Abstract
The (1−x)Ba0.96Ca0.04TiO3-xBa(Mg1/3Nb2/3)O3 (x = 0–0.20) lead-free ceramics were prepared through the chemical-furnace-assisted combustion synthesis (abbreviated as CFACS). The phase structure, microstructure, dielectric, and piezoelectric properties were systematically investigated. Phase analysis revealed the coexistence of orthorhombic and tetragonal phases in the vicinity of x = 0.07. More importantly, the composition with x = 0.07 exhibited optimal overall electrical properties, including a high piezoelectric coefficient (d33) of 495 pC/N, the planar electromechanical coupling factor (Kp) of 41.9%, and the Curie temperature (Tc) of 123.7 °C. In addition, the average grain size was observed to progressively decrease with increasing x.
1. Introduction
Piezoelectric devices have become indispensable core components in modern sensors, drives, and energy harvesting systems derived from their excellent electromechanical conversion capabilities, high sensitivity and rapid response characteristics [1,2,3]. Driven by growing environmental concerns and legislation against lead-based products, BaTiO3 (BT)-based piezoelectric ceramics are considered a prime alternative to the dominant lead zirconate titanate (PZT) systems [4]. Moreover, the various phase structures of BaTiO3 ceramics can cause lattice mismatch and internal stress. This internal stress produces a pinning effect that hinders domain steering in the field, which is not favorable for obtaining excellent piezoelectric properties. Chen et al. found that introducing Ca2+ ions into BaTiO3-based ceramics enhances the piezoelectric properties to 578 pC/N at a Ca content of 0.04. This is attributed so that the suppression of the hexagonal phase can promote the transformation from orthorhombic to tetragonal phase transition, and reduce long-range ferroelectric domain ordering [5]. However, poor temperature-stability of Ba0.96Ca0.04TiO3 (BCT) ceramics restricts its practical application. The introduction of other elements is an effective approach to enhance electric performance [6,7,8,9]. Ba(Mg1/3Nb2/3)O3 (BMN) was reported to be effective to improve dielectric properties in BaTiO3 ceramics [10], as well as Curie temperature (Tc) to 380 °C in (Ba0.85Ca0.15)(Ti0.9Zr0.1) ceramics and to 280 °C in Bi1/2Na1/2TiO3 ceramics, respectively. In addition, the Mg2+ and Nb5+ ions introduced in BMN can induce field-induced strain effects, which is conducive to further improving the piezoelectric performance [11,12]. Noteworthy, the study of microstructural evolution and electrical properties of BT-based composite ceramics doped with BMN is still rare. This is mainly attributed that the introduction of BMN ceramics fails to achieve a small grain size. A large grain size makes it difficult for the electric domain to turn, which is not conducive to further improving the piezoelectric performance.
Conventionally, the preparation of BT-based ceramics relies on the solid-state reaction method, which suffers from disadvantages including extended processing durations and substantial energy requirements [13]. The long-time sintering in high temperature inevitably accompanies grain growth and degradation of the microstructures, which leads to the deterioration of the electrical properties. The CFACS is a furnace-free and energy saving approach to prepare inorganic ceramics [14,15]. Rapid grain growth can be limited by the CFACS process as reported in superconductor material, cermets, and their composites [16,17,18]. The highly exothermic combustion reaction is a self-sustained process that can be completed rapidly in a short period of a few seconds [19,20].
Therefore, in this work, the CFACS method is introduced to synthesize (1−x)Ba0.96Ca0.04TiO3-xBa(Mg1/3Nb2/3)O3 (x = 0–0.20) ceramics. The phase evolution, microstructural characteristics along with electrical performance of composite ceramics were systematically investigated.
2. Experimental Section
The (1−x)Ba0.96Ca0.04TiO3-xBa(Mg1/3Nb2/3)O3 [BCT-xBMN x = 0–0.20] ceramics were fabricated through the CFACS. The BaCO3 (99.0%), CaCO3 (99.0%), MgO (99.7%), TiO2 (99%), and Nb2O5 (99.99%) materials were weighted according to the stoichiometric formula. The first step is to mix the powders in ethanol through a Planetary ball mill for 12 h, followed by drying at 120 °C for 2 h. Subsequently, the dried powders were compacted into pellets with size of Ø20 mm × 3 mm for further measurements.
