Polished 6061Al substrate after FAS modification; (e) LE layer after FAS modification; (f) LE/AAO coating after FAS modification. Figure S2. Surface micromorphology of aluminum alloy samples anodized at 60 V for different durations: (a1–a3) 10 min; (b1–b3) 20 min; (c1–c3) 30 min; (d1–d3) 40 min. Figure S3. Wetting mechanism of samples at different anodization times. (a1–d1) Water contact angle measurements on sample surfaces under different anodization conditions; (a2–d2) Theoretical surface wetting models corresponding to samples with varying anodization parameters.
Author Contributions
Data curation, Q.L.; Writing—original draft, Q.L.; Writing—review and editing, Y.W.; Funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Jiangsu Provincial Outstanding Overseas Talent Project (BX2023029), the Jiangsu Provincial Natural Science Fund Research Project (BK20211344), and the Jiangsu Provincial Postgraduate Research & Practice Innovation Program (SJCX24_2540).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feng, W. Status and development trends for fluorinated carbon in China. New Carbon Mater. 2023, 38, 130–142. [Google Scholar] [CrossRef]
- Sun, H.L.; Bu, Y.B.; Liu, H.; Wang, J.W.; Yang, W.K.; Li, Q.M.; Guo, Z.H.; Liu, C.T.; Shen, C.Y. Superhydrophobic conductive rubber band with synergistic dual conductive layer for wide-range sensitive strain sensor. Sci. Bull. 2022, 67, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.X.; Deng, Q.W.; Yang, P.Y.; Yin, K. Femtosecond laser-chemical hybrid processing for achieving substrate-independent superhydrophobic surfaces. J. Cent. South Univ. 2024, 31, 1–10. [Google Scholar] [CrossRef]
- Cao, X.K.; Sun, X.G.; Cai, G.Y.; Dong, Z.H. Durable Superhydrophobic Surfaces: Theoretical Models, Preparation Strategies, and Evaluation Methods. Prog. Chem. 2021, 33, 1525–1537. [Google Scholar] [CrossRef]
- Zhang, W.B.; Wang, J.N.; Wei, L.F.; Jin, H.; Bao, Y.; Ma, J.Z. Preparation and Application of Functional Polymer-Based Electromagnetic Shielding Materials. Prog. Chem. 2023, 35, 1065–1076. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Tian, Y.; Zhang, D.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P.; Lu, Y. Robust and durable liquid-repellent surfaces. Chem. Soc. Rev. 2022, 51, 8476–8583. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A.K. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Ataei, S.; Khorasani, S.N.; Neisiany, R.E. Biofriendly vegetable oil healing agents used for developing self-healing coatings: A review. Prog. Org. Coat. 2019, 129, 77–95. [Google Scholar] [CrossRef]
- Bake, A.; Merah, N.; Matin, A.; Gondal, M.; Qahtan, T.; Abu-Dheir, N. Preparation of transparent and robust superhydrophobic surfaces for self-cleaning applications. Prog. Org. Coat. 2018, 122, 170–179. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Abu Jarad, N.; Imran, H.; Imani, S.M.; Didar, T.F.; Soleymani, L. Fabrication of Superamphiphobic Surfaces via Spray Coating: A Review. Adv. Mater. Technol. 2022, 7, 2101702. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Yang, Y.; Liu, Z.; Wang, H.; He, Z. Nanostructured Superhydrophobic Titanium-Based Materials: A Novel Preparation Pathway to Attain Superhydrophobicity on TC4 Alloy. Nanomaterials 2022, 12, 2086. [Google Scholar] [CrossRef]
- Douglas, L.D.; O’Loughlin, T.E.; Chalker, C.J.; Cool, N.; Gupta, S.; Batteas, J.D.; Banerjee, S. Three-Dimensional Inverse Opal TiO2 Coatings to Enable the Gliding of Viscous Oils. Energy Fuels 2020, 34, 13606–13613. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Jiang, G.; Luo, X.; Chen, C.; Hu, X.; Zhang, H.; Zhong, M. Spontaneous dewetting transitions of droplets during icing & melting cycle. Nat. Commun. 2022, 13, 47–53. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, B.; Zhang, X.; Tang, L.; Lin, X.; Hu, C.; Ren, K. Enhanced corrosion resistance and self-cleaning properties of superhydrophobic nickel coating fabricated by one-step electrodeposition. Ceram. Int. 2023, 49, 13109–13118. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Wang, Y. AAO-ZnO nanocomposite coatings on aluminum alloy surface with high bonding strength and long-lasting antibacterial properties. Chem. Eng. J. 2024, 497, 154414. [Google Scholar] [CrossRef]
- Jiang, K.D.; Liao, Z.X.; Chen, M.Y.; Liu, S.D.; Tang, J.G. Impact of cooling rate on exfoliation corrosion resistance of a Li-containing 7xxx aluminum alloy. J. Cent. South Univ. 2024, 31, 2225–2236. [Google Scholar] [CrossRef]
- Kang, M.L.; Deng, Y.L.; Lei, J.Q. Effect of Roughness and Intermetallic Particles on Surface Corrosion of A6111 Alloy. Rare Met. Mat. Eng. 2024, 53, 1252–1261. [Google Scholar] [CrossRef]
- Kong, D.J.; Liu, H.; Wang, J.C. Energy Spectrum Scan Analysis of 7475 Aluminum Alloy Surface-Interface by Micro-arc Oxidation. Rare Met. Mat. Eng. 2016, 45, 2587–2592. [Google Scholar]