Specifically, the CFACS occurred in a closed environment, as shown in Figure 1. A total of 100 g of thermite (Ti + C + 2Al + Al2O3 + Fe2O3) powers were placed in a graphite crucible with a diameter of 80 mm, where the disk wrapped with carbon paper was buried. Firstly, the reaction chamber was evacuated and inflated with argon to a pressure of 0.5 MPa. Then, at an electric current of 10 A, the top of the thermite was ignited using a tungsten coil. The thermite reaction kept burning until the powder products generated in a self-sustained process. The powders were naturally cooled down in the furnace and then pressed into particles with a diameter of 12 mm. Finally, the samples were burnt off at 1280 °C for 3 h in air.
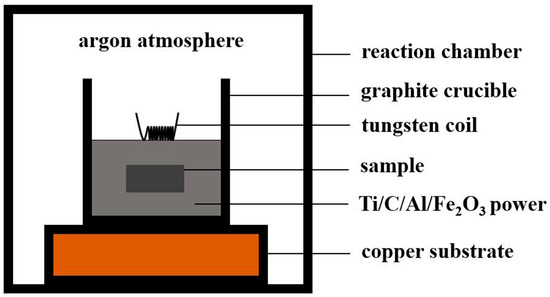
Figure 1.
Diagram of the experimental apparatus for assisted combustion synthesis in chemical furnace.
The phase structure of the samples was investigated using an X-ray diffraction (Japan Rigaku, D/max-2500/PC, Cu Kα1, λ = 1.5406 Å, Tokyo, Japan). The Raman spectrum was recorded through Raman spectrometer with a wavelength of 514 nm (InVia Refex, Renishaw, London, UK). The surface morphology was observed using scanning electron microscopy (SEM; S-4800, Hitachi, Tokyo, Japan). The bulk density of the sample was determined via the Archimedes method. Processing of the sample for the electrical performance test: the sample was polished, then both sides were coated with silver pastes, and finally fired at 650 °C for 30 min to obtain electrodes. The TH2818 Automatic Component Analyzer (Changzhou Tonghui Electronic Co., Ltd., Changzhou, China) was used to measure dielectric properties. A Radiant Precision Premier LC ferroelectric material test system (Radiant Technologies Inc., Albuquerque, NM, USA) was employed to record the polarization-electric field (P-E) hysteresis loops. The piezoelectric constants of the samples were characterized by quasi-static d33 m (ZJ-3AN, Institute of Acoustics, Chinese Academy of Sciences, Beijing, China).
3. Results and Discussions
Figure 2a illustrates the reaction temperature profile of the (1−x)Ba0.96Ca0.04TiO3-xBa(Mg1/3Nb2/3)O3 (x = 0.07) powders fabricated by the CFACS. The temperature of the reacting curve rises quickly to above 1600 °C for about 10 s corresponding to the reaction initiating, then the temperature drops quickly, and the reaction lasts for about 200 s above 1200 °C. The XRD pattern of the (1−x)BCT-xBMN (x = 0.07) powders by the CFACS at around 1200 °C is shown in Figure 2b. The sample exhibits mainly perovskite structure. Some additional peaks arose from inadequately reacted powders during the rapid reaction process. The SEM diagram inserted in Figure 2b shows that the microstructure of (1−x)BCT-xBMN (x = 0.07) powder is uniformly distributed with an average particle size of approximately 300 nm. Because of the faster heating and cooling process during the chemical synthesis, the prepared powder exhibited smaller particle size than that fabricated by traditional preparation method [21]. Such a process is too fast for the particles to grow, and fine powers are naturally associated with high sintering ability.
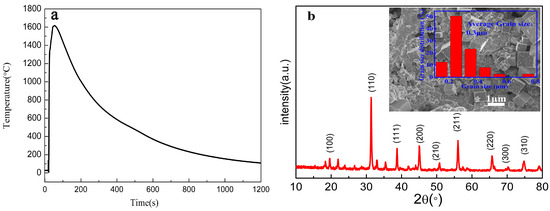
Figure 2.
(a) The reaction temperature profile of the prepared sample (1−x)Ba0.96Ca0.04TiO3-xBa(Mg1/3Nb2/3)O3 (x = 0.07). (b) The XRD patterns of the corresponding powders (illustration is SEM image and grain distribution).
Figure 3a displays the XRD patterns of the BCT-xBMN ceramics. All compositions crystallize in a pure perovskite structure, with the absence of secondary phases confirming the formation of a solid solution as Mg2+ and Nb5+ ions dissolve into the BaTiO3 lattice. As illustrated in Figure 3b, the samples exhibit a single peak with x = 0 to 0.05 at 2θ around 45°, then split into two peaks with further x increasing. This indicates that the samples exhibit single orthorhombic phase at 0 ≤ x< 0.07, and the coexistence of orthorhombic and tetragonal phase (PDF#79-2264) at 0.07 ≤ x < 0.20. Moreover, Figure 3c shows that all diffraction peaks consistently shift to lower 2θ values with increasing x. This peak shift indicates an expansion of the unit cell volume, which is ascribed to the replacement of smaller Ti4+ (0.605 Å) ions by the larger (Mg1/3Nb2/3)4+ (0.693 Å) complex at the B-site of the B-O6 octahedra [22,23,24].
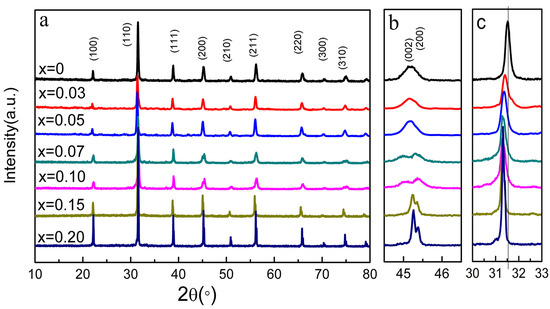
Figure 3.
(a) XRD patterns of the BCT-xBMN ceramics at x = 0–0.2. (b) The enlarged patterns from 44° to 47°. (c) The enlarged patterns from 30°–33°.
To further characterize the structure evolution, Raman spectra were acquired for all the samples. Figure 4 shows room-temperature Raman spectra of the BCT-xBMN (x = 0–0.2) ceramics in the frequency range of 100–1000 cm−1. The Raman spectra of the ceramics are similar to that of BaTiO3, suggesting that these compositions share the basic unit structure [25]. The Raman peaks located near 717, 518, 306, and 270 cm−1 are the characteristic vibrational modes of the ferroelectric orthorhombic phase of BaTiO3 at room temperature [26]. It is noteworthy that the peak located at around 188 cm−1 undergoes significant broadening with increasing doping. This phenomenon is attributed to the intensified tilting or rotation of BO6 octahedra, which induces local structural disorder [27]. The enhancement of this structural disorder weakens the original long-range ferroelectric order in the crystal, manifesting macroscopically as a reduction in the orthorhombic phase content. The evolution is consistent with the XRD results shown in Figure 3.
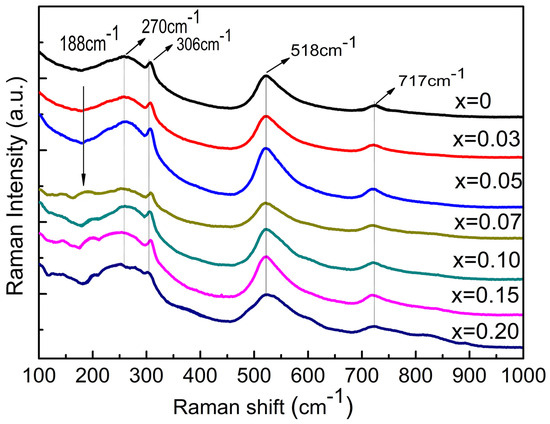
Figure 4.
Raman spectra of the (1−x)BCT-xBMN (x = 0–0.2) ceramics at room temperature.
Figure 5 exhibits SEM micrographs of the BCT-xBMN ceramics. The microstructures were observed to be more homogeneous with x increasing. For the undoped composition, the sample exhibits a relatively small grain size of approximately 1.31 μm, which can be attributed to the ultra-fast crystallization process of the powder products synthesized during the CFACS. As shown in the inset of Figure 5, the average grain size of each composition is 1.31 μm, 1.21 μm, 1.14 μm, 1.10 μm, 0.96 μm, 0.92 μm, 0.86 μm, respectively. The reduction in grain size is likely governed by the solute drag mechanism. The disparity in ionic radii between the larger dopant ions (Mg2+ 0.72 Å, Nb5+ 0.64 Å) and the smaller host ion (Ti4+, 0.605 Å) generates significant lattice strain energy (ΔGstrain). As these dopants occupy the Ti4+ sites, the accumulation of this strain energy impedes grain boundary migration, thereby suppressing grain growth. As can be seen from Figure 5h, the corresponding relative density increases from 88.7% (x = 0) to a maximum value of 93.7% (x = 0.07). The high densification of the ceramic in this range reduces surface defects, which in turn mitigates charge concentration and contributes to an enhanced polarization. However, as x increases further, the density subsequently decreases to 84.6% at x = 0.2. This phenomenon is closely correlated with the complexities of domain switching and domain wall mobility at higher dopant concentrations [12].