- Liu, S.; Chen, J.; Zhang, D.; Wang, Y.; He, Z.; Guo, P. Properties of Micro-Arc Oxidation Coatings on 5052 Al Alloy Sealed by SiO2 Nanoparticles. Coatings 2022, 12, 373. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, L.; He, Z.; Tan, J.; Singh, H.; Hayat, M.D.; Yao, C. Preparation and characterisation of AAO/Ni/Ni superhydrophobic coatings on aluminium alloys. Surf. Eng. 2021, 37, 1246–1254. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; He, Z.; Chen, J.; Gao, W.; Cao, P. Superhydrophobic Ni nanocone surface prepared by electrodeposition and its overall performance. Surf. Coat. Technol. 2023, 464, 129548. [Google Scholar] [CrossRef]
- Dong, J.; Han, J.; Ouyang, X.; Gao, W. How voltage dictates anodic TiO2 formation. Scr. Mater. 2015, 94, 32–35. [Google Scholar] [CrossRef]
- Gao, P.H.; Li, J.P.; Yang, Z.; Guo, Y.C.; Wang, Y.R. Preparation of Al/SiC Composite Coatings on Surface of Aluminum Alloy by Atmospheric Plasma Spraying. Rare Met. Mat. Eng. 2015, 44, 2396–2400. [Google Scholar]
- Neelakantan, N.K.; Weisensee, P.B.; Overcash, J.W.; Torrealba, E.J.; King, W.P.; Suslick, K.S. Spray-on omniphobic ZnO coatings. RSC Adv. 2015, 5, 69243–69250. [Google Scholar] [CrossRef]
- Tong, Q.; Fan, Z.; Wang, B.; Liu, Q.; Bo, Y.; Qian, L. Preparation and Application of Superhydrophobic Copper Mesh by Chemical Etching and In-situ Growth. Front. Chem. 2021, 9, 737550. [Google Scholar] [CrossRef]
- Tong, W.; Xiong, D. Direct laser texturing technique for metal surfaces to achieve superhydrophobicity. Mater. Today Phys. 2022, 23, 100651. [Google Scholar] [CrossRef]
- Xin, G.; Wu, C.; Liu, W.; Rong, Y.; Huang, Y. Anti-corrosion superhydrophobic surfaces of Al alloy based on micro-protrusion array structure fabricated by laser direct writing. J. Alloys Compd. 2021, 881, 160649. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, F.; Ouyang, C. Electroless Ni–P alloys on nanoporous ATO surface of Ti substrate. J. Mater. Sci. 2017, 53, 2812–2829. [Google Scholar] [CrossRef]
- Liang, G.; Lu, F.; Zhang, B. Robust superhydrophobic composite coating with dual-sized particles for self-cleaning and anti-corrosion of 5083 aluminum alloy. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 136020. [Google Scholar] [CrossRef]
- Verro, V.; Di Franco, F.; Zaffora, A.; Santamaria, M. Enhancing corrosion resistance of anodized AA7075 alloys by electrodeposition of superhydrophobic coatings. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132040. [Google Scholar] [CrossRef]
- Masoud Emarati, S.; Mozammel, M. Theoretical, fundamental and experimental study of Liquid-repellency and corrosion resistance of fabricated superamphiphobic surface on Al alloy 2024. Chem. Eng. J. 2020, 387, 124046. [Google Scholar] [CrossRef]
- Fan, P.; Bai, B.; Zhong, M.; Zhang, H.; Long, J.; Han, J.; Wang, W.; Jin, G. General Strategy toward Dual-Scale-Controlled Metallic Micro–Nano Hybrid Structures with Ultralow Reflectance. ACS Nano 2017, 11, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.G.; Zhu, Y.C.; Zhang, X.; Luo, B.P. Effects of Superhydrophobic Surface on Tribological Properties: Mechanism, Status and Prospects. Prog. Chem. 2020, 32, 320–330. [Google Scholar] [CrossRef]
- Linklater, D.P.; Ivanova, E.P. Nanostructured antibacterial surfaces—What can be achieved? Nano Today 2022, 43, 101404. [Google Scholar] [CrossRef]
- Qu, M.N.; Hou, L.G.; He, J.M.; Ma, X.R.; Yuan, M.J.; Liu, X.R. Research and Development of Functional Superhydrophobic Materials. Prog. Chem. 2016, 28, 1774–1787. [Google Scholar] [CrossRef]
- Wang, B.; An, W.; Wang, L.; Jiao, L.; Zhang, H.; Song, H.; Liu, S. Superhydrophobic and Antibacterial Hierarchical Surface Fabricated by Femtosecond Laser. Sustainability 2022, 14, 12412. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Zhao, N.; Lu, X.Y.; Zhang, X.Y.; Liu, H.Y.; Tan, S.X.; Xu, J. Progress in superhydrophobic surfaces. Prog. Chem. 2007, 19, 860–871. [Google Scholar]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. Fabrication of superhydrophobic surface on stainless steel by two-step chemical etching. Chem. Phys. Lett. 2022, 797, 139567. [Google Scholar] [CrossRef]
- da Silva, R.G.C.; Malta, M.I.C.; de Carvalho, L.A.P.; da Silva, J.J.; da Silva Filho, W.L.C.; Oliveira, S.H.; de Araújo, E.G.; Urtiga Filho, S.L.; Vieira, M.R.S. Low-cost superhydrophobic coating on aluminum alloy with self-cleaning and repellency to water-based mixed liquids for anti-corrosive applications. Surf. Coat. Technol. 2023, 457, 129293. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.-H. A durable, fluorine-free, and repairable superhydrophobic aluminum surface with hierarchical micro/nanostructures and its application for continuous oil-water separation. J. Membr. Sci. 2021, 618, 118716. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Liu, Z.; Zheng, S.; Zhu, L.; He, Z. Robust and durable superhydrophobic coating prepared via a combined method of laser marking and electrodeposition. J. Mater. Res. Technol. 2023, 26, 477–486. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).