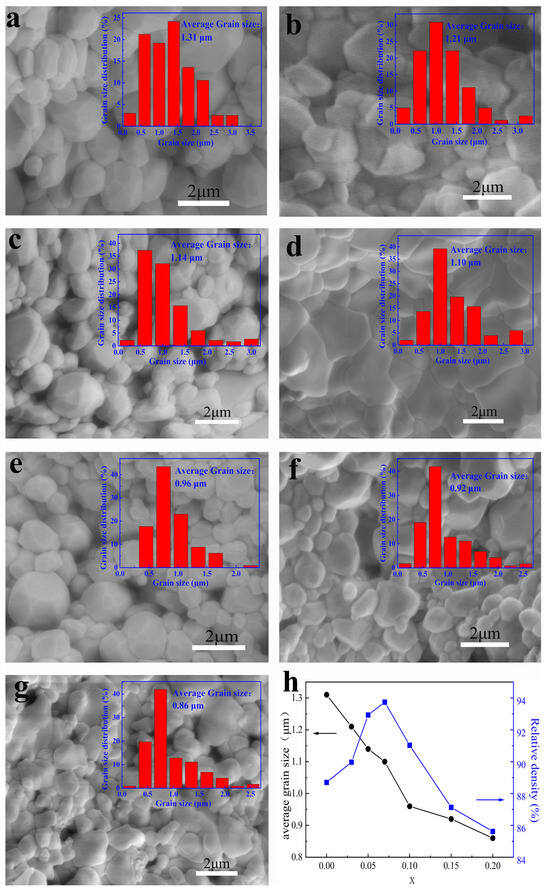
Figure 5.
(a–g) SEM micrographs of the BCT-xBMN ceramics at x = 0–0.2. The illustration in the upper right corner represents the grain size distribution of each composition; (h) variations in the grain size and relative density of ceramics with x.
Figure 6 shows the temperature dependence of the dielectric permittivity (εr) for the BCT-xBMN ceramics at 100 kHz. With the increase in x, the dielectric peak widens gradually, suggesting polarization fluctuations in the ceramic volume or fluctuations in spatial components. To quantitatively evaluate this peak broadening, the Full Width at Half Maximum (FWHM) from the dielectric-temperature spectrum is employed, which can be expressed based on the following equation: FWHM = T2 − T1, where T1 and T2 represent the temperatures on either side of the dielectric peak corresponding to half its maximum value. The calculated FWHM values of each component are 62, 67, 77, 92, 101, 107, and 109, respectively, indicating excellent relaxation properties in the ceramics during the transition from ferroelectric phase to paraelectric phase. The dielectric constant increases first and then decreases with the increase in x, and reaches a peak value at x = 0.07. This is related to the transformation of phase structure. Compared with the tetragonal structure, the orthorhombic structure has more polarization vectors, so it can obtain higher dielectric constant. With the further increase in x, the dielectric constant decreases slightly, which is related to the small grain size and the change in density [26,28]. Moreover, the εr decreasing with further addition of x content is derived from the increased volume fraction of the grain boundaries. A similar decrease in grain size and the εr on BMN doping was reported in the BT and (K,Na)NbO3 (KNN) system [29]. At the same time, it can be seen from the inset that the Tc of the BCT-xBMN ceramics increases gradually from 107 °C (x = 0) to 124.7 °C (x = 0.10), then decreases to 110 °C (x = 0.20). Under rapid reaction conditions, the limited diffusion time prevents the complete penetration of BMN into the BaTiO3 grains, leading to the formation of a core–shell structure with a BaTiO3-rich core and a BMN-rich shell [10]. The results show that the ferroelectric-paraelectric transition temperature caused by dipole polarization is above 110 °C [30].
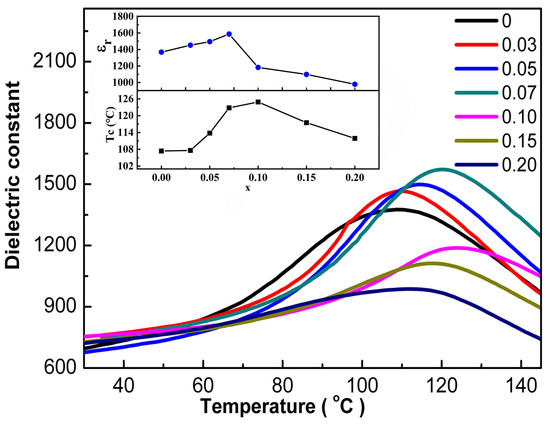
Figure 6.
Dielectric temperature spectrum of BCT-xBMN ceramics. The illustration represents the Tc and εr with different BMN contents.
In order to further analyze the dielectric relaxation performance, the experimental data can be fitted using a modified Curie–Weiss law: 1/ε – 1/εm = (T − Tm)γ/C (T > Tm), where the exponent γ, εm, T, Tm, and C represent the dielectric relaxation, maximum value of dielectric permittivity, temperature, temperature for εm, and Curie-like constant, respectively [23]. Figure 7 exhibits that the values of γ are 1.84, 1.75, 1.68, 1.69, 1.83, 1.78, 1.81 for the samples of the BCT-xBMN ceramics with x = 0–0.2, respectively. The introduction of BMN into the BCT ceramic matrix serves to break down the long-range ferroelectric order. The mismatch in both valence state and ionic radius from the BMN dopants induces the fragmentation of large domains into a dense arrangement of finer nanodomains. This refined domain configuration is a key factor in the enhancement of the material’s electrical properties. In addition, small grain size can inhibit domain steering and obtain strong relaxation ferroelectric behavior.
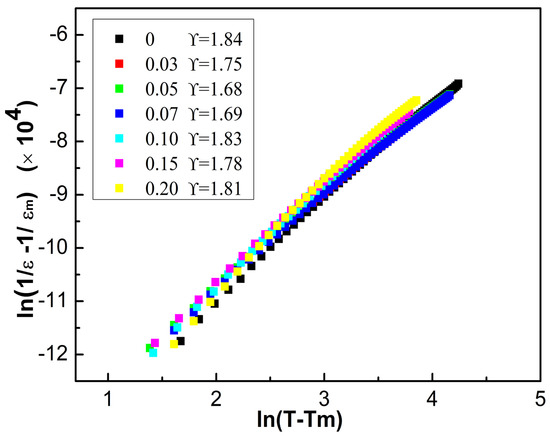
Figure 7.
Dispersion coefficient of the BCT-xBMN ceramics.
Figure 8 shows d33 and Kp of the BCT-xBMN ceramics with x = 0–0.2. The d33 and Kp of samples were greatly improved at 0 < x ≤ 0.07, but then exhibit a decreasing trend with further increases in x. The optimal piezoelectric properties are achieved at the x = 0.07, with a peak d33 value of 495 pC/N and a Kp of 41.9%. This significant enhancement is primarily attributed to the presence of a morphotropic phase boundary (MPB) near this composition, coupled with the microstructural effects arising from the orthorhombic-to-tetragonal phase transition [11]. A comparison of the piezoelectric constant and the plane electromechanical coupling coefficient achieved in this work with those of other lead-free ceramics by other techniques, illustrating excellent d33 and Kp of theBCT-xBMN ceramics [4,9,31,32,33,34]. It is notable that the decrease of d33 and Kp with further x increasing corresponds to the grain sizes decrease, which is related to the domain wall size [35]. The moving of the domain walls becomes difficult with the domain size decreasing, consequently, leading to the reduction in piezoelectric performance [15].
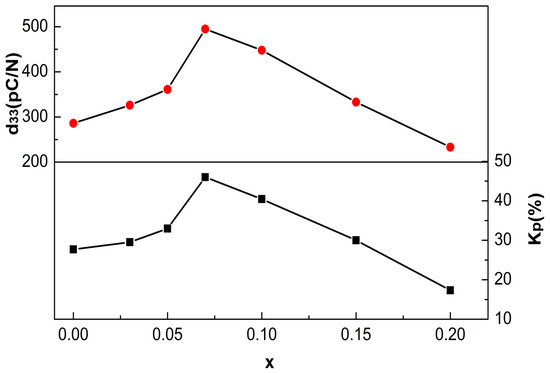
Figure 8.
The poling electric field dependence of piezoelectric properties of the BCT-xBMN ceramics.
Figure 9 displays the P-E diagrams of BCT-xBMN samples at 30 kV/cm and 1 Hz at room temperature. All the hysteresis loops possess well-saturated characteristics. The inset shows the Pr and Ec under different contents. The Pr and Ec of the BCT-xBMN ceramics decrease gradually with x increasing. This can be explained by the Avrami model for ferroelectrics proposed by Orihara [33]. The decrease of Pr derived from the decrease in grain size, which can deteriorate the polarization reverses. The Ec of the samples decreases gradually from 3.05 kV/cm (x = 0) to 0.82 kV/cm (x = 0.2), indicating that ferroelectric properties of BCT-based samples can be enhanced through BMN-doped. The low value of Ec (<1.77 kV/cm) shows quite “soft” feature in 0.05 < x < 0.2. This trend indicates that the excellent piezoelectric properties of samples can be obtained through tailoring the poling condition.
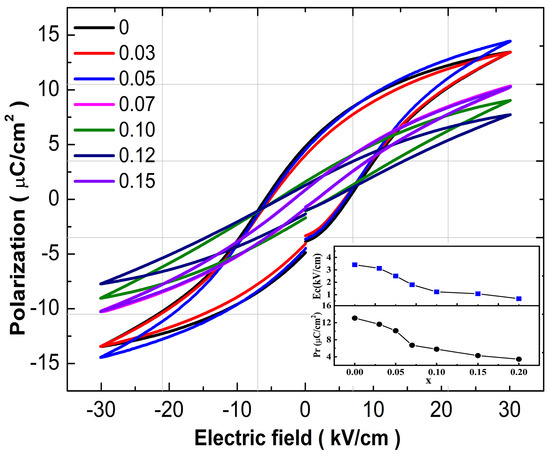
Figure 9.
The hysteresis loop (P-E) of the BCT-xBMN ceramics at 30 kV/cm and 1 Hz with x = 0–0.02. Illustrations represent Pr and Ec.
4. Conclusions
The (1−x)Ba0.96Ca0.04TiO3-xBa(Mg1/3Nb2/3)O3 (x = 0–0.20) ceramics were synthesized via the chemical-furnace-assisted combustion synthesis. The effects of Ba(Mg1/3Nb2/3)O3 (BMN) doping content on the phase structure, microstructure, and electrical properties of the ceramics were systematically investigated. Phase analysis revealed the formation of a morphotropic phase boundary (MPB) at around x = 0.07, characterized by the coexistence of orthorhombic and tetragonal phases. Fine-grained and homogeneous microstructures were formed by the CFACS process, and doping of BMN further inhibits the grain growth of grains and form the homogeneous microstructures. Consequently, the ceramic at x = 0.07 demonstrated optimal comprehensive electrical properties with d33 = 495 pC/N, Kp = 41.9%, Tc = 123.7 °C. The CFACS is proved to be a good fabrication method to piezoelectric ceramics.
Author Contributions
Conceptualization, H.D.; Methodology, H.D. and J.W.; Software, T.Q. and L.C.; Validation, H.D.; Formal analysis, H.D., T.Q., L.C., G.J. (Guang Ji) and Z.L.; Investigation, H.D.; Resources, G.J. (Guodong Jia); Data curation, H.D. and J.W.; Writing—original draft, H.D.; Writing—review & editing, H.D.; Visualization, G.J. (Guodong Jia) and G.J. (Guang Ji); Funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (24KJB430035), Nantong Natural Science Foundation (JCZ2024010) and Nantong Social Livelihood Science and Technology Project (MSZ2024064).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Jun Wang was employed by the company Zhejiang Cfmoto Power Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to the existing affiliation information. This change does not affect the scientific content of the article.
References
- Peng, R.B.; Zhang, B.T.; Dong, G.H.; Wang, Y.H.; Yang, G.N.; Zhang, J.; Peng, B.; Zhao, Y.N.; Liu, M. Enhanced Piezoelectric Energy Harvester by employing freestanding single-crystal BaTiO3 Films in PVDF-TrFE Based composites. Adv. Funct. Mater. 2024, 34, 2316519. [Google Scholar] [CrossRef]
- Sheng, J.; Qiao, Y.; Zhang, W.; Cao, W.; Gao, C.; Li, W. Enhanced piezoelectric properties and depolarization temperature in NBT-based ceramics by doping BT nanowires. J. Alloy. Compd. 2020, 819, 153045. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.; Li, H.; Ye, H.; Cheng, J.; Wu, S.; He, X.; Jian, B.; Tao, R.; Ge, Q. High-precision BaTiO3 piezoelectric ceramics via vat photopolymerization 3D printing. J. Eur. Ceram. Soc. 2024, 44, 116706. [Google Scholar] [CrossRef]
- Nallagatla, V.R.; Maier, C.; Glettler, J.; Feteira, A.; Reichmann, K.; Deluca, M. Synergistic homovalent and heterovalent substitution effects on piezoelectric and relaxor behavior in lead-free BaTiO3 ceramics. J. Eur. Ceram. Soc. 2024, 44, 116689. [Google Scholar] [CrossRef]
- Sharma, P.; Berwal, N.; Ahlawat, N.; Maan, A.; Punia, R. Study of structural, dielectric, ferroelectric and magnetic properties of vanadium doped BCT ceramics. Ceram. Int. 2019, 45, 20368–20378. [Google Scholar] [CrossRef]
- Sharma, M.; Halder, A.; Vaish, R. Effect of Ce on piezo/photocatalytic effects of Ba0.9Ca0.1CexTi1-xO3 ceramics for dye/pharmaceutical waste water treatment. Mater. Res. Bull. 2020, 122, 110647. [Google Scholar] [CrossRef]
- Puli, V.S.; Pradhan, D.K.; Riggs, B.C.; Chrisey, D.B.; Katiyar, R.S. Investigations on structure, ferroelectric, piezoelectric and energy storage properties of barium calcium titanate (BCT) ceramics. J. Alloy. Compd. 2014, 584, 369–373. [Google Scholar] [CrossRef]
- Zhu, L.-F.; Zhang, B.-P.; Zhao, L.; Li, S.; Zhou, Y.; Shi, X.-C.; Wang, N. Large piezoelectric effect of (Ba,Ca)TiO3-xBa(Sn,Ti)O3 lead-free Ceramics. J. Eur. Ceram. Soc. 2016, 36, 1017–1024. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, B.; Thong, H.-C.; Wu, J. Improved temperature stability and high piezoelectricity in lead-free barium titanate-based ceramics. J. Eur. Ceram. Soc. 2018, 38, 5411–5419. [Google Scholar] [CrossRef]
- Munpakdee, A.; Pengpat, K.; Tontrakoon, J.; Tunkasiri, T. The study of dielectric diffuseness in the Ba(Mg1/3Nb2/3)O3-BaTiO3 ceramic system. Smart Mater. Struct. 2006, 15, 1255–1259. [Google Scholar] [CrossRef]
- Huang, Z.H.; Lai, Y.M.; Guan, W.M.; Zeng, Y.M.; Wei, Y.X.; Wu, S.; Han, J.; Mao, Y.Y.; Xiang, Y. Structure, dielectric and ferroelectric properties of lead-free (1-x)(Ba0.85Ca0.15)(Ti0.9Zr0.1)O3-xBa(Mg1/3Nb2/3)O3 ceramics. Mater. Let. 2016, 178, 163–165. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, C.L.; Liu, X.Y.; Li, Y.; Chen, G.H.; Li, X.Q.; Luo, F.H. Electric field-induced strains, conductivities and energy-storage properties in Na1/2Bi1/2TiO3-Ba(Mg1/3Nb2/3)O3 ceramics. J. Mater. Sci. Mater. Electron. 2017, 28, 4788–4795. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Veena, E.; Kumar, N.S.; Naidu, K.C.B.; Mallikarjuna, A.; Basha, D.B. Areview on piezoelectric materials and their applications. Cryst. Res. Technol. 2023, 58, 2200130. [Google Scholar] [CrossRef]
- Liu, G.; Chen, K.; Li, J. Combustion synthesis: An effective tool for preparing inorganic materials. Scr. Mater. 2018, 157, 167–173. [Google Scholar] [CrossRef]
- Liu, G.; Chen, K.; Li, J.; Li, Y.; Zhou, M.; Li, L. Combustion synthesis of Cu2SnSe3 thermoelectric materials. J. Eur. Ceram. Soc. 2016, 36, 1407–1415. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Chen, K. Combustion synthesis of refractory and hard materials: A review. Int. J. Refract. Met. Hard. Mater. 2013, 39, 90–102. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, T.; Ma, J.; Boey, F. Progress in synthesis of ferroelectric ceramic materials via high-energy mechanochemical technique. Prog. Mater. Sci. 2008, 53, 207–322. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Chen, K.; Yang, Z.; He, G.; Chen, Y. Combustion synthesis of TiB2-TiC-WB powders by coupling weak with strong exothermic reactions. Ceram. Int. 2017, 43, 12992–12995. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskay, I.P. Historical retrospective of SHS: An autoreview. J. Self Propag. High. Temp. Synth. 2008, 17, 242–265. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion synthesis of advanced materials: Principles and applications. Adv. Chem. Eng. 1998, 24, 79–226. [Google Scholar]
- Ding, Y.; Wang, Y.; Liu, W.; Pan, Y.; Yang, P.; Meng, D.; Zheng, T.; Wu, J. Shear-structured piezoelectric accelerometers based on KNN lead-free ceramics for vibration monitoring. J. Mater. Chem. C 2024, 10, 1039. [Google Scholar] [CrossRef]
- Chen, Z.H.; Li, Z.W.; Qiu, J.H.; Zhao, T.X.; Ding, J.N.; Jia, X.G.; Zhu, W.Q.; Xu, J.J. Y2O3 doped Ba0.9Ca0.1Ti0.9Sn0.1O3 ceramics with improved piezoelectric properties. J. Eur. Ceram. Soc. 2018, 38, 1349–1355. [Google Scholar]
- Perry, R.H.; Green, D. Perry’s Chemical Engineer’s Handbook; McGraw Hill: Tokyo, Japan, 1984. [Google Scholar]
- Lin, Y.T.; Ou, S.F.; Lin, M.H.; Song, Y.R. Effect of MgO addition on the microstructure and dielectric properties of BaTiO3 ceramics. Ceram. Int. 2018, 44, 3531–3535. [Google Scholar]
- Wang, Y.R.; Pu, Y.P.; Li, X.; Zheng, H.Y. Structural evolution, relaxation behaviors and dielectric properties of BaTiO3-BiAlO3 perovskite solid solutions. J. Mater. Sci. Mater. Electron. 2016, 27, 11565–11571. [Google Scholar] [CrossRef]
- Strathdee, T.; Luisman, L.; Feteira, A.; Reichmann, K. Ferroelectric to relaxor crossover in (1-x) BaTiO3-xBiYbO3 (0 ≤x≤0.08) ceramics. J. Am. Ceram. Soc. 2011, 94, 2292–2295. [Google Scholar] [CrossRef]
- Xie, T.B.; Huang, R.X.; Tan, J.H.; Luo, Y.N.; Lin, H.T.; Dai, Y.J. The impact of co-doping Ce, W, and Mn on the microstructure and piezoelectric performance of high-temperature piezoelectric ceramics based on CaBi2Nb2O9. Ceram. Int. 2024, 50, 17204–17213. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Wang, Z.; Zhang, L.; Chen, P.; Wei, F.; Wang, Y.; Yu, K.; Yan, Y.; Jin, L.; et al. Dielectric, ferroelectric and energy storage properties of lead-free (1−x)Ba0.9Sr0.1TiO3-xBi(Zn0.5Zr0.5)O3 ferroelectric ceramics sintered at lower temperature. Ceram. Int. 2019, 45, 15556–15565. [Google Scholar] [CrossRef]
- Su, H.H.; Hong, C.S.; Tsai, C.C.; Chu, S.Y. Dielectric behaviors of Ba(Mg1/3Nb2/3)O3 modified (Na0.5K0.5)NbO3 ceramics. Ceram. Int. 2018, 44, 7955–7962. [Google Scholar] [CrossRef]
- Luo, Z.; Hao, H.; Chen, C.; Zhang, L.; Yao, Z.; Cao, M.; Emmanuel, M.; Liu, H. Dielectric behaviors of Ba(Mg1/3Nb2/3)O3 modified (Na0.5K0.5)NbO3 ceramics. J. Alloy. Compd. 2019, 794, 358–364. [Google Scholar] [CrossRef]
- Cao, W.W.; Randall, C.A. Grain size and domain size relations in bulk ceramic ferroelectric materials. J. Phys. Chem. Solids 1996, 57, 1499–1505. [Google Scholar] [CrossRef]
- Orihara, H.; Hashimoto, S.; Ishibashi, Y. A theory of D-E hysteresis loop based on the avrami model. J. Phys. Soc. Jpn. 1994, 63, 103–1035. [Google Scholar] [CrossRef]
- Wu, M.; Yao, R.; Jin, C.; Xu, Y.; Xu, J.; He, L.; Yang, Y.; Liu, Y.; Zhong, L.; Gao, J. Significantly enhanced piezoelectric properties of BaTiO3-based ceramics with unchanged curie temperature via local chemical inhomogeneity. Chem. Eng. J. 2025, 518, 164844. [Google Scholar] [CrossRef]
- Tin, G.A.; Puneth, P.; Adhikary, G.D.; Ranjan, R. Simultaneous increase in piezoelectric response and Curie point in BaTiO3 based Pb-free piezoceramic. Scripta Mater. 2024, 243, 115994. [Google Scholar] [CrossRef]
- Keswani, B.C.; Patil, S.I.; James, A.R.; Kolekar, Y.D.; Ramana, C.V. Correlation between structural, ferroelectric, piezoelectric and dielectric properties of Ba0.7Ca0.3TiO3-xBaTi0.8Zr0.2O3 (x = 0.45, 0.55) ceramics. Ceram. Int. 2018, 44, 20921–20928. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).